Decrease in the Starting Temperature of the Reaction for Fabricating Carbides of Refractory Metals When Using Carbon Nanoparticles as Precursors
Abstract
:1. Introduction
2. Materials, Equipment, and Research Methods
3. Results and Discussion
3.1. Niobium Composites
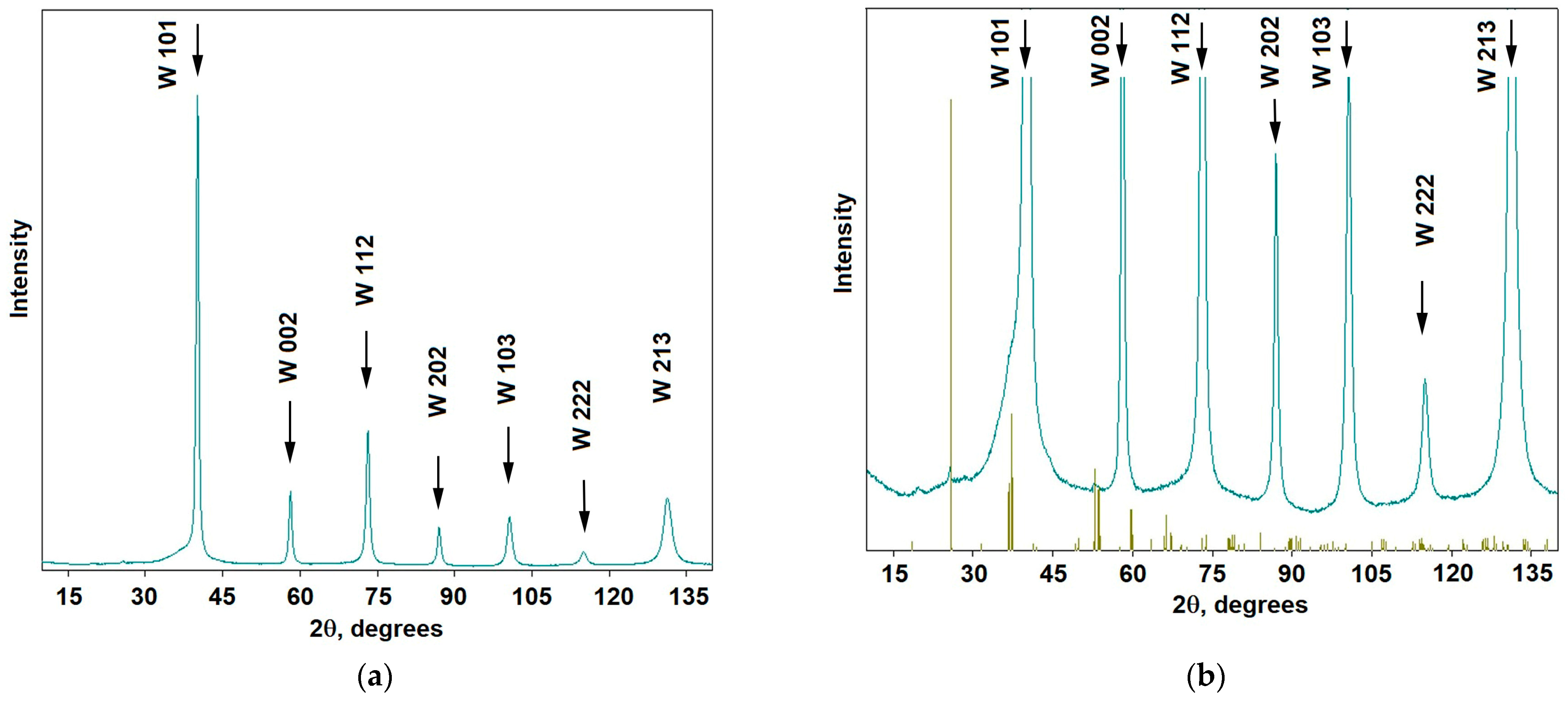
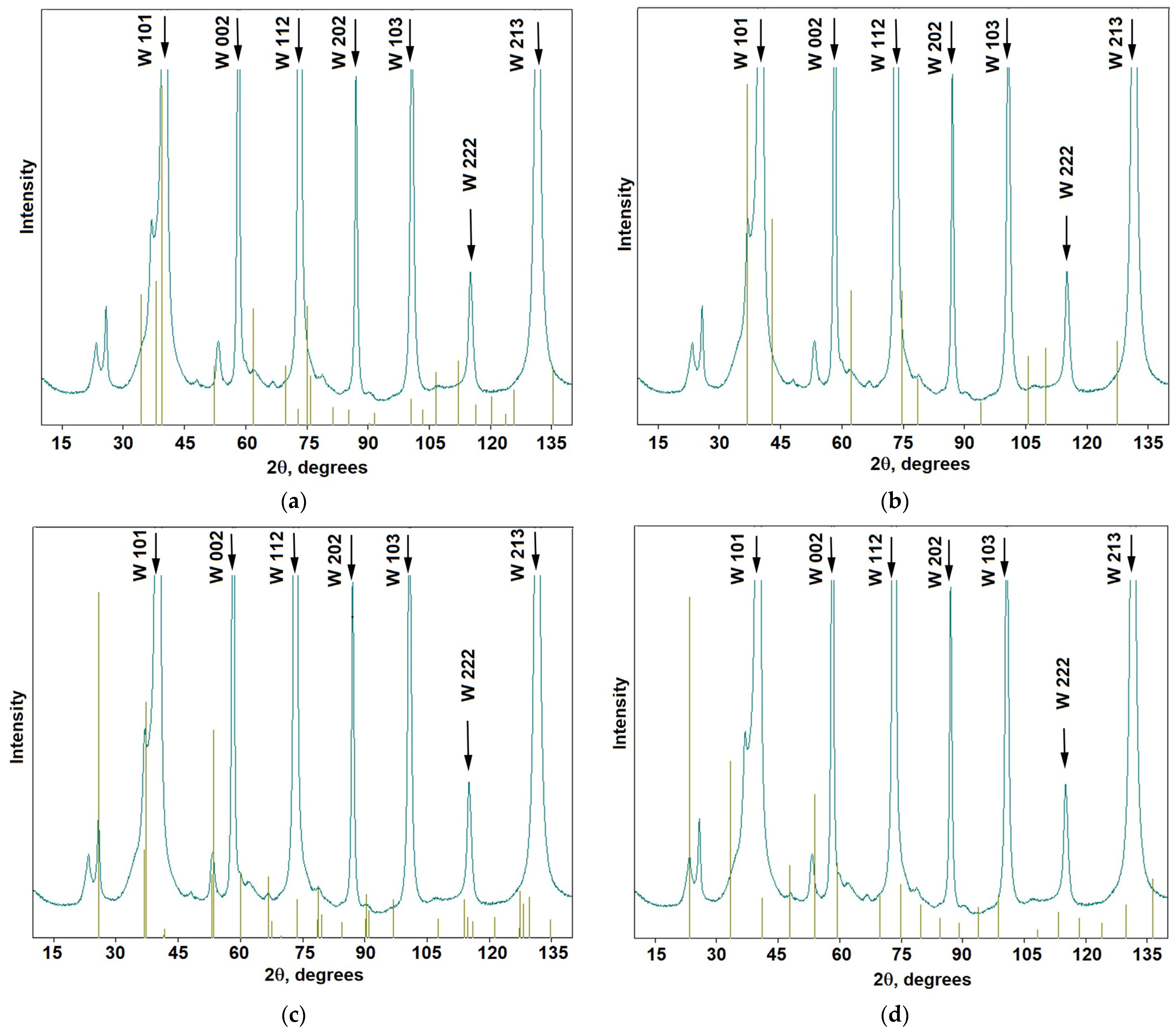
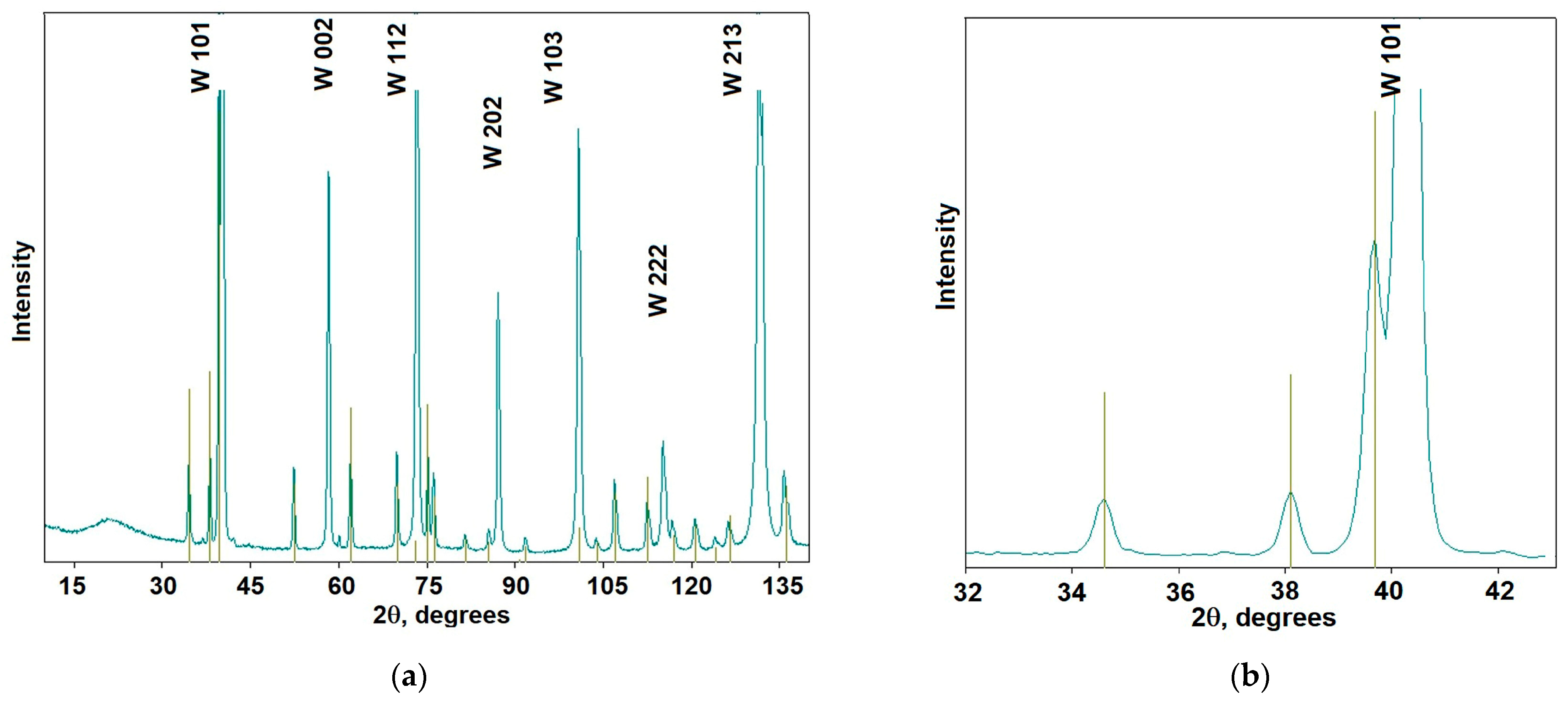
3.2. Tungsten Carbides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, A.; Sharma, V.K.; Mausam, K. An analytical study of nano carbon materials for developing metal matrix nano compo-sites. Mater. Today Proc. 2021, 45, 2867–2870. [Google Scholar] [CrossRef]
- Tan, W.; Jiang, X.; Shao, Z.; Sun, H.; Fang, Y.; Shu, F. Fabrication and mechanical properties of nano carbon reinforced lam-inated Cu matrix composites. Powder Technol. 2022, 395, 377–390. [Google Scholar] [CrossRef]
- Popov, V.; Borunova, A.; Shelekhov, E.; Khodos, I.; Senatilin, B.; Matveev, D.; Versinina, E. Peculiarities of chemical interaction of some carbon nanoreinforcements with aluminum matrix in metal matrix composite (MMC). Mater. Werkst. 2022, 53, 602–607. [Google Scholar] [CrossRef]
- Reddy, S.T.; Manohar, H.; Anand, S. Effect of carbon black nano-fillers on tribological properties of Al6061-Aluminium metal matrix composites. Mater. Today Proc. 2019, 20, 202–207. [Google Scholar] [CrossRef]
- Romanova, V.S.; Khotina, I.A.; Kushakova, N.S.; Kovalev, A.I.; Kharitonova, V.G.; Kupriyanova, D.V.; Naumkin, A.V. Compound based on star-shaped oligophenylene and fullerene C60. Mendeleev Commun. 2022, 32, 783–785. [Google Scholar] [CrossRef]
- Popov, V.A.; Borunova, A.B.; Senatulin, B.R.; Shelekhov, E.V.; Kirichenko, A.N. Peculiarities of fullerenes and carbon onions application for reinforcing the aluminum matrix in the metal matrix composites. Surf. Interface Anal. 2019, 52, 127–131. [Google Scholar] [CrossRef]
- Dhand, V.; Yadav, M.; Kim, S.H.; Rhee, K.Y. A comprehensive review on the prospects of multi-functional carbon nano onions as an effective, high- performance energy storage material. Carbon 2020, 175, 534–575. [Google Scholar] [CrossRef]
- Volkov, D.S.; Krivoshein, P.K.; Mikheev, I.V.; Proskurnin, M.A. Pristine detonation nanodiamonds as regenerable adsorbents for metal cations. Diam. Relat. Mater. 2020, 110, 108121. [Google Scholar] [CrossRef]
- Popov, V. Several Aspects of Application of Nanodiamonds as Reinforcements for Metal Matrix Composites. Appl. Sci. 2021, 11, 4695. [Google Scholar] [CrossRef]
- Qin, J.-X.; Yang, X.-G.; Lv, C.-F.; Li, Y.-Z.; Liu, K.-K.; Zang, J.-H.; Yang, X.; Dong, L.; Shan, C.-X. Nanodiamonds: Synthesis, properties, and applications in nanomedicine. Mater. Des. 2021, 210, 110091. [Google Scholar] [CrossRef]
- Parker, D.M.; Lineweaver, A.J.; Quast, A.D.; Zharov, I.; Shumaker-Parry, J.S. Thiol-terminated nanodiamond powders for support of gold nanoparticle catalysts. Diam. Relat. Mater. 2021, 116, 108449. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Aleksandrov, M.N.; Zagoruiko, I.V.; Chuvilin, A.L.; Moroz, E.M.; Kolomiichuk, V.N.; Likholobov, V.A.; Brylyakov, P.M.; Sakovitch, G.V. Study of ultradispersed diamond powders obtained using explosion energy. Carbon 1991, 29, 665–668. [Google Scholar] [CrossRef]
- Leonard, K.J.; Busby, J.; Zinkle, S. Aging effects on microstructural and mechanical properties of select refractory metal alloys for space-reactor applications. J. Nucl. Mater. 2007, 366, 336–352. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, Z.; Song, L.; Wang, W.; Chen, J.; Ma, S. Inductively coupled plasma etching of bulk tungsten for MEMS applications. Sens. Actuators A Phys. 2022, 345, 113825. [Google Scholar] [CrossRef]
- Yu, M.; Pu, G.; Xue, Y.; Wang, S.; Chen, S.; Wang, Y.; Yang, L.; Wang, Z.; Zhu, T.; Tan, T.; et al. The oxidation behaviors of high-purity niobium for superconducting radio-frequency cavity application in vacuum heat treatment. Vacuum 2022, 203, 111258. [Google Scholar] [CrossRef]
- Lavigne, O.; Luzin, V.; Labonne, M.; Missiaen, J.-M. Neutron diffraction characterizations of NbC-Ni cemented carbides thermal residual stresses. Int. J. Refract. Met. Hard Mater. 2022, 109, 105966. [Google Scholar] [CrossRef]
- Graboś, A.; Rutkowski, P.; Huebner, J.; Kozień, D.; Zhang, S.; Kuo, Y.-L.; Kata, D.; Hayashi, S. Oxidation performance of spark plasma sintered Inconel 625-NbC metal matrix composites. Corros. Sci. 2022, 205, 110453. [Google Scholar] [CrossRef]
- Sanad, M.M.S.; El-Sadek, M.H. Porous niobium carbide as promising anode for high performance lithium-ions batteries via cost-effective processing. Diam. Relat. Mater. 2022, 121, 108722. [Google Scholar] [CrossRef]
- Dai, W.; Yue, B.; Chang, S.; Bai, H.; Liu, B. Mechanical properties and microstructural characteristics of WC-bronze-based impregnated diamond composite reinforced by nano-NbC. Tribol. Int. 2022, 174, 107777. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, H.; Du, Y.; Chen, G.; Zhang, J. Sulfur and nitrogen codoped Nb2C MXene for dendrite-free lithium metal battery. Electrochim. Acta 2021, 390, 138812. [Google Scholar] [CrossRef]
- Arif, N.; Gul, S.; Sohail, M.; Rizwan, S.; Iqbal, M. Synthesis and characterization of layered Nb2C MXene/ZnS nanocomposites for highly selective electrochemical sensing of dopamine. Ceram. Int. 2020, 47, 2388–2396. [Google Scholar] [CrossRef]
- Babar, Z.U.D.; Zheng, R.-K.; Mumtaz, M.; Rizwan, S. Magneto-transport of mechanically-pressed niobium carbide (Nb2C) distorted MXene. Mater. Lett. 2020, 285, 129210. [Google Scholar] [CrossRef]
- Zou, Q.; Jiao, Z.; Li, Y.; He, W.; Li, S.; Luo, Y. Effects of TiC0.4 on microstructure and properties of WC composites. J. Alloys Compd. 2022, 925, 166588. [Google Scholar] [CrossRef]
- Han, L.; Liu, Z.; Yu, L.; Ma, Z.; Huang, Y.; Liu, Y.; Wang, Z. Effect of WC nanoparticles on the thermal stability and mechanical performance of dispersion-reinforced Cu composites. Scr. Mater. 2023, 222, 115030. [Google Scholar] [CrossRef]
- Shen, Q.; Ge, S.; Zhao, A.; Sun, Y.; Zhang, J.; Zhou, D.; Luo, G.; Zhang, L. Microstructural evolution and mechanical behavior of porous W reinforced by in-situ W2C. J. Alloys Compd. 2019, 797, 1106–1114. [Google Scholar] [CrossRef]
- Garcia-Ayala, E.; Tarancon, S.; Ferrari, B.; Pastor, J.; Sanchez-Herencia, A. Thermomechanical behaviour of WC-W2C composites at first wall in fusion conditions. Int. J. Refract. Met. Hard Mater. 2021, 98, 105565. [Google Scholar] [CrossRef]
- Šestan, A.; Sreekala, L.; Markelj, S.; Kelemen, M.; Zavašnik, J.; Liebscher, C.H.; Dehm, G.; Hickel, T.; Čeh, M.; Novak, S.; et al. Non-uniform He bubble formation in W/W2C composite: Experimental and ab-initio study. Acta Mater. 2022, 226, 117608. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, L.; Huang, Y.; Xu, S. Enrichment of in-situ synthesized WC by partial dissolution of ex-situ eutectoid-structured WC/W2C particle in the coatings produced by laser hot-wire deposition. Mater. Lett. 2020, 281, 128641. [Google Scholar] [CrossRef]
- Tungsten Carbide. Available online: https://en.wikipedia.org/wiki/Tungsten_carbide (accessed on 1 August 2022).
- Niobium Carbide. Available online: https://en.wikipedia.org/wiki/Niobium_carbide (accessed on 1 August 2022).
- Ma, J.; Wu, M.; Du, Y.; Chen, S.; Jin, W.; Fu, L.; Yang, Q.; Wen, A. Formation of nanocrystalline niobium carbide (NbC) with a convenient route at low temperature. J. Alloys Compd. 2009, 475, 415–417. [Google Scholar] [CrossRef]
- Popov, V. The impact of the diamond reinforcing particle size on their interaction with the aluminum matrix of composites in the course of heating. Surf. Interface Anal. 2018, 50, 1106–1109. [Google Scholar] [CrossRef]
- Popov, V.; Borunova, A.; Shelekhov, E.; Cheverikin, V.; Khodos, I. Several Aspects of Interaction between Chrome and Nanodiamond Particles in Metal Matrix Composites When Being Heated. Inventions 2022, 7, 75. [Google Scholar] [CrossRef]
- Benjamin, J.S.; Volin, T.E. The mechanism of mechanical alloying. Met. Mater. Trans. B 1974, 5, 1929–1934. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Fabrication of Nanocomposite Materials. In Mechanical Alloying: For Fabrication of Advanced Engineering Materials; Noyes Publications/William Andrew Publishing: Norwich, NY, USA, 2001; pp. 45–61. [Google Scholar] [CrossRef]
- Jain, H.; Shadangi, Y.; Chakravarty, D.; Dubey, A.K.; Mukhopadhyay, N. High entropy steel processed through mechanical alloying and spark plasma sintering: Alloying behaviour, thermal stability, and mechanical properties. Mater. Sci. Eng. A 2022, 856, 144029. [Google Scholar] [CrossRef]
- Lu, L.; Lai, M.O. Mechanical Alloying. In Kluwer; Academic Publisher: Cambridge, MA, USA, 1998; 276p. [Google Scholar]
- Höhne, G.W.H.; Hemminger, W.F.; Flammersheim, H.-J. Differential Scanning Calorimetry; Springer: Berlin/Heidelberg, Germany, 2003; 298p. [Google Scholar] [CrossRef]
- Spathara, D.; Sergeev, D.; Kobertz, D.; Müller, M.; Putman, D.; Warnken, N. Thermodynamic study of single crystal, Ni-based superalloys in the γ+γ′ two-phase region using Knudsen Effusion Mass Spectrometry, DSC and SEM. J. Alloys Compd. 2021, 870, 159295. [Google Scholar] [CrossRef]
- Toda, A. Kinetics of enthalpy recovery studied by temperature-modulated differential scanning calorimetry. Thermochim. Acta 2022, 716, 179330. [Google Scholar] [CrossRef]
- Gonçalves, R.L.; Robustillo, M.D.; Filho, P.D.A.P. A simplified two-resistance model for the description of melting curves obtained through differential scanning calorimetry. Thermochim. Acta 2022, 714, 179269. [Google Scholar] [CrossRef]
- Couturier, L.; De Geuser, F.; Deschamps, A. A comparative study of Fe-Cr unmixing using differential scanning calorimetry and small-angle scattering. Mater. Charact. 2021, 173, 110934. [Google Scholar] [CrossRef]
- Shelekhov, E.V.; Sviridova, T.A. Programs for X-ray analysis of polycrystals. Met. Sci. Heat Treat. 2000, 42, 309–313. [Google Scholar] [CrossRef]
- Kwon, Y.-S.; Gerasimov, K.B.; Yoon, S.-K. Ball temperatures during mechanical alloying in planetary mills. J. Alloys Compd. 2002, 346, 276–281. [Google Scholar] [CrossRef]
- Popov, V.A. X-ray micro-absorption enhancement for non-agglomerated nanodiamonds in mechanically alloyed aluminium matrix composites. Phys. Status Solidi (A) 2015, 212, 2722–2726. [Google Scholar] [CrossRef]
- Wirtz, G.P.; Sis, L.B.; Wheeler, J.S. Sublimation of MoO3 from WO3-MoO3 catalysts during the oxidation of toluene. J. Catal. 1975, 38, 196–205. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Yao, J.; Loo, B. Simulation of the sublimation process in the preparation of photochromic WO3 film by laser microprobe mass spectrometry. J. Non-Cryst. Solids 2000, 272, 71–74. [Google Scholar] [CrossRef]

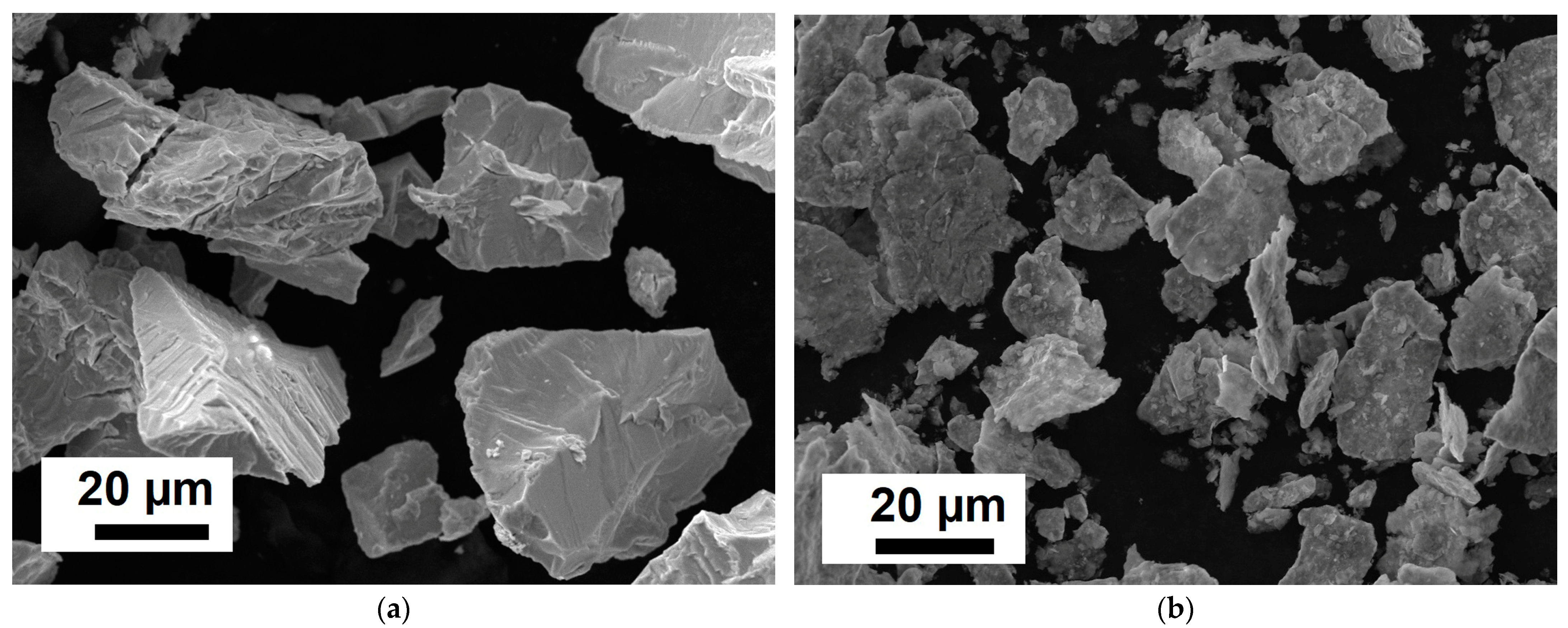
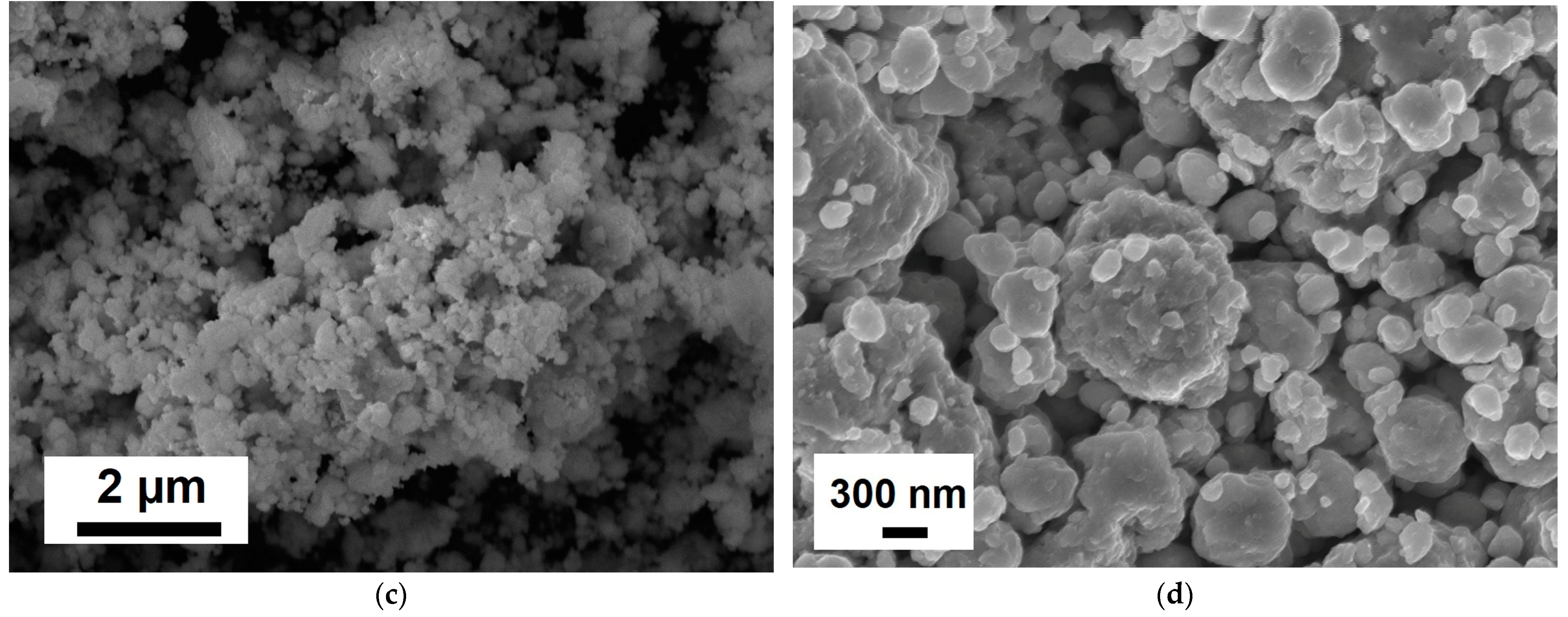

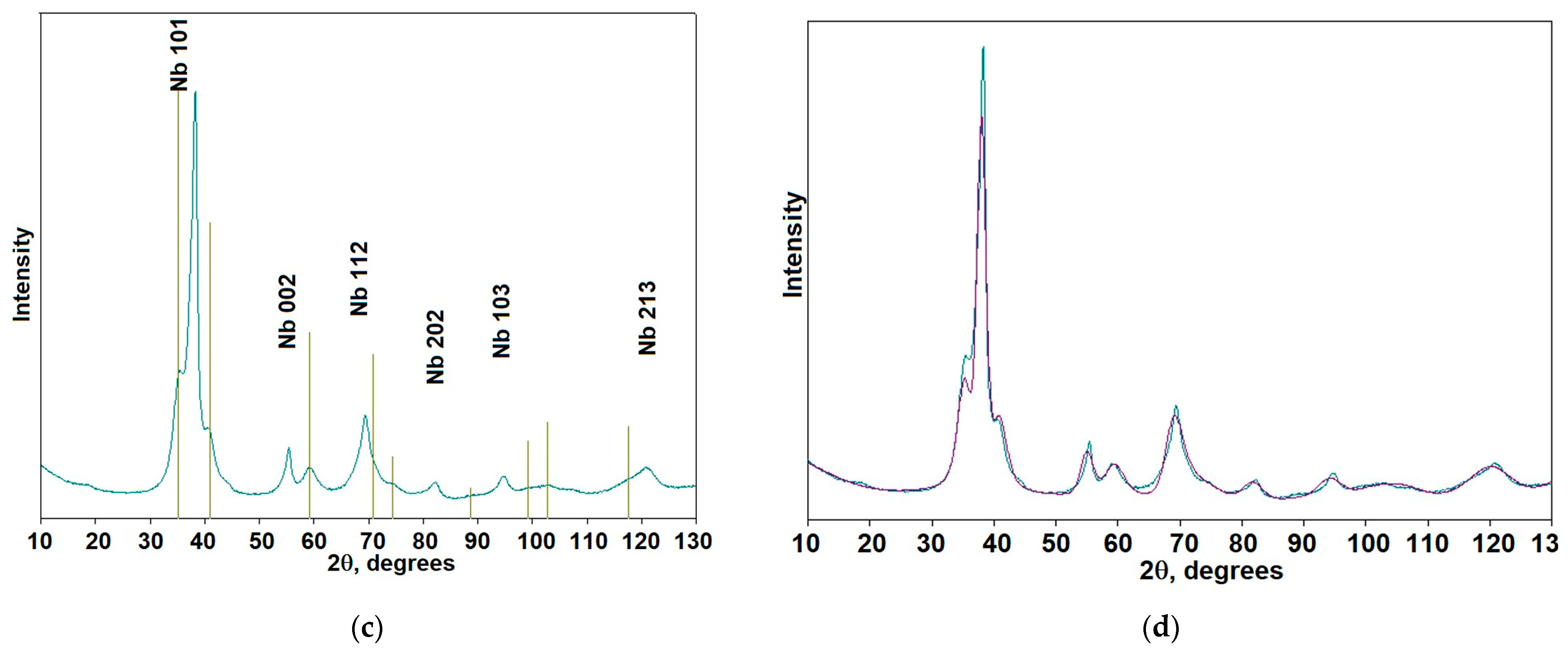
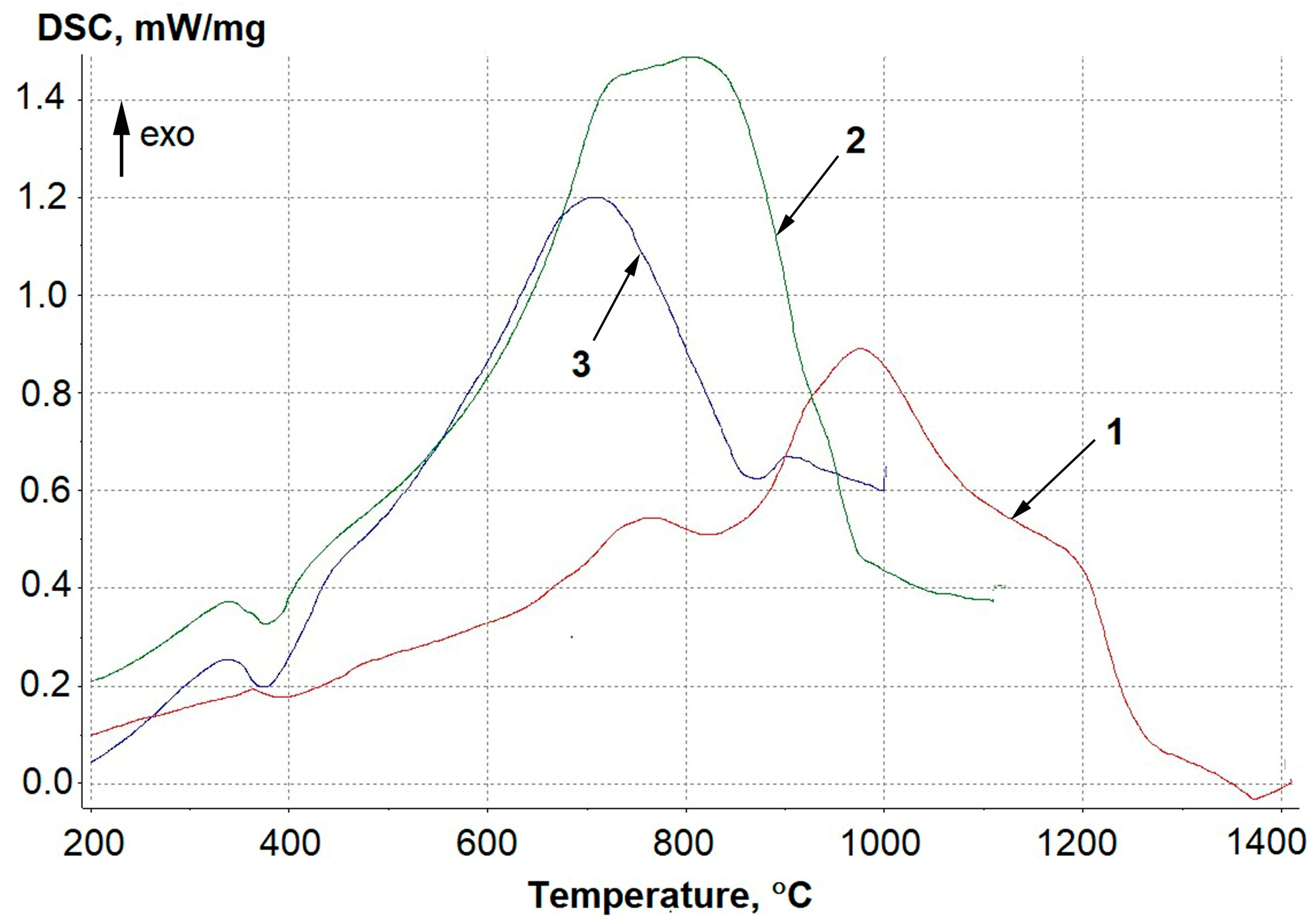


| Element | Ta | Si | Fe | W | Mo | C | NbH | Other Impurities | Nb |
|---|---|---|---|---|---|---|---|---|---|
| Content, %mass | 0.05 | 0.03 | 0.02 | 0.02 | 0.02 | 0.1 | 3.0 | 0.11 | Balance |
| Element | Fe | Ni | Mo | K | Na | C | Other Impurities | W |
|---|---|---|---|---|---|---|---|---|
| Content, %mass | 0.1 | 0.06 | 0.1 | 0.02 | 0.02 | 0.1 | 0.1 | Balance |
| Number of Specimen | Type and Mass of Metal, g | Mass of Nanodiamonds, g | Content of Nanodiamonds, %mass | Time of Mechanical Alloying, h |
|---|---|---|---|---|
| 1-Nb | Nb, 9.296 | 0.653 | 6.56 | 0.5 |
| 2-Nb | Nb, 9.302 | 0.699 | 6.98 | 1.2 |
| 3-Nb | Nb, 9.307 | 0.699 | 6.98 | 6 |
| 4-W | W, 20.092 | 0.653 | 3.25 | 9 |
| Temperature, °C | Phase | Volume Fraction, % | Mass Fraction, % |
|---|---|---|---|
| As milled | NbC0.77 (B1, cF8) Balance–Nb | 49.4 | 47.8 |
| 350 | NbC0.77 (B1, cF8) Balance–Nb | 66.1 | 65.6 |
| 275 | NbC0.77 (B1, cF8) Balance–Nb | 67.1 | 66.0 |
| 417 | NbC0.77 (B1, cF8) Balance–Nb | 69.7 | 68.0 |
| 760 | NbC0.77 (B1, cF8) Nb2C (L’3, hP4) Nb2C (oP12) Balance–niobium oxide | 65.3 8.0 21.4 | 64.7 8.1 21.3 |
| Phase | Volume Fraction, % | Mass Fraction, % |
|---|---|---|
| W (A2, cI2) | 61.2 | 69.0 |
| W2C (C6, hP3) | 14.6 | 14.7 |
| WC0.82 (B1, cF8) | 6.9 | 7.0 |
| WO2 (C4, tP6) | 9.4 | 6.0 |
| WO3 (D0.9, cP4) | 7.9 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, V.; Borunova, A.; Shelekhov, E.; Koplak, O.; Dvoretskaya, E.; Matveev, D.; Prosviryakov, A.; Vershinina, E.; Cheverikin, V. Decrease in the Starting Temperature of the Reaction for Fabricating Carbides of Refractory Metals When Using Carbon Nanoparticles as Precursors. Inventions 2022, 7, 120. https://doi.org/10.3390/inventions7040120
Popov V, Borunova A, Shelekhov E, Koplak O, Dvoretskaya E, Matveev D, Prosviryakov A, Vershinina E, Cheverikin V. Decrease in the Starting Temperature of the Reaction for Fabricating Carbides of Refractory Metals When Using Carbon Nanoparticles as Precursors. Inventions. 2022; 7(4):120. https://doi.org/10.3390/inventions7040120
Chicago/Turabian StylePopov, Vladimir, Anna Borunova, Evgeny Shelekhov, Oksana Koplak, Elizaveta Dvoretskaya, Danila Matveev, Alexey Prosviryakov, Ekaterina Vershinina, and Vladimir Cheverikin. 2022. "Decrease in the Starting Temperature of the Reaction for Fabricating Carbides of Refractory Metals When Using Carbon Nanoparticles as Precursors" Inventions 7, no. 4: 120. https://doi.org/10.3390/inventions7040120
APA StylePopov, V., Borunova, A., Shelekhov, E., Koplak, O., Dvoretskaya, E., Matveev, D., Prosviryakov, A., Vershinina, E., & Cheverikin, V. (2022). Decrease in the Starting Temperature of the Reaction for Fabricating Carbides of Refractory Metals When Using Carbon Nanoparticles as Precursors. Inventions, 7(4), 120. https://doi.org/10.3390/inventions7040120








