Laser Fabrication of Gold–sp-Carbon Films
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
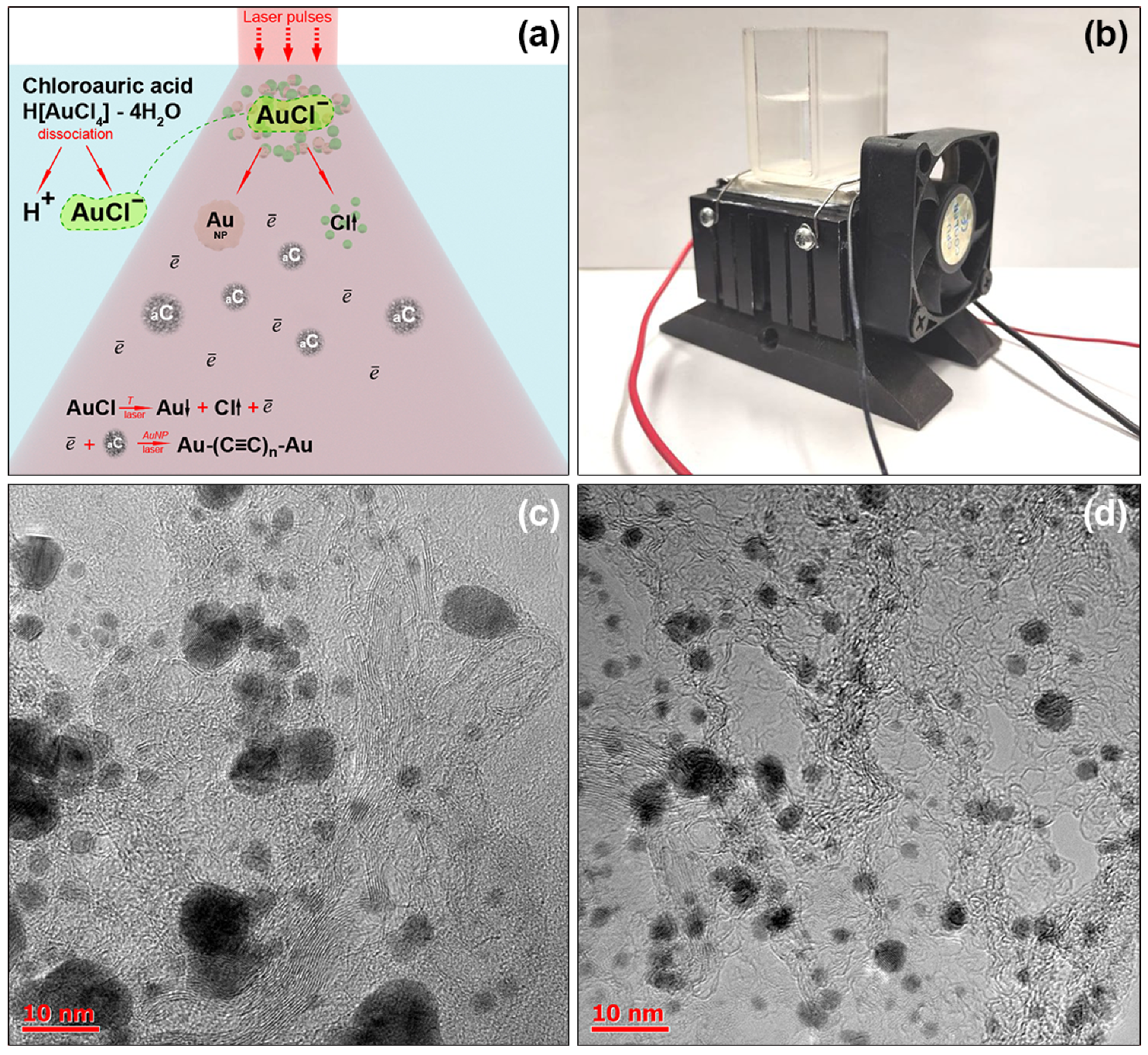
2.2. Linear Chain Formation Method
2.3. Apparatus
3. Results and Discussion
3.1. Characterization of Raman Spectra
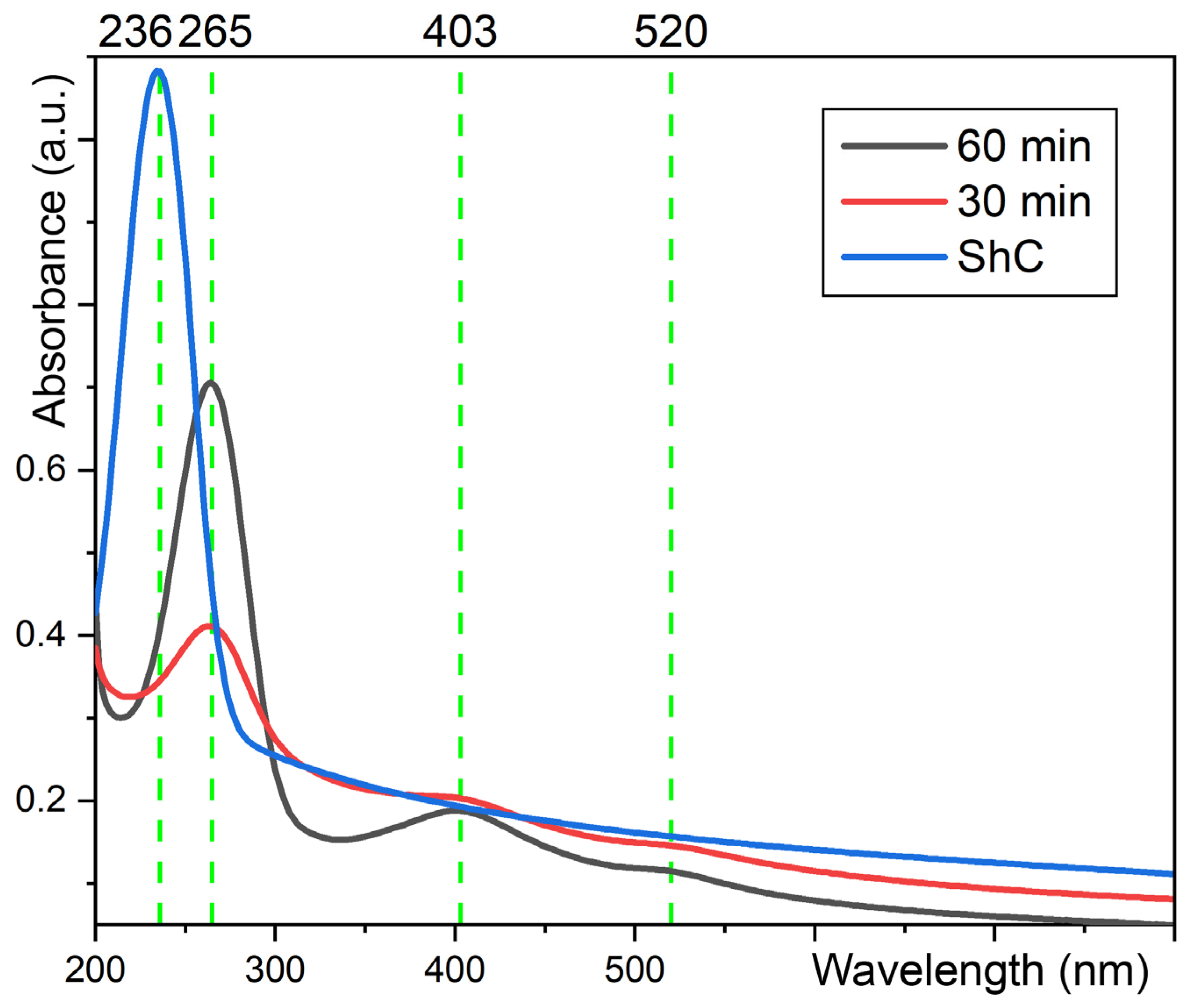
3.2. The Optical Absorption of the Carbyne Bundles Stabilized by Au NPs
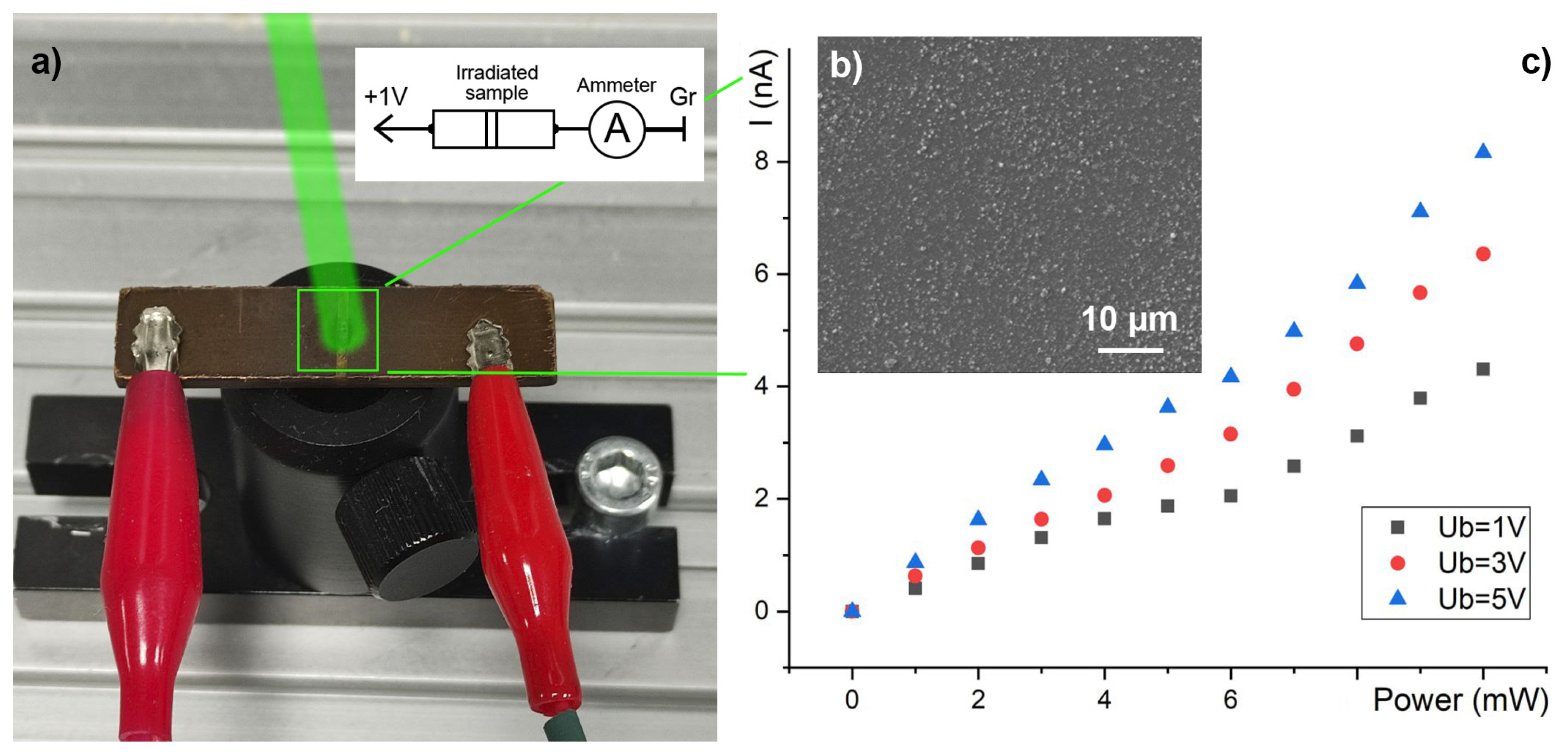
3.3. The Electroconductivity Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, G. Synthesis, properties, and applications of carbyne nanocrystals. Mater. Sci. Eng. R 2022, 151, 100692. [Google Scholar]
- Lv, C.F.; Yang, X.G.; Shan, C.X. One-dimensional sp carbon: Synthesis, properties, and modifications. Chin. Phys. B 2022, 31, 128103. [Google Scholar]
- Pan, B.; Xiao, J.; Li, J.; Liu, P.; Wang, C.; Yang, G. Carbyne with finite length: The one-dimensional sp carbon. Sci. Adv. 2015, 1, e1500857. [Google Scholar] [PubMed]
- Orekhov, N.; Logunov, M. Atomistic structure and anomalous heat capacity of low-density liquid carbon: Molecular dynamics study with machine learning potential. Carbon 2022, 192, 179–186. [Google Scholar]
- Ramberger, B.; Kresse, D. New insights into the 1D carbon chain through the RPA. Phys. Chem. Chem. Phys. 2021, 23, 5254. [Google Scholar]
- Chalifoux, W.A.; Tykwinski, R.R. Synthesis of polyynes to model the sp-carbon allotrope carbyne. Nat. Chem. 2010, 2, 967. [Google Scholar] [PubMed]
- Shi, L.; Rohringer, P.; Suenaga, K.; Niimi, Y.; Kotakoski, J.; Meyer, J.C.; Peterlik, H.; Wanko, M.; Cahangirov, S.; Rubio, A.; et al. Conned linear carbon chains as a route to bulk carbyne. Nat. Mater. 2016, 15, 634–639. [Google Scholar]
- Khanna, R.; Ikram-Ul-Haq, M.; Rawal, A.; Rajarao, R.; Sahajalla, V.; Cayumil, R.; Mikherjee, P.S. Formation of carbyne-like materials during low temperature pyrolysis of lignocellulosic biomass: A natural resource of linear sp carbons. Sci. Rep. 2017, 7, 16832. [Google Scholar] [CrossRef]
- Marabotti, P.; Peggiani, S.; Vidale, A.; Casari, C.S. Pulsed laser ablation in liquid of sp-carbon chains: Status and recent advances. arXiv 2022, arXiv:2206.01238. [Google Scholar]
- Sun, A.; Lauher, J.W.; Goroff, N.S. Preparation of poly(diiododiacetylene), an ordered conjugated polymer of carbon and iodine. Science 2006, 312, 1030–1033. [Google Scholar]
- Zavidovskii, I.A.; Streletskii, O.A.; Nishchak, O.Y.; Khaidarov, A.A.; Pavlikov, A.V. Resistivity of Thin Carbon Films with Different sp-Bonds Fractions. Tech. Phys. 2020, 65, 139–144. [Google Scholar] [CrossRef]
- Samyshkin, V.; Lelekova, A.; Osipov, A.; Bukharov, D.; Skryabin, I.; Arakelian, S.; Kucherik, A.; Kutrovskaya, S. Photosensitive free-standing ultra-thin carbyne-gold films. Opt. Quantum Electron. 2019, 51, 394. [Google Scholar] [CrossRef]
- Kucherik, A.O.; Arakelian, S.M.; Garnov, S.V.; Kutrovskaya, S.V.; Nogtev, D.S.; Osipov, A.V.; Khor’kov, K.S. Two-stage laser-induced synthesis of linear carbon chains. Quantum Electron. 2016, 46, 627. [Google Scholar] [CrossRef]
- Kutrovskaya, S.; Osipov, A.; Baryshev, S.; Zasedatelev, A.; Samyshkin, V.; Demirchyan, S.; Pulci, O.; Grassano, D.; Gontrani, L.; Hartmann, R.; et al. Excitonic Fine Structure in Emission of Linear Carbon Chains. Nano Lett. 2020, 20, 6502–6509. [Google Scholar] [CrossRef] [PubMed]
- Al-Jishi, R.; Dresselhaus, G. Lattice-dynamical model for graphite. Phys. Rev. B 1982, 26, 4514. [Google Scholar]
- Nemanich, R.J.; Solin, S.A. First-and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392. [Google Scholar] [CrossRef]
- Ravagnan, L.; Siviero, F.; Lenardi, C.; Piseri, P.; Barborini, E.; Milani, P.; Casari, C.S.; Bassi, A.L.; Bottani, C.E. Cluster-Beam Deposition and in situ Characterization of Carbyne-Rich Carbon Films. Phys. Rev. Lett. 2002, 89, 285506. [Google Scholar] [CrossRef]
- Kucherik, A.; Arakelian, S.; Vartanyan, T.; Kutrovskaya, S.; Osipov, A.; Povolotskaya, A.; Povolotskii, A.; Man’shina, A. Laser-induced synthesis of metalcarbon materials for implementing surface enhanced Raman scattering. Opt. Spectrosc. 2016, 121, 263–270. [Google Scholar] [CrossRef]
- Rozhkova, N.; Kovalchuk, A.; Goryunov, A.; Borisova, A.; Osipov, A.; Kucherik, A.; Rozhkov, S. Thin film coatings from aqueous dispersion of graphene-based nanocarbon and its hybrids with metal nanoparticles. Coatings 2022, 12, 600. [Google Scholar] [CrossRef]
- Cui, W.; Saito, T.; Ayala, P.; Pichler, T.; Shi, L. Oxidation stability of confined linear carbon chains, carbon nanotubes, and graphene nanoribbons as 1D nanocarbons. Nanoscale 2019, 11, 15253–15258. [Google Scholar] [PubMed]
- Sadezky, A.; Muchkenhuber, H.; Grothe, H.; Niessner, R.; Poschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731. [Google Scholar] [CrossRef]
- Karekin, S.; Esmeryan, D.; Castano, E.C.; Bressler, H.A.; Abolghasemibizaki, M.; Fergusson, P.C.; Roberts, A.; Mohammadi, R. Kinetically driven graphite-like to diamond-like carbon transformation in low temperature laminar diffusion flames. Diam. Relat. Mater 2017, 75, 58. [Google Scholar]
- Sidorov, A.; Leks, E.; Podsvirov, O.; Vinogradov, A. Crystallization and silicon carbide formation in two-layer amorphous silicon-carbon films during electron irradiation. Tech. Phys. 2022, 11, 1475. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 64, 075414. [Google Scholar] [CrossRef]
- Condorelli, M.; D’Urso, L.; Compagnini, G.; Fazio, E.; Neri, F.; Litvinenko, V.; Kurmaev, E.; Zhidkov, I. The catalytic role of platinum nanoparticles in laser generated nanocarbons. Appl. Surf. Sci. 2021, 558, 149890. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.; Pardanaud, C. A guide to and review of the use of multiwavelength Raman spectroscopy for characterizing defective aromatic carbon solids: From graphene to amorphous carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Moura, T.A.; Neves, W.Q.; Alencar, R.S.; Kim, Y.; Endo, M.; Vasconcelos, T.L.; Costa, D.G.; Candiotto, G.; Capaz, R.B.; Araujo, P.T.; et al. Resonance Raman spectroscopy characterization of linear carbon chains encapsulated by multi-walled carbon nanotubes. Carbon 2023, 212, 118123. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Zhou, J.; Liu, R.; Du, R.; Xu, H.; Liu, Z.; Zhang, J.; Liu, Z. Identifying sp–sp2 carbon materials by Raman and infrared spectroscopies. Phys. Chem. Chem. Phys. 2014, 16, 11303–11309. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Barbieri, V.; Facibeni, A.; Russo, V.; Bassi, A.; Lucotti, A.; Tommasini, M.; Tzirakis, M.; Diederich, F.; Casari, C.S. Structure modulated charge transfer in carbon atomic wires. Sci. Rep. 2019, 9, 1648. [Google Scholar] [CrossRef]
- Kutrovskaya, S.; Chestnov, I.; Osipov, A.; Samyshkin, V.; Sapegina, I.; Kavokin, A.; Kucherik, A. Electric field assisted alignment of monoatomic carbon chains. Sci. Rep. 2020, 10, 9709. [Google Scholar] [CrossRef]
- Eisler, S.; Slepkov, A.D.; Elliott, E.; Luu, T.; McDonald, R.; Hegmann, F.A.; Tykwinski, R.R. Polyynes as a Model for Carbyne: Synthesis, Physical Properties, and Nonlinear Optical Response. J. Am. Chem. Soc. 2005, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Arutyunyan, N.; Kononenko, V.; Gololobov, V.; Obraztsova, E. Resonant Effects in SERS Spectra of Linear Carbon Chains. Phys. Status Solidi B 2017, 255, 1700254. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavokina, S.; Osipov, A.; Samyshkin, V.; Abramov, A.; Rozhkova, N.; Kononenko, V.; Konov, V.; Kucherik, A. Laser Fabrication of Gold–sp-Carbon Films. Condens. Matter 2023, 8, 96. https://doi.org/10.3390/condmat8040096
Kavokina S, Osipov A, Samyshkin V, Abramov A, Rozhkova N, Kononenko V, Konov V, Kucherik A. Laser Fabrication of Gold–sp-Carbon Films. Condensed Matter. 2023; 8(4):96. https://doi.org/10.3390/condmat8040096
Chicago/Turabian StyleKavokina, Stella, Anton Osipov, Vlad Samyshkin, Andrey Abramov, Natalia Rozhkova, Vitali Kononenko, Vitali Konov, and Alexey Kucherik. 2023. "Laser Fabrication of Gold–sp-Carbon Films" Condensed Matter 8, no. 4: 96. https://doi.org/10.3390/condmat8040096
APA StyleKavokina, S., Osipov, A., Samyshkin, V., Abramov, A., Rozhkova, N., Kononenko, V., Konov, V., & Kucherik, A. (2023). Laser Fabrication of Gold–sp-Carbon Films. Condensed Matter, 8(4), 96. https://doi.org/10.3390/condmat8040096






