Contactless Blood Pressure Estimation System Using a Computer Vision System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Ethics and Participants
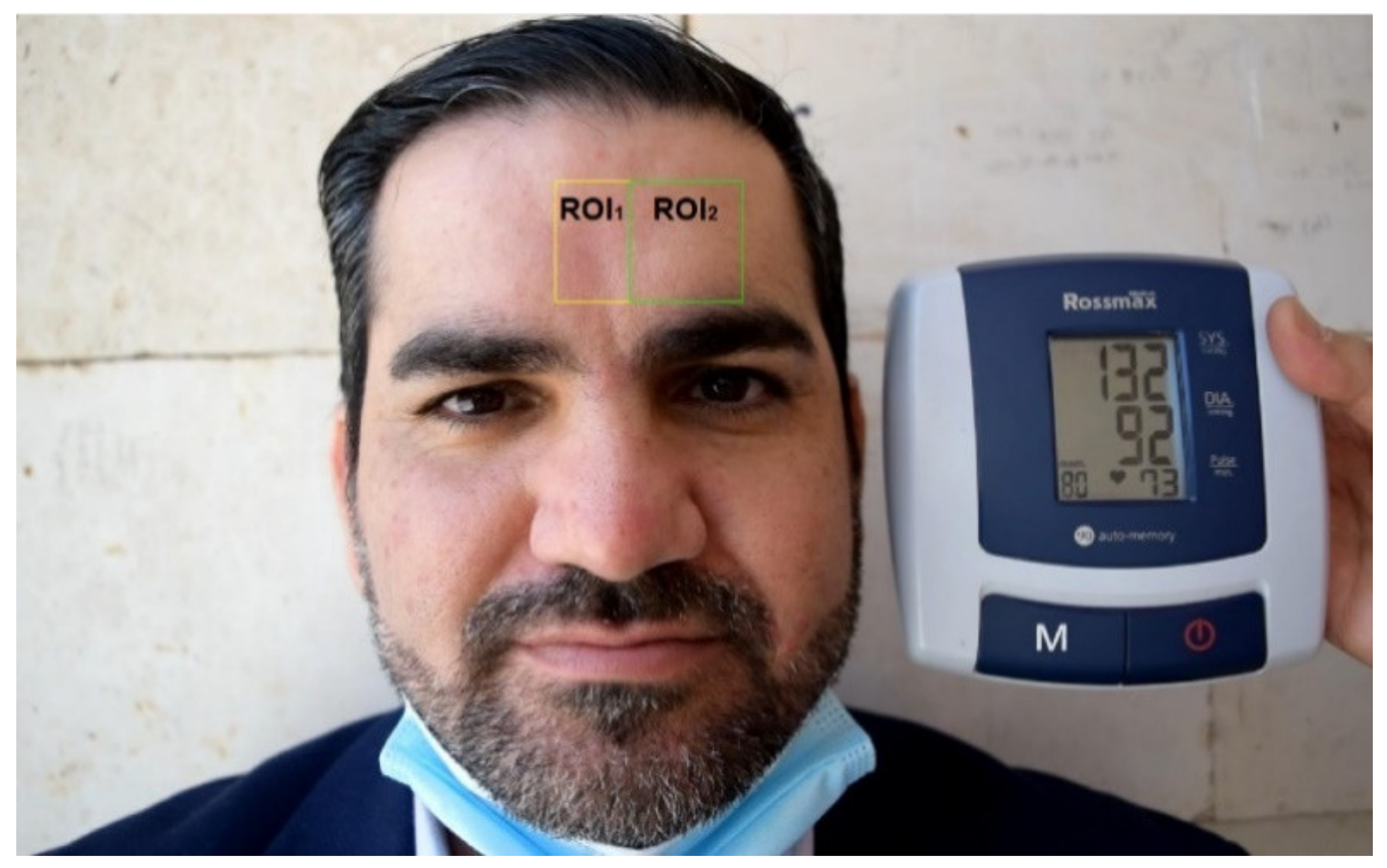
2.2. Experimental Setup
2.3. System Overview
3. Experimental Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 10 July 2022).
- WHO. More Than 700 Million People with Untreated Hypertension. Available online: https://www.who.int/news/item/25-08-2021-more-than-700-million-people-with-untreated-hypertension (accessed on 13 July 2022).
- Zhou, Y.; Ni, H.; Zhang, Q.; Wu, Q. The noninvasive blood pressure measurement based on facial images processing. IEEE Sens. J. 2019, 19, 10624–10634. [Google Scholar] [CrossRef]
- Dawes, M.; Beerman, S.; Gelfer, M.; Hobson, B.; Khan, N.; Kuyper, L.; Mangat, B.; Tran, K.; Wilson, M.G.; Kaczorowski, J. The challenges of measuring blood pressure during COVID-19: How to integrate and support home blood pressure measurements. Can. Fam. Physician 2021, 67, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, X.; Yang, F.; Yin, S.; Zhang, X.; Meng, M.Q.-H. Implementation of cuff-less continuous blood pressure measurement system based on Android. In Proceedings of the 2012 IEEE International Conference on Information and Automation, Shenyang, China, 5–8 June 2012; pp. 552–556. [Google Scholar]
- Junior, A.D.; Murali, S.; Rincon, F.; Atienza, D. Estimation of blood pressure and pulse transit time using your smartphone. In Proceedings of the 2015 Euromicro Conference on Digital System Design, Madeira, Portugal, 26–28 August 2015; pp. 173–180. [Google Scholar]
- Li, H.; Zhao, H. Systolic blood pressure estimation using Android smart phones. In Proceedings of the 2013 6th International Conference on Biomedical Engineering and Informatics, Hangzhou, China, 16–18 December 2013; pp. 260–264. [Google Scholar]
- Sagirova, Z.; Kuznetsova, N.; Gogiberidze, N.; Gognieva, D.; Suvorov, A.; Chomakhidze, P.; Omboni, S.; Saner, H.; Kopylov, P. Cuffless blood pressure measurement using a smartphone-case based ECG monitor with photoplethysmography in hypertensive patients. Sensors 2021, 21, 3525. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.; Luu, L.; Cao, T. Blood pressure measurement using finger ECG and photoplethysmogram for IoT. In Proceedings of the International Conference on the Development of Biomedical Engineering in Vietnam, Ho Chi Minh, Vietnam, 27–29 June 2017; pp. 83–89. [Google Scholar]
- Wang, E.J.; Zhu, J.; Jain, M.; Lee, T.-J.; Saba, E.; Nachman, L.; Patel, S.N. Seismo: Blood pressure monitoring using built-in smartphone accelerometer and camera. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems, Montreal, QC, Canada, 21–26 April 2018; pp. 1–9. [Google Scholar]
- Djeldjli, D.; Bousefsaf, F.; Maaoui, C.; Bereksi-Reguig, F.; Pruski, A. Remote estimation of pulse wave features related to arterial stiffness and blood pressure using a camera. Biomed. Signal Process. Control 2021, 64, 102242. [Google Scholar] [CrossRef]
- Gambi, E.; Ricciuti, M.; Spinsante, S. Sensitivity of the contactless videoplethysmography-based heart rate detection to different measurement conditions. In Proceedings of the 2018 26th European Signal Processing Conference (EUSIPCO), Rome, Italy, 3–7 September 2018; pp. 767–771. [Google Scholar]
- Al-Naji, A.; Khalid, G.A.; Mahdi, J.F.; Chahl, J. Non-contact SpO2 prediction system based on a digital camera. Appl. Sci. 2021, 11, 4255. [Google Scholar] [CrossRef]
- Al-Naji, A.; Gibson, K.; Lee, S.-H.; Chahl, J. Monitoring of cardiorespiratory signal: Principles of remote measurements and review of methods. IEEE Access 2017, 5, 15776–15790. [Google Scholar] [CrossRef]
- McDuff, D.; Gontarek, S.; Picard, R.W. Remote detection of photoplethysmographic systolic and diastolic peaks using a digital camera. IEEE Trans. Biomed. Eng. 2014, 61, 2948–2954. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yoshioka, M.; Ozawa, J. Non-contact pulse transit time measurement using imaging camera, and its relation to blood pressure. In Proceedings of the 2015 14th IAPR International Conference on Machine Vision Applications (MVA), Tokyo, Japan, 18–22 May 2015; pp. 414–417. [Google Scholar]
- Secerbegovic, A.; Bergsland, J.; Halvorsen, P.S.; Suljanovic, N.; Mujcic, A.; Balasingham, I. Blood pressure estimation using video plethysmography. In Proceedings of the 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI), Prague, Czech Republic, 3–16 April 2016; pp. 461–464. [Google Scholar]
- Patil, O.R.; Gao, Y.; Li, B.; Jin, Z. CamBP: A camera-based, non-contact blood pressure monitor. In Proceedings of the 2017 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2017 ACM International Symposium on Wearable Computers, Maui, HI, USA, 11–15 September 2017; pp. 524–529. [Google Scholar]
- Sugita, N.; Yoshizawa, M.; Abe, M.; Tanaka, A.; Homma, N.; Yambe, T. Contactless technique for measuring blood-pressure variability from one region in video plethysmography. J. Med. Biol. Eng. 2019, 39, 76–85. [Google Scholar] [CrossRef]
- Fan, X.; Ye, Q.; Yang, X.; Choudhury, S.D. Robust blood pressure estimation using an RGB camera. J. Ambient Intell. Humaniz. Comput. 2020, 11, 4329–4336. [Google Scholar] [CrossRef]
- Zou, J.; Zhou, S.; Ge, B.; Yang, X. Non-Contact Blood Pressure Measurement Based on IPPG. J. New Media 2021, 3, 41. [Google Scholar] [CrossRef]
- Iuchi, K.; Miyazaki, R.; Cardoso, G.C.; Ogawa-Ochiai, K.; Tsumura, N. Remote Estimation of Continuous Blood Pressure by a Convolutional Neural Network Trained on Spatial Patterns of Facial Pulse Waves. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, New Orleans, LA, USA, 19–20 June 2022; pp. 2139–2145. [Google Scholar]
- Cheng, J.; Chen, X.; Xu, L.; Wang, Z.J. Illumination variation-resistant video-based heart rate measurement using joint blind source separation and ensemble empirical mode decomposition. IEEE J. Biomed. Health Inform. 2017, 21, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Viola, P.; Jones, M. Rapid object detection using a boosted cascade of simple features. In Proceedings of the 2001 IEEE Computer Society Conference on Computer Vision and Pattern Recognition, CVPR 2001, Kauai, HI, USA, 8–14 December 2001; pp. 511–518I. [Google Scholar]
- Lahiani, H.; Kherallah, M.; Neji, M. Hand pose estimation system based on Viola-Jones algorithm for android devices. In Proceedings of the 2016 IEEE/ACS 13th International Conference of Computer Systems and Applications (AICCSA), Agadir, Marocco, 29 November–2 December 2016; pp. 1–6. [Google Scholar]
- Rabiha, S.G.; Kurniawan, A.; Moniaga, J.; Wilson, E.; Wahyudi, D.I. Face Authentication in E-Learning using Local Binary Pattern and Haar Cascade. In Proceedings of the 2018 International Seminar on Research of Information Technology and Intelligent Systems (ISRITI), Yogyakarta, Indonesia, 21–22 November 2018; pp. 28–32. [Google Scholar]
- Khanam, F.-T.-Z.; Perera, A.G.; Alnaji, A.; Gibson, K.; Chahl, J. Non-contact automatic vital signs monitoring of infants in a neonatal intensive care unit based on neural networks. J. Imaging 2021, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Poh, M.-Z.; McDuff, D.J.; Picard, R.W. Advancements in noncontact, multiparameter physiological measurements using a webcam. IEEE Trans. Biomed. Eng. 2010, 58, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Poh, M.-Z.; McDuff, D.J.; Picard, R.W. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt. Express 2010, 18, 10762–10774. [Google Scholar] [CrossRef] [PubMed]
- Khanam, F.-T.-Z.; Al-Naji, A.; Perera, A.G.; Gibson, K.; Chahl, J. Non-contact automatic vital signs monitoring of neonates in NICU using video camera imaging. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2022, 1–8. [Google Scholar] [CrossRef]
- Colominas, M.A.; Schlotthauer, G.; Torres, M.E. Improved complete ensemble EMD: A suitable tool for biomedical signal processing. Biomed. Signal Process. Control 2014, 14, 19–29. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Wang, J.-J.; Lin, K.-Y.; Chang, H.-H.; Wu, H.-K.; Chen, Y.-S.; Lee, S.-Y. Image Sensor-Based Heart Rate Evaluation from Face Reflectance Using Hilbert–Huang Transform. IEEE Sens. J. 2015, 15, 618–627. [Google Scholar] [CrossRef]
- Al-Naji, A.; Perera, A.G.; Chahl, J. Remote monitoring of cardiorespiratory signals from a hovering unmanned aerial vehicle. Biomed. Eng. Online 2017, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.-C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, N.E. Ensemble empirical mode decomposition: A noise-assisted data analysis method. Adv. Adapt. Data Anal. 2009, 1, 1–41. [Google Scholar] [CrossRef]
- Torres, M.E.; Colominas, M.A.; Schlotthauer, G.; Flandrin, P. A complete ensemble empirical mode decomposition with adaptive noise. In Proceedings of the 2011 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Prague, Czech Republic, 22–27 May 2011; pp. 4144–4147. [Google Scholar]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Naji, A.; Fakhri, A.B.; Mahmood, M.F.; Chahl, J. Contactless Blood Pressure Estimation System Using a Computer Vision System. Inventions 2022, 7, 84. https://doi.org/10.3390/inventions7030084
Al-Naji A, Fakhri AB, Mahmood MF, Chahl J. Contactless Blood Pressure Estimation System Using a Computer Vision System. Inventions. 2022; 7(3):84. https://doi.org/10.3390/inventions7030084
Chicago/Turabian StyleAl-Naji, Ali, Ahmed Bashar Fakhri, Mustafa F. Mahmood, and Javaan Chahl. 2022. "Contactless Blood Pressure Estimation System Using a Computer Vision System" Inventions 7, no. 3: 84. https://doi.org/10.3390/inventions7030084
APA StyleAl-Naji, A., Fakhri, A. B., Mahmood, M. F., & Chahl, J. (2022). Contactless Blood Pressure Estimation System Using a Computer Vision System. Inventions, 7(3), 84. https://doi.org/10.3390/inventions7030084








