A Review of Methane Gas Detection Sensors: Recent Developments and Future Perspectives
Abstract
:1. Introduction
2. Optical Sensors
Recent Research Developments
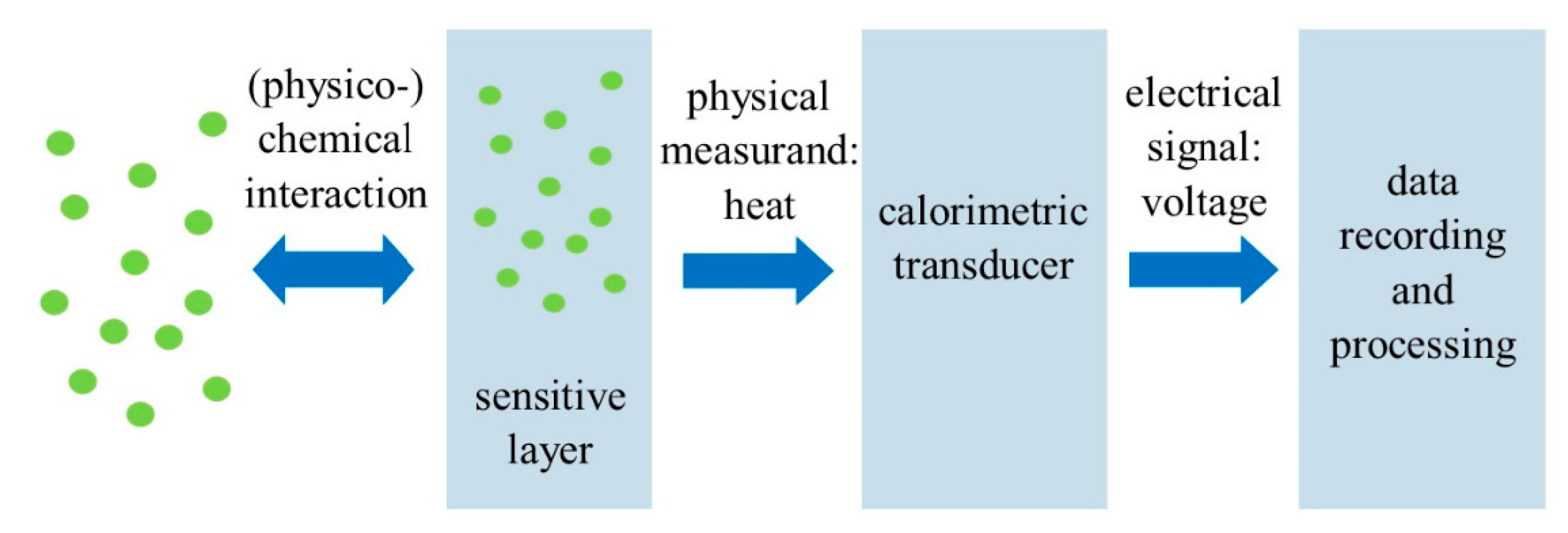
3. Calorimetric Sensors
Recent Research Developments
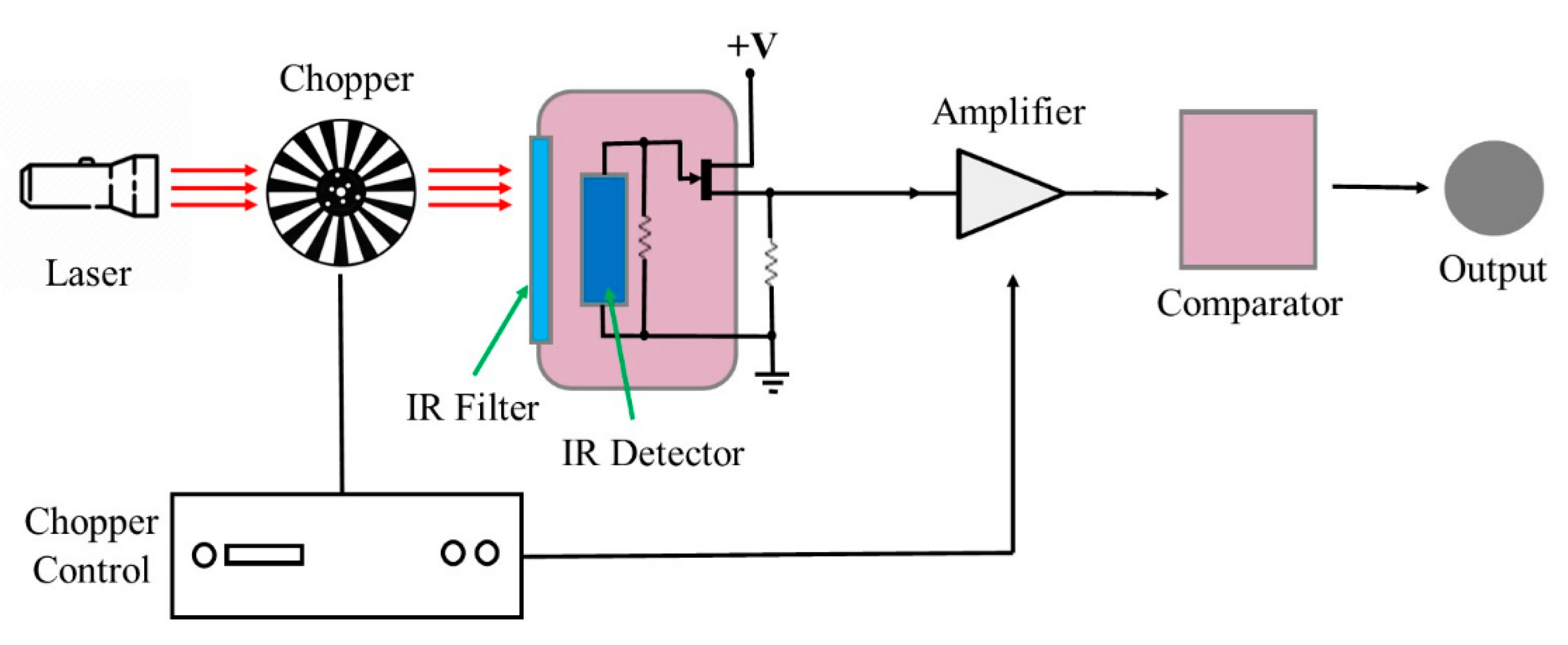
4. Pyroelectric Sensors
Recent Research Developments
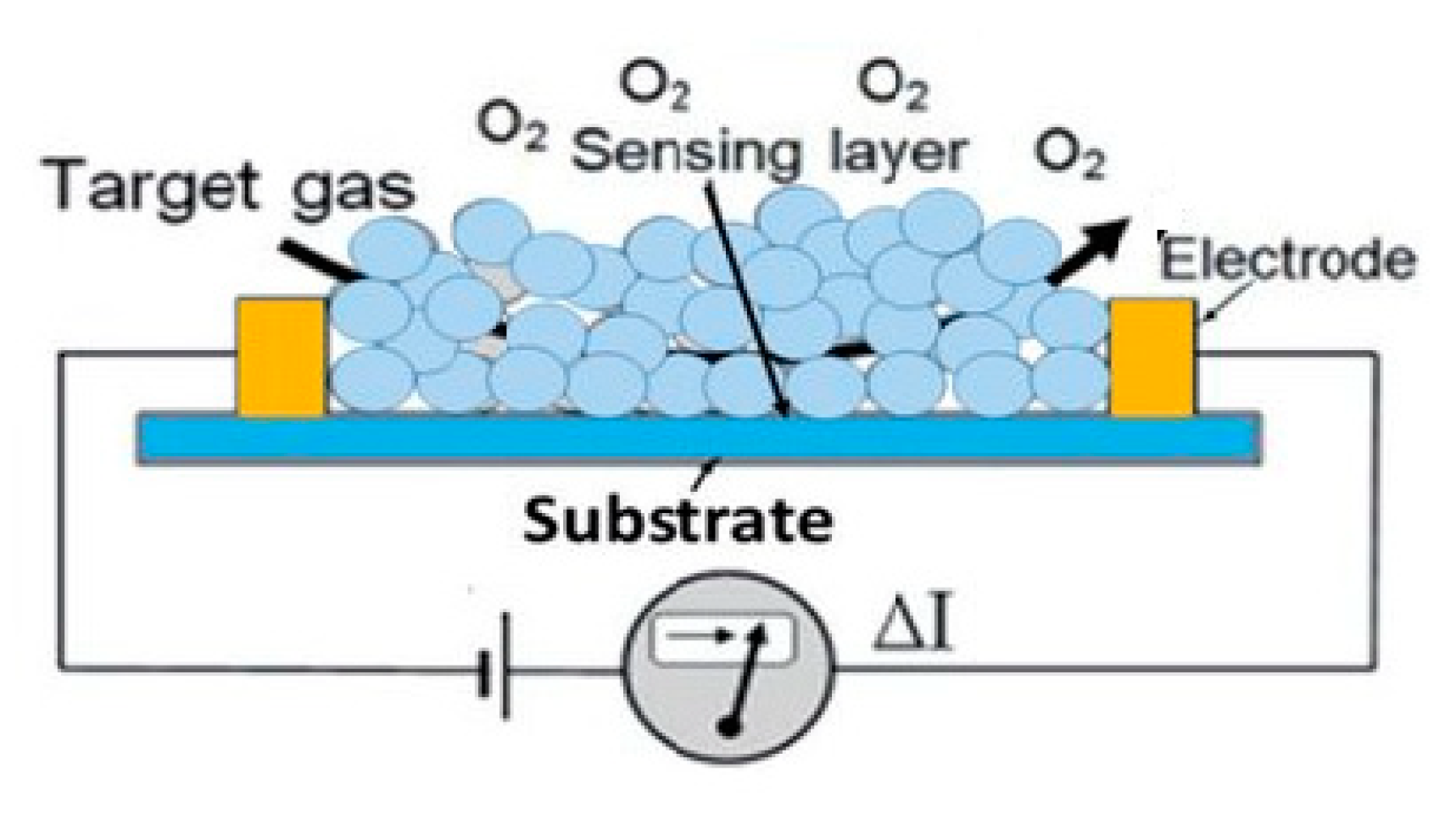
5. Semiconducting Metal Oxide Sensors
Recent Research Developments
6. Electrochemical Sensors
Recent Research Developments
7. Comparison of Methane Sensors and Discussion of Future Challenges
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Methane Initiative. Global Methane Emissions and Mitigation Opportunities. 2011. Available online: www.globalmethane.org (accessed on 12 December 2019).
- Turner, A.J.; Frankenberg, C.; Kort, E.A. Interpreting contemporary trends in atmospheric methane. Proc. Nat. Acad. Sci. USA 2019, 116, 2805–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Government of Canada. About Methane Emissions. 2014. Available online: https://www.canada.ca/en/environment-climate-change/services/climate-change/global-methane-initiative/about-methane-emissions.html (accessed on 23 November 2019).
- Stocker, T.F.; Dahe, Q.; Plattner, G.K.; Tignor, M.B.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P. Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Tran, M.-K.; Fowler, M. A Review of Lithium-Ion Battery Fault Diagnostic Algorithms: Current Progress and Future Challenges. Algorithms 2020, 13, 62. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo, P.; Griffin, W.M.; Matthews, H.S. Comparative analysis of the production costs and life-cycle GHG emissions of FT liquid fuels from coal and natural gas. Environ. Sci. Technol. 2008, 42, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagarin, H.; Sridhar, S.; Lange, I.; Bazilian, M. Considering non-power generation uses of coal in the United States. Renew. Sustain. Energy Rev. 2020, 124, 109790. [Google Scholar] [CrossRef]
- Maroufmashat, A.; Fowler, M. Transition of future energy system infrastructure; through power-to-gas pathways. Energies 2017, 10, 1089. [Google Scholar] [CrossRef] [Green Version]
- EIA. How Much Carbon Dioxide Is Produced When Different Fuels are Burned? 2018. Available online: https://www.eia.gov/tools/faqs/faq.php?id1/473&t1/411 (accessed on 25 November 2019).
- King, G.E.; King, D.E. Environmental risk arising from well-construction failure—Differences between barrier and well failure, and estimates of failure frequency across common well types, locations, and well Age. SPE Prod. Oper. 2013, 28, 323–344. [Google Scholar]
- Bachu, S. Analysis of gas leakage occurrence along wells in Alberta, Canada, from a GHG perspective—Gas migration outside well casing. Int. J. Greenh. Gas Control 2017, 61, 146–154. [Google Scholar] [CrossRef]
- Olmer, N.; Comer, B.; Roy, B.; Mao, X.; Rutherford, D. Greenhouse Gas Emissions from Global Shipping, 2013–2015. The International Council on Clean Transportation 2017. Available online: https://www.theicct.org/publications/GHG-emissions-globalshipping-2013-2015 (accessed on 25 November 2019).
- Ingraffea, A.; Wawrzynek, P.A.; Santoro, R.; Wells, M.T. Reported methane emissions from active oil and gas wells in pennsylvania, 2014–2018. Environ. Sci. Technol. 2020, 54, 5783–5789. [Google Scholar] [CrossRef]
- Wisen, J.; Chesnaux, R.; Werring, J.; Wendling, G.; Baudron, P.; Barbecot, F. A portrait of wellbore leakage in northeastern British Columbia, Canada. Proc. Natl. Acad. Sci. USA 2020, 117, 913–922. [Google Scholar] [CrossRef]
- Government of Canada. Federal Regulations to Reduce Methane Emissions in the Oil and Gas Sector. Available online: https://www.canada.ca/en/environment-climate-change/news/2018/04/federal-regulations-to-reduce-methane-emissions-in-the-oil-and-gas-sector.html#targetText=Greenhouse%20gas%20emission%20reductions,the%20road%20for%20a%20year (accessed on 10 December 2019).
- Lu, H.; Iseley, T.; Behbahani, S.; Fu, L. Leakage detection techniques for oil and gas pipelines: State-of-the-art. Tunn. Undergr. Space Technol. 2020, 98, 103249. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, B.; Yu, C.; Rao, Y. Optical graphene gas sensors based on microfibers: A review. Sensors 2018, 18, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korotcenkov, G. Handbook of Gas Sensor Materials: Properties, Advantages and Shortcomings for Applications Volume 1: Conventional Approaches; Springer New York; Springer e-books; Imprint; Springer: New York, NY, USA, 2013. [Google Scholar]
- Jaaniso, R.; Tan, O.K. Semiconductor Gas Sensors; Woodhead Pub: Cambridge, UK, 2013. [Google Scholar]
- Spain, E.; Venkatanarayanan, A. Review of physical principles of sensing and types of sensing materials. In Sensor Materials, Technologies and Applications; ScienceDirect: Dublin, Ireland, 2014; pp. 5–46. [Google Scholar]
- Triki, M.; Nguyen, B.T.; Vicet, A. Compact sensor for methane detection in the mid infrared region based on quartz enhanced photoacoustic spectroscopy. Infrared Phys. Technol. 2015, 69, 74–80. [Google Scholar] [CrossRef]
- Butt, M.A.; Degtyarev, S.A.; Khonina, S.N.; Kazanskiy, N.L. An evanescent field absorption gas sensor at mid-IR 3.39 μm wavelength. J. Mod. Opt. 2017, 64, 1892–1897. [Google Scholar] [CrossRef]
- Massie, C.; Stewart, G.; McGregor, G.; Gilchrist, J.R. Design of a portable optical sensor for methane gas detection. Sens. Actuators B Chem. 2016, 113, 830–836. [Google Scholar] [CrossRef]
- Gershon, J.S.; Ballinger, J.T. Spectroscopy. In Chemical Technicians’ Ready Reference Handbook, 5th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors. Anal. Chem. 2002, 74, 2663–2678. [Google Scholar] [CrossRef] [PubMed]
- Shemshad, J.; Aminossadati, S.M.; Kizil, M.S. A review of developments in near infrared methane detection based on tunable diode laser. Sens. Actuators B Chem. 2012, 171, 77–92. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, L.; Huang, J.; Tao, C.; Li, X.; Chen, W. Sensitivity enhancing of transition mode long-period fiber grating as methane sensor using high refractive index polycarbonate/cryptophane A overlay deposition. Sens. Actuators B Chem. 2015, 207, 477–480. [Google Scholar] [CrossRef]
- Ryoko, Y.; Masaki, K.; Koji, F.; Tadashi, S.; Yoshihisa, S. Highly sensitive laser based trace-gas sensor technology and its application to stable isotope ratio analysis. NTT Tech. Rev. 2014, 12, 4. [Google Scholar]
- Zheng, C.; Ye, W.; Sanchez, N.P.; Li, C.; Dong, L.; Wang, Y.; Griffin, R.J.; Tittel, F.K. Development and field deployment of a mid-infrared methane sensor without pressure control using interband cascade laser absorption spectroscopy. Sens. Actuators B Chem. 2017, 244, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Yin, W.; Ma, W.; Zhang, L.; Jia, S. High-sensitivity, large dynamic range, auto-calibration methane optical sensor using a short confocal Fabry–Perot cavity. Sens. Actuators B Chem. 2007, 127, 350–357. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Ding, E.J.; Xu, S.C.; Wang, X.X.; Song, F. Sensitization of an Optical Fiber Methane Sensor with graphene. Opt. Fiber Technol. 2017, 37, 26–29. [Google Scholar] [CrossRef]
- Tombez, L.; Zhang, E.J.; Orcutt, J.S.; Kamlapurkar, S.; Green, W.M.J. Methane absorption spectroscopy on a silicon photonic chip. Optica 2017, 4, 1322. [Google Scholar] [CrossRef]
- Campanella, C.E.; de Carlo, M.; Cuccovillo, A.; de Leonardis, F.; Passaro, V.M.N. Methane gas photonic sensor based on resonant coupled cavities. Sensors 2019, 19, 5171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismaeel, R.; Beaton, A.; Donko, A.; Talataisong, W.; Lee, T.; Brotin, T.; Brambilla, G. high sensitivity all-fibre methane sensor with gas permeable Teflon/Cryptophane-A membrane. In Proceedings of the 2019 Conference on Lasers and Electro-Optics Europe & European Quantum Electronics Conference (CLEO/Europe-EQEC), Munich, Germany, 23–27 June 2019; p. 1. [Google Scholar]
- Huang, X.; Sheng, P.; Tu, Z.; Zhang, F.; Wang, J.; Geng, H.; Zou, Y.; Di, C.; Yi, Y.; Sun, Y.; et al. A two-dimensional π–d conjugated coordination polymer with extremely high electrical conductivity and ambipolar transport behaviour. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Pennetta, R.; Russell, P.S.J. Self-alignment of glass fiber nanospike by optomechanical back-action in hollow-core photonic crystal fiber. Optica 2016, 3, 277–282. [Google Scholar] [CrossRef]
- Kohl, C.D.; Wagner, T. Gas Sensing Fundamentals; Springer: Berlin/Heidelberg, Germany, 2014; Volume 15. [Google Scholar]
- Chou, J. Hazardous Gas Monitors: A Practical Guide to Selection, Operation and Applications; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Park, N.H.; Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. Calorimetric thermoelectric gas sensor for the detection of hydrogen, methane and mixed gases. Sensors 2014, 14, 8350–8362. [Google Scholar] [CrossRef]
- Dücso, C.; Ádám, M.; Fürjes, P.; Hirschfelder, M.; Kulinyi, S.; Bársony, I. Explosion-proof monitoring of hydrocarbons by mechanically stabilised, integrable calorimetric microsensors. Sens. Actuators B Chem. 2003, 95, 189–194. [Google Scholar]
- Yamauchi, S. Chemical Sensor Technology; Kodansha; Elsevier Science: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Schierbaum, K.D.; Weimar, U.; Göpel, W.; Kowalkowski, R. Conductance, work function and catalytic activity of SnO2-based gas sensors. Sens. Actuators B Chem. 1991, 3, 205–214. [Google Scholar] [CrossRef]
- Ho, C.K.; Itamura, M.T.; Kelley, M.J.; Hughes, R.C. Review of chemical sensors for in-situ monitoring of volatile contaminants. Sandia Rep. 2001, 28. [Google Scholar] [CrossRef] [Green Version]
- Sberveglieri, G. Gas Sensors: Principles, Operation and Developments; Springer International Publishing: Cham, Switzerland, 1992. [Google Scholar]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, A.M.; Akbar, S.A.; Mhaisalkar, S.G.; Birkefeld, L.D.; Goto, K.S. Solid-state gas sensors—A review. J. Electrochem. Soc. 1992, 139, 3690–3704. [Google Scholar] [CrossRef]
- Krebs, P.; Grisel, A. A low-power integrated catalytic gas sensor. Sens. Actuators B Chem. 1993, 13, 155–158. [Google Scholar] [CrossRef]
- Bíró, F.; Dücső, C.; Radnóczi, G.Z.; Baji, Z.; Takács, M.; Bársony, I. ALD nano-catalyst for micro-calorimetric detection of hydrocarbons. Sens. Actuators B Chem. 2017, 247, 617–625. [Google Scholar] [CrossRef]
- Alpert, B.; Ferri, E.; Bennett, D. Algorithms for identification of nearly-coincident events in calorimetric sensors. J. Low Temp. Phys. 2016, 184, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Gardner, E.L.; Luca, A.D.; Falco, C.; Udrea, F. Geometrical optimisation of diode-based calorimetric thermal flow sensors through multiphysics finite element modelling. Proceedings 2017, 1, 280. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.; Zheng, X.; Zhang, Y.; Wang, R. The Designed MEMS Methane Sensor Based on Pulse Power Supply. IOP Conf. Ser.: Earth Environ. Sci. 2019, 300, 42029. [Google Scholar] [CrossRef]
- Fraden, J. Handbook of Modern Sensors: Physics, Designs, and Applications, 5th ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Jun, L.; Qiulin, T.; Wendong, Z.; Chenyang, X.; Tao, G.; Jijun, X. Miniature low-power IR monitor for methane detection. Measurement 2011, 44, 823–831. [Google Scholar] [CrossRef]
- Alexander, B. Mathematical Processing of a Pyroelectric Detector; Ivanchenko: Volodymyr Dahl, East Ukrainian, 2019. [Google Scholar]
- Yukinori, Y.; Toshiaki, S.; Kenichi. The pyroelectric sensor. Jpn. J. Appl. Phys. 1981, 20, 221–224. [Google Scholar]
- Dorojkine, L.M. The non-catalytic thermal wave-based chemical gas sensor for methane and natural gas. Sens. Actuators B Chem. 2003, 89, 76–85. [Google Scholar] [CrossRef]
- Qiu-lin, T.; Wen-dong, Z.; Chen-yang, X.; Ji-jun, X.; Jun, L.; Jun-hong, L.; Ting, L. Design, fabrication and characterization of pyroelectric thin film and its application for infrared gas sensors. Microelectron. J. 2009, 40, 58–62. [Google Scholar] [CrossRef]
- Shikha, S.; Zheng, X.; Zhang, Y. Upconversion nanoparticles-encoded hydrogel microbeads-based multiplexed protein detection. Nano-Micro Lett. 2018, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Song, M.K.; Rhee, H.W. Ultraviolet-cured polyethylene glycol diacrylate/polyvinylidene fluoride blend gel polymer electrolytes. Electrochem. Solid-State Lett. 2001, 4, A105. [Google Scholar] [CrossRef]
- Acton, Q.A. Ethylene Glycols-Advances in Research and Application; Scholarly Editions: Atlanta, GA, USA, 2013. [Google Scholar]
- Song, M.K.; Kim, Y.T.; Kim, Y.T.; Cho, B.W.; Popov, B.N.; Rhee, H.W. Thermally stable gel polymer electrolytes. J. Electrochem. Soc. 2003, 150, A439. [Google Scholar] [CrossRef] [Green Version]
- Wamer, J.H.; Schaffel, F.; Rummeli, M.; Bachmatiuk, A. Graphene: Fundamentals and Emergent Applications; Elsevier: Kidlington, Oxford, UK, 2012. [Google Scholar]
- Querner, Y.; Norkus, V.; Gerlach, G. High-sensitive pyroelectric detectors with internal thermal amplification. Sens. Actuators B Chem. 2011, 172, 169–174. [Google Scholar] [CrossRef]
- Dong, M.; Zheng, C.; Miao, S.; Zhang, Y.; Du, Q.; Wang, Y.; Tittel, F. Development and measurements of a mid-infrared multi-gas sensor system for CO, CO2 and CH4 Detection. Sensors 2017, 17, 2221. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; He, Q.; Zheng, C.; Wang, Y. Development of a portable mid-infrared methane detection device. Optoelectron. Lett. 2017, 13, 100–103. [Google Scholar] [CrossRef]
- Hu, L.; Zheng, C.; Zhang, M.; Yao, D.; Zheng, J.; Zhang, Y.; Wang, Y.; Tittel, F.K. Quartz-enhanced photoacoustic spectroscopic methane sensor system using a quartz tuning fork-embedded, double-pass and off-beam configuration. Photoacoustics 2020, 18, 100174. [Google Scholar] [CrossRef]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A New detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Prabakaran, S.; John, B.; Balaguru, R. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases. A review. Sci. Lett. J. 2015, 4, 1262015. [Google Scholar]
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-oxide-semiconductor based gas sensors: Screening, preparation, and integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Rajaković, L.; Milosavljević, D. Possibilities of application of piezoelectric sensors for the detection of explosives based on nitroaromatic compounds. Vojnoteh. Glas. 1996, 44, 570–576. [Google Scholar] [CrossRef]
- Kanan, M.S.; El-Kadri, M.O.; Abu-Yousef, A.I.; Kanan, C.M. Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef] [Green Version]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Basu, S. Multilayer Thin Films: Versatile Applications for Materials Engineering; IntechOpen: London, UK, 2020. [Google Scholar]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [Green Version]
- Xue, D.; Wang, P.; Zhang, Z.; Wang, Y. Enhanced methane sensing property of flower-like SnO2 doped by Pt nanoparticles: A combined experimental and first-principle study. Sens. Actuators B Chem. 2019, 296, 126710. [Google Scholar] [CrossRef]
- Ghanbari, R.; Safaiee, R.; Sheikhi, M.H.; Golshan, M.M.; Horastani, Z.K. Graphene decorated with silver nanoparticles as a low-temperature methane gas sensor. ACS Appl. Mater. Interfaces 2019, 11, 21795–21806. [Google Scholar] [CrossRef]
- Fedorenko, G.; Oleksenko, L.; Maksymovych, N.; Skolyar, G.; Ripko, O. Semiconductor gas sensors based on Pd/SnO2 nanomaterials for methane detection in air. Nanoscale Res. Lett. 2017, 12, 329. [Google Scholar] [CrossRef] [Green Version]
- Oleksenko, L.P.; Fedorenko, G.V.; Maksymovych, N.P. Highly sensitive to methane sensor materials based on Nano-Pd/SnO2. Theor. Exp. Chem. 2019, 55, 1–5. [Google Scholar] [CrossRef]
- Oleksenko, L.P.; Fedorenko, G.V.; Maksymovych, N.P. Platinum-containing adsorption-semiconductor sensors based on nanosized tin dioxide for methane detection. Theor. Exp. Chem. 2017, 53, 259–264. [Google Scholar] [CrossRef]
- Moalaghi, M.; Gharesi, M.; Ranjkesh, A.; Hossein-Babaei, F. Tin oxide gas sensor on tin oxide microheater for high-temperature methane sensing. Mater. Lett. 2019, 263, 127196. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Moharram, A.H.; Abdel-Rahim, M.A. Promising methane gas sensor synthesized by microwave-assisted Co3O4 nanoparticles. Mater. Sci. Semicond. Process. 2016, 46, 1–5. [Google Scholar] [CrossRef]
- Stoytcheva, M.; Zlatev, R. Electrochemical sensors for environmental analysis. In Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; pp. 1–515. [Google Scholar]
- Coillier, A. Gas Detection—An Introduction to Electrochemical Sensor Technology 2015. Available online: https://www.azosensors.com/article.aspx?ArticleID=618 (accessed on 21 December 2019).
- Tsiplakides, D. Electrochemical sensor of gaseous contaminants. In Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; pp. 379–893. [Google Scholar]
- Mari, C.M.; Barbi, G.B. Electrochemical Gas Sensors. In Gas Sensors: Principles, Operation and Developments; Springer International Publishing: Cham, Switzerland, 1992; pp. 329–364. [Google Scholar]
- Wang, Z.; Kumar, A.; Sevilla, M.D.; Zeng, X. Anaerobic oxidation of methane to methyl radical in NTf2-based ionic liquids. J. Phys. Chem. C 2016, 120, 13466–13473. [Google Scholar] [CrossRef]
- Chaudoy, V.; Ghamouss, F.; Luais, E.; Tran-Van, F. Cross-Linked polymer electrolytes for li-based batteries: From solid to gel electrolytes. Ind. Eng. Chem. Res. 2016, 55, 9925–9933. [Google Scholar] [CrossRef]
- Willem, L.; Driessen, J.; Dunbar, K.; Pence, L. Solid solvates: The use of weak ligands in coordination chemistry. Inorg. Synth. 1992, 29, 111–118. [Google Scholar]
- Zhang, J.X.J.; Hoshino, K. Molecular Sensors and Nanodevices 2e: Principles, Designs and Applications in Biomedical Engineering, 2nd ed.; Elsevier Science & Technology: San Diego, CA, USA, 2018. [Google Scholar]
- Fleischer, M.; Meixner, H. Sensitive, selective and stable CH4 detection using semiconducting Ga2O3 thin films. Sens. Actuators B Chem. 1995, 26, 81–84. [Google Scholar] [CrossRef]
- Otagawa, T. Electrochemical oxidation of methane in nonaqueous electrolytes at room temperature. J. Electrochem. Soc. 1985, 132, 2951. [Google Scholar] [CrossRef]
- Hsieh, S.Y. Anodic oxidation of methane. J. Electrochem. Soc. 1977, 124, 1171. [Google Scholar] [CrossRef]
- Weingärtner, H. An introduction to aqueous electrolyte solutions. ChemPhysChem 2008, 9, 1482. [Google Scholar] [CrossRef]
- Ashcroft, A.; Cheetham, A.; Green, M. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, M.; Baker, G.A.; Stetter, J.R.; Lin, L.; Mason, A.J.; Zeng, X. Methane–oxygen electrochemical coupling in an ionic liquid: A robust sensor for simultaneous quantification. Analyst 2014, 139, 5140–5147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, X.; Wang, Z.; Guo, M.; Zeng, X.; Mason, A.J. Fabrication of a miniaturized room temperature ionic liquid gas sensor for human health and safety monitoring. In Proceedings of the 2012 IEEE Biomedical Circuits and Systems Conference (BioCAS), Zhubei City, Taiwan, 28–30 November 2012. [Google Scholar]
- Rehman, A.; Zeng, X. Ionic liquids as green solvents and electrolytes for robust chemical sensor development. Acc. Chem. Res. 2012, 45, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Dosi, M.; Lau, I.; Zhuang, Y.; Simakov, D.S.A.; Fowler, M.W.; Pope, M.A. Ultra-sensitive electrochemical methane sensors based on solid polymer electrolyte-infused laser-induced graphene. ACS Appl. Mater. Interfaces 2019, 11, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.T.; Stetter, J.R.; Findlay, M.W.; Patel, V. Printed amperometric gas sensors. ECS Trans. 2013, 50, 211–220. [Google Scholar] [CrossRef]
- Yao, S.; Stetter, J.R. Solid Electrolyte Sensors for Emissions Monitoring; BCPS Department, Chemistry, Illinois Institute of Technology: Chicago, IL, USA, 2012. [Google Scholar]
- Nádherná, M.; Opekar, F.; Reiter, J. Ionic liquid–polymer electrolyte for amperometric solid-state NO2 sensor. Electrochim. Acta 2011, 56, 5650–5655. [Google Scholar] [CrossRef]
- Narayanan, B.K.; Akbar, S.A.; Dutta, P.K. A phosphate-based proton conducting solid electrolyte hydrocarbon gas sensor. Sens. Actuators B Chem. 2002, 87, 480–486. [Google Scholar] [CrossRef]
- Alberti, G.; Carbone, A.; Palombari, R. Solid state potentiometric sensor at medium temperatures (150–300 °C) for detecting oxidable gaseous species in air. Sens. Actuators B Chem. 2001, 75, 125–128. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, X. Bis(trifluoromethylsulfonyl)imide (NTf2)-Based ionic liquids for facile methane electro-oxidation on Pt. J. Electrochem. Soc. 2013, 160, H604–H611. [Google Scholar] [CrossRef]
- Yin, H.; Wan, H.; Lin, L.; Zeng, X.; Mason, A.J. Miniaturized planar RTIL-based electrochemical gas sensor for real-time point-of-exposure monitoring. In Proceedings of the 2016 IEEE Healthcare Innovation Point-Of-Care Technologies Conference (HI-POCT), Cancun, Mexico, 9–11 November 2016. [Google Scholar]
- Wan, H.; Yin, H.; Lin, L.; Zeng, X.; Mason, A.J. Miniaturized planar room temperature ionic liquid electrochemical gas sensor for rapid multiple gas pollutants monitoring. Sens. Actuators B Chem. 2018, 255, 638–646. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Kysar, J.; Brosha, E.L.; Kreller, C.R. Development and testing of an electrochemical methane sensor. Sens. Actuators B Chem. 2016, 228, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Gross, P.A.; Jaramillo, T.; Pruitt, B. Cyclic-Voltammetry-Based Solid-State Gas Sensor for Methane and Other VOC Detection. Anal. Chem. 2018, 90, 6102–6108. [Google Scholar] [CrossRef]
- Yang, B.; Xu, J.; Wang, C.; Xiao, J. A potentiometric sensor based on SmMn2O5 sensing electrode for methane detection. Mater. Chem. Phys. 2020, 245, 122679. [Google Scholar] [CrossRef]
- Favard, A.; Yan, X.; Anguille, S.; Moulin, P.; Seguin, J.L.; Aguir, K.; Bendahan, M. Sensors & transducers humidity impact reduction on WO 3 gas microsensor response using new filters based on ionic liquid. In Proceedings of the Third International Conference on Advances in Sensors, Rome, Italy, 25–29 March 2018. [Google Scholar]
- Vasiliev, A.A.; Varfolomeev, A.E.; Volkov, I.A.; Simonenko, N.P.; Arsenov, P.V.; Vlasov, I.S.; Ivanov, V.V.; Pislyakov, A.V.; Lagutin, A.S.; Jahatspanian, I.E.; et al. Reducing humidity response of gas sensors for medical applications: Use of spark discharge synthesis of metal oxide nanoparticles. Sensors 2018, 18, 2600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, W.; You, L.; Yuan, G.; Zhao, Y.; Jiang, Z. Back propagation neural network model for temperature and humidity compensation of a non-dispersive infrared methane sensor. Instrum. Sci. Technol. 2013, 41, 608–618. [Google Scholar] [CrossRef]


| Methane Sensor Types | Working Mechanisms | Advantages | Disadvantages | Related Research |
|---|---|---|---|---|
| Optical sensors | Detect changes in light waves that result from an interaction of the analyte with the receptor part. | Non-destructive method; Immune to electromagnetic interference; Operate without oxygen. | High cost; High power consumption; Lack of significance and distinctiveness of methane optical absorption region. | [28,30,31,32,33,34,35] |
| Calorimetric sensors | Measure the heat produced from a reaction and correlate the value to the reactant concentration. | Low cost; Simplistic design; Portable; Easy to manufacture; Good selectivity for methane; Can operate in harsh environmental conditions. | Low detection accuracy; Susceptible to cracking, catalyst poisoning and oversaturation; High power consumption; Short lifespan; Require high temperature. | [40,48,49,50,51,52] |
| Pyroelectric sensors | Convert thermal energy into electrical energy based on the phenomenon of pyroelectricity. | Non-destructive; Operate without oxygen; Good sensitivity and responsivity; Wide measuring range; Operate at room temperature. | High cost; High power consumption; Immobile; Difficult to manufacture. | [57,58,64,65,66,67] |
| Semiconducting metal oxide sensors | Absorption of gas on the surface of a metal oxide changes its conductivity, which is then quantified to obtain the gas concentration. | Low cost; Lightweight and robust; Long lifespan; Resistant to poisoning. | Poor selectivity; Small and high operational temperature range; Slow recovery rate; Significant additive dependency; Affected by temperature; Susceptible to degradation; Sensitive to changes in humidity | [77,78,79,80,81,82,83] |
| Electrochemical sensors | Measure the target gas concentration by oxidizing or reducing the gas at an electrode and measuring the resulting current. | AE-based: Low cost. IL-based: Non-hazardous materials; High boiling points and low volatility; Good selectivity for methane; Can detect small leaks. SE-based: No leakage; Safe; Robust; Good selectivity for methane; Can detect small leaks. | AE-based: Susceptible to leakage and evaporation; Hazardous materials; Slow response time. IL-based: Susceptible to leakage; Slow response time. SE-based: Require high temperature; Unable to detect low gas concentrations; Susceptible to degradation or loss of electrolyte. | [97,100,106,107,108,109,110,111] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldhafeeri, T.; Tran, M.-K.; Vrolyk, R.; Pope, M.; Fowler, M. A Review of Methane Gas Detection Sensors: Recent Developments and Future Perspectives. Inventions 2020, 5, 28. https://doi.org/10.3390/inventions5030028
Aldhafeeri T, Tran M-K, Vrolyk R, Pope M, Fowler M. A Review of Methane Gas Detection Sensors: Recent Developments and Future Perspectives. Inventions. 2020; 5(3):28. https://doi.org/10.3390/inventions5030028
Chicago/Turabian StyleAldhafeeri, Tahani, Manh-Kien Tran, Reid Vrolyk, Michael Pope, and Michael Fowler. 2020. "A Review of Methane Gas Detection Sensors: Recent Developments and Future Perspectives" Inventions 5, no. 3: 28. https://doi.org/10.3390/inventions5030028
APA StyleAldhafeeri, T., Tran, M.-K., Vrolyk, R., Pope, M., & Fowler, M. (2020). A Review of Methane Gas Detection Sensors: Recent Developments and Future Perspectives. Inventions, 5(3), 28. https://doi.org/10.3390/inventions5030028







