Abstract
Today, most of the electrical energy in the world is generated by fossil fuel incineration. This causes significant emissions of harmful substances into the atmosphere. The noted problem can be solved by switching to power plants with zero emissions, operating in semi-closed cycles, and producing electricity through oxygen combustion of fuel. A significant drawback of most of the known oxygen–fuel cycles is the lack of useful utilization of various sources of low-grade heat, which is especially typical for power plants operating on gasified coal fuel; as a result of the gasification process, a significant amount of excess heat is released into the atmosphere. This paper presents the results of the development and study of oxygen–fuel cycle thermal schemes of increased efficiency with coal gasification. It was determined that the modernization of the scheme using the carbon dioxide Rankine cycle for the utilization of low-grade heat makes it possible to achieve an increase in the net electrical efficiency equal to 1.2%.
1. Introduction
1.1. Modern Promising Directions of Carbon Dioxide Emissions Control
Today, the main global trend in the development of energy is to reduce the quantity of toxic substances and greenhouse gases emissions into the atmosphere. The relevance of this area of development is confirmed by the adopted international agreements [1], European Union plans to introduce a trans-border carbon tax [2], and when taking into account ESG principles when formulating the development strategies of large energy companies [3,4].
A number of scientific papers written over the past decades have been devoted to the issue of reducing emissions of harmful substances from power plants. Many of the developed methods are successfully used today at thermal power plants. In particular, methods of controls of atmospheric pollutants, such as nitrogen and sulfur oxides, have been widely used [5,6,7]. At the same time, the prevention of carbon dioxide emissions, which are formed in large quantities during the combustion of fossil fuels, still causes difficulties [8,9,10]. The introduction of carbon dioxide capture technologies leads to a significant increase in the cost of electricity produced [11,12] and, therefore, the issue of creating environmentally friendly and economically viable high-capacity energy systems remains open.
There are four main ways to reduce carbon emissions, as follows:
- Reducing the use of fossil fuels by increasing the efficiency of energy blocks, reducing the consumption of electrical energy, using technologies without CO2 emissions (renewable energy sources, nuclear fuel, environmentally friendly hydrogen), or changing a low carbon and hydrogen ratio C/H (coal, oil products) to gaseous fuels (natural gas);
- Capture of carbon dioxide released during the combustion process and its disposal and beneficial use in oil producing enterprises (intensification of crude oil production, increase in the oil recovery factor);
- Limitation of deforestation processes, thereby increasing the accumulation of CO2 in biomass.
Utilization and storage of carbon is one of the topical areas for the development of traditional energy facilities. There are three main technologies for capturing CO2 emissions (pre-, post-, and oxy-combustion) as follows:
- Pre-combustion carbon capture takes place prior to the combustion process (by gasification of the fuel with oxygen, e.g., integrated coal gasification technology);
- Post-combustion carbon capture takes place after the combustion process (recovery of CO2 from flue gases, e.g., by chemical absorption, physical adsorption, membrane separation, or the use of a chemical circuit);
- Carbon capture by oxy-combustion occurs after the combustion process in an oxygen atmosphere by separating the CO2 produced in the oxy-combustion process. An oxygen atmosphere can be obtained by removing nitrogen from the air before the combustion process in an air separation unit (ASU).
The most promising direction was the development of technologies for the oxygen–fuel generation of electricity, which are based on the combustion of hydrocarbon fuels in pure oxygen, followed by the capture of carbon dioxide and its disposal.
At the moment, more than thirty cycles with oxygen fuel combustion are known [13,14,15]. Among them, one of the most effective is the Allam cycle, the net electrical efficiency of which can exceed 50% at an initial temperature of the working flow of about 1100 °C [16,17]. At the same time, there is a significant potential for improving the energy efficiency of this cycle.
Most of the research on the Allam cycle has been carried out using natural gas, which has less than 76 years of proven reserves. This factor forces us to consider the operation of traditional units on other energy raw materials. For example, according to rough estimates [18], coal fuel will last for more than 400 years, at the current rate of energy consumption, which is an attractive prospect in the current energy crisis. Because of this, in the last 10 years, research has been actively carried out to improve the efficiency of oxygen–fuel energy complexes with the gasification of solid fuel. However, the use of coal fuel gasification technologies will lead to a direct increase in the complex’s own needs and, accordingly, to a decrease in the net efficiency of the power unit, which leads to the need for the emergence of technologies that increase the energy efficiency of thermal power plants operating on solid fuel [16].
1.2. Ways to Improve the Energy Efficiency of Oxygen–Fuel Power Plants
There are many works devoted to the development of circuit solutions aimed at increasing the energy efficiency of the Allam cycle. In particular, there is a known method for increasing the efficiency by adding an organic Rankine cycle or a Brayton cycle to utilize low-grade heat. Studies show [19,20,21,22] that the introduction of such cycles can lead to an increase in thermal efficiency up to 1.5%. According to various estimates [23,24,25,26,27], depending on temperatures and parameters, the net efficiency of the Rankine cycle on a low-boiling coolant can reach 18–25%, while the net efficiency of the Brayton cycle can reach 30–42%.
Another way to increase the efficiency of the Allam cycle is the production of liquid oxygen in the ASU with its storage in storage tanks. Research [21] shows that this solution makes it possible to expand the control range of the energy complex by varying the power of the air separation unit (ASU), to increase the net efficiency at the moments of ASU shutdown, and that it is able to use the cold from oxygen evaporation.
The introduction of closed-type nitrogen cooling for carbon dioxide turbine blades makes it possible not only to increase the initial parameters of the cycle, but also to direct the heated nitrogen flow to perform useful work in additional nitrogen turbines [28].
The injection of water into the carbon dioxide turbine coolant flow, due to an increase in the heat capacity of the cooling flow, reduces its consumption, which leads to a decrease in energy costs for pumping the coolant. The use of this technology makes it possible to increase the net efficiency of the Allam cycle by 2.2%, but its use has a significant drawback, namely the possible condensation of water vapor, followed by the formation of carbon dioxide [29].
The cooling of the coolant with the help of an intermediate heat exchanger has no drawback associated with the formation of aggressive media in the flow path of the turbine, and its use increases the net efficiency of the Allam cycle by 3.2%. However, this method requires additional economic costs, which can significantly affect the payback of the station [29].
Furthermore, to increase energy efficiency, condensation of the carbon dioxide flow is used before compression, which makes it possible to increase the net cycle efficiency up to 2% by reducing the cost of compressing the working flow. However, the implementation of this method requires a constant source of energy with a low temperature of the cooling flow, and the operation of the pump in the vicinity of the critical zone is dangerous due to the possibility of a surge [30].
Based on the results of the review of various ways to increase the efficiency of the Allam cycle presented in the open literature sources, Table 1 has been compiled, which summarizes information on the amount of increase in net efficiency, as well as the advantages and disadvantages of various methods.

Table 1.
Comparison of different methods to improve the efficiency of the Allam cycle.
Thus, among the considered options for improving the energy efficiency of oxygen–fuel power plants (OFPP), one of the most promising technologies is the use of energy cycles with the utilization of low-grade heat, the use of which can provide an increase in the net electrical efficiency equal to 1.51. However, to achieve such a high increase in efficiency, it is necessary to carry out thermodynamic optimization, which includes a comparative analysis of coolants, as well as a determination of the optimal structure and parameters of the thermal schemes. In this regard, the purpose of this work was the development and study of circuit solutions aimed at improving the energy efficiency of complexes operating on the Allam cycle with coal gasification.
2. Materials and Methods
2.1. Research Object
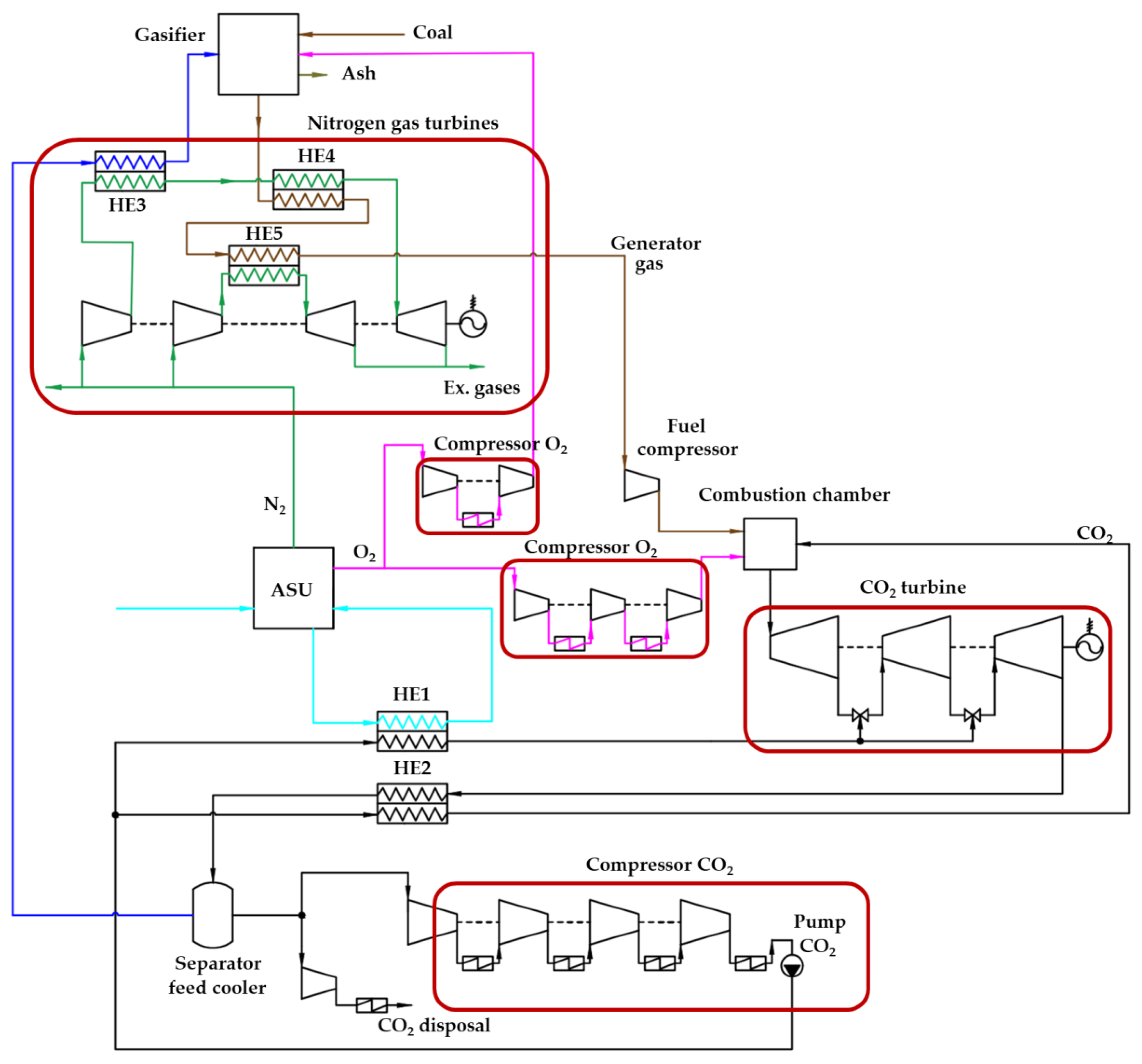
A schematic diagram of the Allam cycle, in which synthesis gas is cooled by the working fluid of two nitrogen gas turbine units, is shown in Figure 1. Nitrogen is a by-product of the high purity oxygen production in ASU, which ensures the operation of nitrogen turbines. This circuit solution allows us to reduce the heat of the synthesis gas and thereby reduce the cost of the oxygen–fuel power unit for its own needs. At the same time, the use of the physical heat of the generator gas for heating nitrogen in front of the turbines makes it possible to generate additional electrical power, which leads to an increase in the efficiency of the entire cycle.
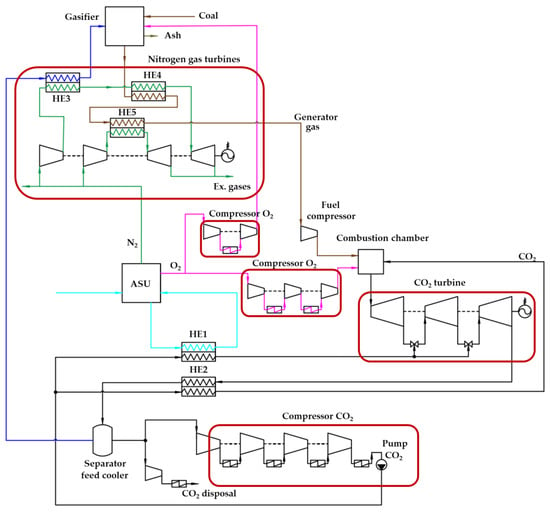
Figure 1.
Scheme of the Allam cycle with coal gasification and additional nitrogen gas turbine units.
The principle of operation of the Allam cycle scheme with coal gasification and additional gas turbine units is as follows. The process of converting solid fuel into generator gas (synthesis gas) takes place in the gasification unit, into which coal is supplied, and the oxygen produced in the ASP and steam are blowing agents. Steam is produced by heating water in a nitrogen–water heat exchanger (HE3) using nitrogen compressed in a compressor. In this case, water is taken from the separator feed cooler (SC) due to the condensation of water vapor. After the gas generator, the synthesis gas enters the heat exchanger (HE4), in which it gives heat off to the nitrogen flow. Then, it enters the heat exchanger (HE5), where it gives its heat off to the nitrogen of the second gas turbine and thereby cools to the minimum temperature at which condensation of the synthesis gas components does not yet occur. The cooled generator gas enters the fuel booster compressor and further into the combustion chamber. Oxygen combustion of fuel takes place in the combustion chamber and heat is transferred to the main flow of carbon dioxide. The flow is subsequently directed to the carbon dioxide turbine, where, after expansion, regeneration takes place in the heat exchanger (HE2). Then, the working flow enters the separator feed cooler, in which the water formed during the combustion of the fuel is condensed. Excess carbon dioxide formed during the combustion of fuel is compressed and cooled to a liquid phase, after which it is sent to disposal. In the heat exchanger (HE1), the air flow entering the ASU after compression in the air compressor is cooled.
The initial data for the analysis of the cycle scheme are given in Table 2. Table 3 shows the fuel composition adopted according to [30]. The initial temperature and pressure at the inlet and outlet of the carbon dioxide turbine are close to the optimal values described in [14]. Synthesis gas is burned in oxygen of 95.6% purity obtained in a low-pressure cryogenic ASU.

Table 2.
Initial data for modeling.

Table 3.
Composition of coal fuel.
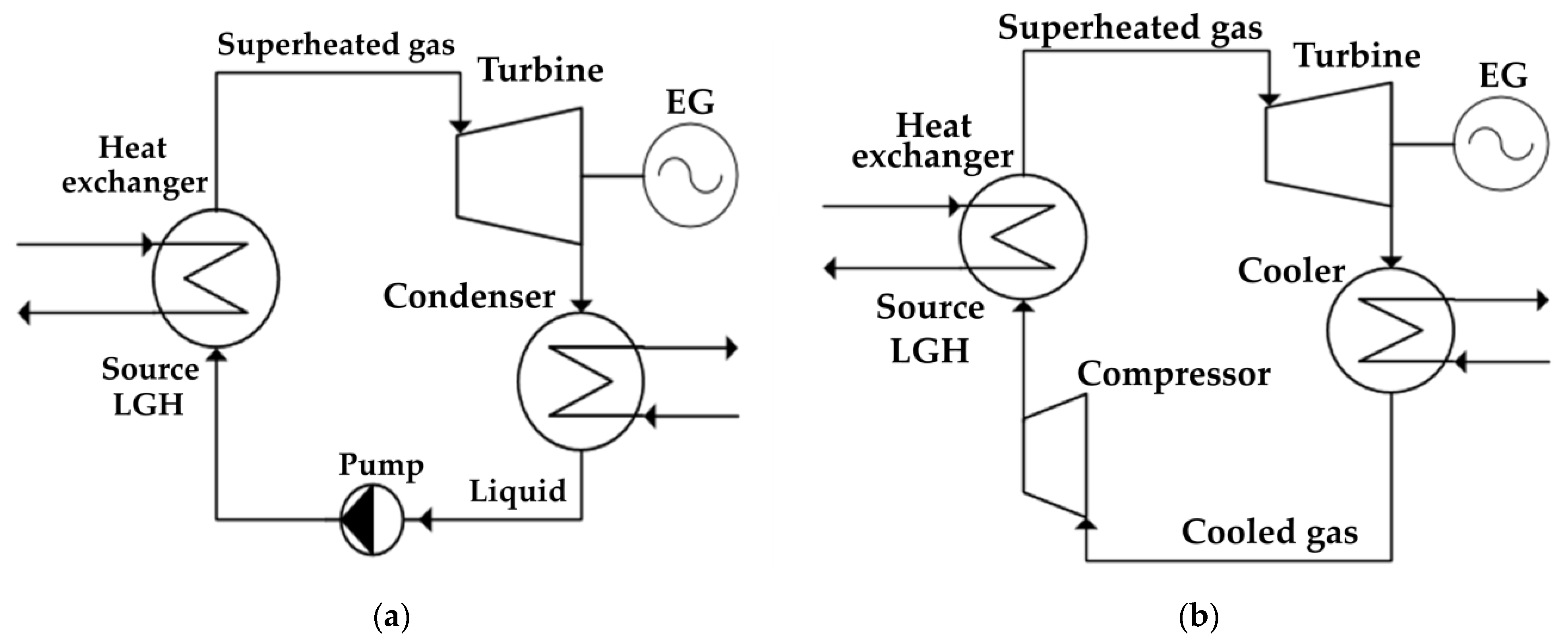
Figure 2a shows a diagram of the simplest organic Rankine cycle, which is used to utilize the low-grade heat of the Allam cycle with gasification. The working flow evaporates and overheats in the heat exchanger due to the heat of a low-grade source, after which it is sent to the turbine, where it expands and performs work. Next, the coolant is sent to the condenser, where it passes into the liquid phase due to the flow cooling, after which the flow enters the pump, where the pressure necessary for circulation in a closed circuit is pumped, after which the flow enters back into the heat exchanger [31,32]. The fundamental difference between the Brayton cycle shown in Figure 2b and the Rankine cycle is the absence of a phase transition—after the turbine, the working fluid does not condense, but cools, after which it is compressed by the compressor and overheated from the heat of a low-potential source, and then it returns to the turbine [33,34].
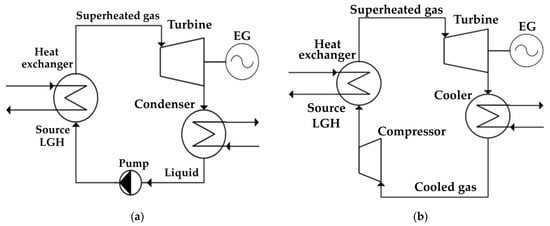
Figure 2.
Schemes of cycles with a low-boiling coolant: (a) Rankine cycle; (b) Brayton cycle.
2.2. Modeling Method
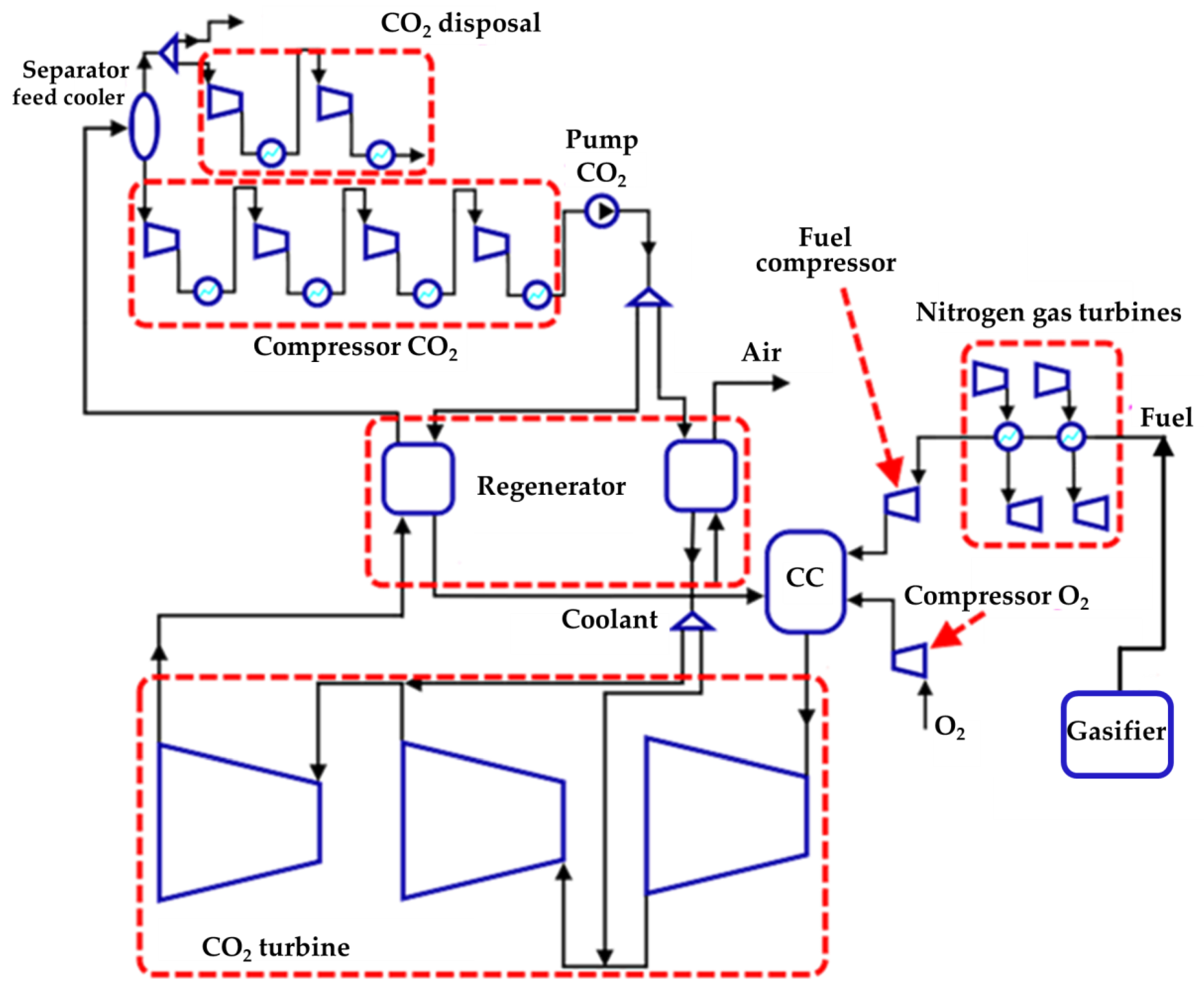
Thermodynamic studies of oxy–fuel combustion energy cycles were carried out using the AspenONE code, created to develop mathematical models of thermal circuits [35]. The NIST REFPROP database was used to determine the thermophysical properties of liquids. The simulation model of the object consists of three sub-models, namely the Allam cycle, the ASU block, and the gasification block (Figure 3).
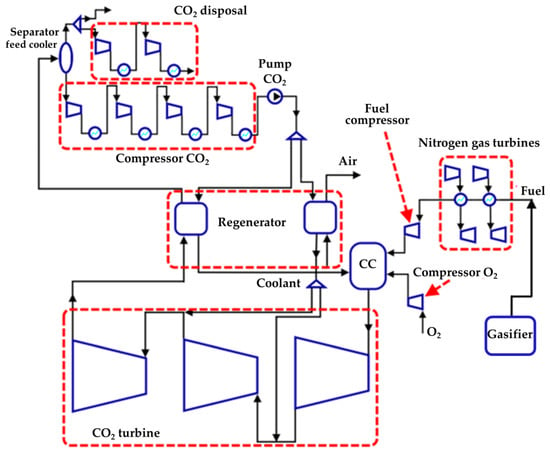
Figure 3.
Simulation block diagram.
The initial data for the study of the steam–oxygen blast gasification plant are as follows: coal parameters, oxygen parameters at the ASU outlet, and water parameters from the Allam cycle. The outlet synthesis gas parameters are calculated according to the method described in [33]. The generator gas temperature is determined iteratively. Chemical and thermal efficiency of generator gas are calculated by Formulas (1) and (2), as follows:
where Qggc—chemical heat of generator gas, kJ/(kg of fuel);
Qfc—chemical heat of gasified fuel, kJ/(kg of fuel);
Qggf—physical heat of generator gas, kJ/(kg of fuel);
Qff—physical heat of gasified fuel, kJ/(kg of fuel);
Qo—heat of the oxidizer, kJ/(kg of fuel).
The Allam cycle model uses a flow with the parameters of the generator gas and an oxygen flow at the outlet of the ASU. The energy generated in the Allam cycle is partially spent on the separation process in the ASU. The air parameters were also used to calculate the ASU model according to the method presented in [36].
The calculation of the thermal scheme was carried out according to the following algorithm. Initially, the composition and parameters of the generator gas were determined at the optimal proportions of oxygen and steam supplied to carry out the gasification process. Then, the dew point temperature for the generator gas was determined, which is the minimum allowable temperature at the outlet of the nitrogen gas turbine unit. Next, the values of the mass flow rates of the generator gas and oxygen sent to the combustion chamber were selected, at which the temperature at the inlet to the cooled gas turbine was reached, equal to 1100 °C with the condition that the combustion reaction proceeds at a stoichiometric ratio. The value of the mass flow rate of air sent to the air separation unit was determined by taking into account the need for an oxygen supply for burning generator gas in the combustion chamber, as well as for the process of gasification of solid fuel. The value of the mass flow rate of nitrogen at the inlet to the first nitrogen gas turbine plant was determined with the condition that the temperature at the inlet to the nitrogen turbine be 750 °C, while the maximum value of the nitrogen mass flow rate was limited by the ASU performance. The degree of pressure increase for the first nitrogen compressor was determined from the condition of heating the working flow in the nitrogen–water heat exchanger to the temperature of 300 °C. The degree of pressure increase for the second nitrogen gas turbine plant was determined based on the condition that the generator gas leaves the heat exchanger of the second nitrogen gas turbine plant with the lowest possible temperature, below which condensation of the generator gas components occurs.
The developed mathematical model of the Allam cycle with gasification has the following assumptions:
- The influence of friction forces in pipelines on the efficiency of the station is not taken into account;
- The model does not take into account the supply of cooling carbon dioxide after each cooled nozzle and blade of the gas turbine;
- In the combustion chamber stoichiometric combustion of synthesis gas;
- The relative internal efficiencies of the compressors, the pump, the CO2 turbine, and the pressure loss in the combustion chamber were assumed to be constant.
The power of the carbon dioxide turbine is calculated by the following Formula (3):
where NCO2.T1, NCO2.T2, and NCO2.T3 are the electric power of the first, second and third stages of the CO2 turbine, W, respectively.
The power of the i-th stage is determined by the following Formula (4):
where GCO2i is the consumption of carbon dioxide in the i-th stage of the turbine, kg/s; hinlet.i and houtlet.i are enthalpy at the inlet and outlet of the i-th stage of the turbine, J/kg, respectively. Stoichiometric combustion of synthesis gas occurs according to the following Formulas (5)–(7):
The Low heating value of the fuel is determined by the following Formula (8):
where C, H, O, S, and W are the content of carbon, hydrogen, oxygen, volatile sulfur and moisture in the working mass of the fuel, %, respectively.
The heat released in the combustion chamber is calculated by the following Formula (9):
where B is fuel flow rate, kg/s;
The net power of the oxy–fuel combustion energy cycle was calculated using Equation (10), as follows:
where NCO2.T, NN2.T1, and NN2.T2 are the power of the carbon dioxide and nitrogen turbines, W; ηme and ηgen are the mechanical efficiency and efficiency of the electric generator, respectively; NCO2.C, NO2.C, Nfuel.C, NN2.C1, NN2.C2, and NST.CO2.C are the power of carbon dioxide, oxygen, fuel, nitrogen compressors, and disposal compressor, W, respectively; NASU is the power spent on the air separation unit, W.
The net efficiency of the oxy–fuel combustion energy cycle is calculated using Equation (11), as follows:
3. Results and Discussion
3.1. Study of Low-Potential Heat Sources in the Allam Cycle with Gasification
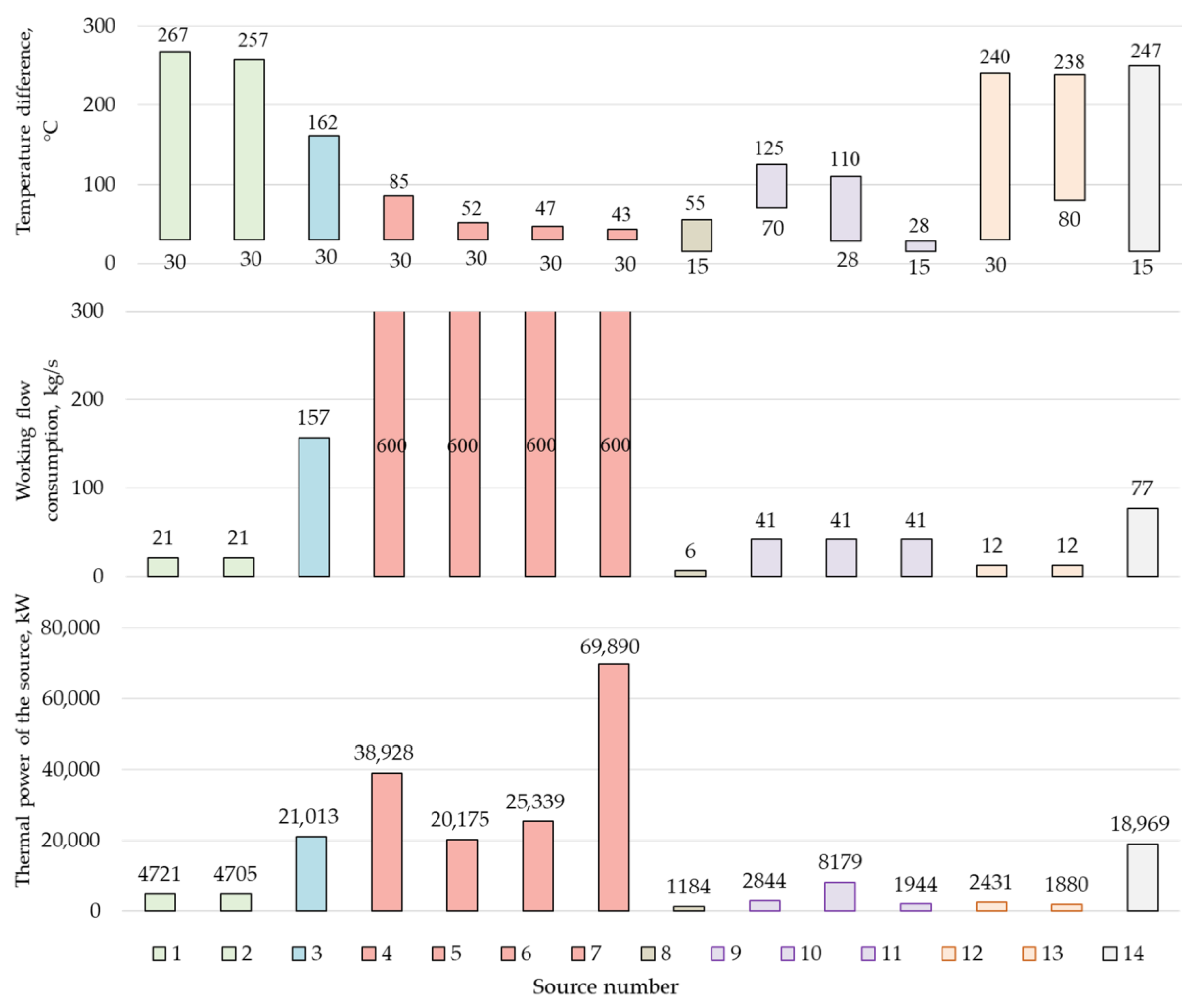
The developed scheme of an oxygen–fuel energy complex with coal gasification (Figure 1) has many sources of low-grade heat (LGH) (Figure 4), which may be useful to utilize in order to increase the efficiency of the cycle.
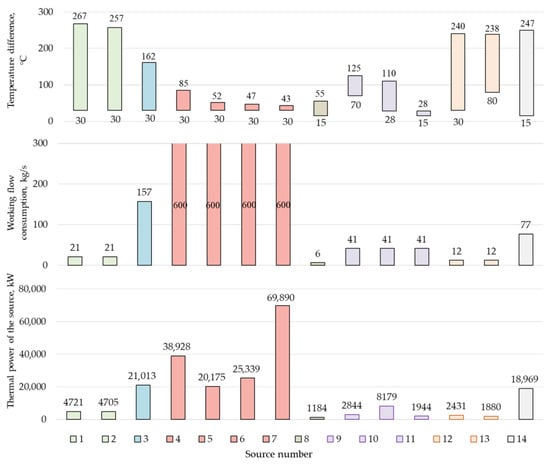
Figure 4.
Thermal potential of the Allam cycle low-grade heat source, as follows: 1—intermediate cooler of the O2 compressor first stage for the combustion chamber (CC); 2—intermediate cooler of the second stage of the O2 compressor for the CC; 3—air cooler in front of the ASU; 4—intermediate cooler of the first stage of the CO2 compressor; 5—intermediate cooler of the second stage of the CO2 compressor; 6—intermediate cooler of the third stage of the CO2 compressor; 7—CO2 flow cooler in front of the carbon dioxide pump; 8—heat loss with H2O; 9—intercooler of CO2 disposal compressor; 10—CO2 flow cooler before disposal; 11—heat losses with CO2 disposal; 12—intermediate cooler of the O2 compressor for gasification; 13—O2 flow cooler before gasification; 14—heat loss to the environment with the exhaust gases of the nitrogen gas turbine unit (GTU).
Several heat sources have the greatest potential for utilization, including the compressed air flow in front of the ASU and the exhaust gases of nitrogen gas turbines. The criterion for the LGH sources selection was the presence of a temperature difference of at least 60 °C and a source of thermal power of more than 10 MW. The limitation on the source temperature is due to the fact that, at a low temperature difference, the required heat exchange area and, accordingly, the cost of heat exchange equipment, will greatly increase.
In the production of oxygen in an air separation plant, the air is pre-compressed, since the separation of oxygen and nitrogen takes place at a pressure of about 6 bar. As a result of compression, the air flow heats up to an average of 241 °C. To increase the efficiency of the Allam cycle, the heated air flow is directed to the cycle regeneration system, where, due to the utilization of air heat, the CO2 flow is heated, which is directed to cool the gas turbine. However, due to the fixed temperature value (200 °C) and the coolant flow, complete heat recovery becomes impossible. At the same time, it is necessary to supply air with a temperature not higher than 30 °C to the ASU and, therefore, about 21 MW is emitted into the atmosphere.
Another source of low-potential heat is the flow of exhaust gases from additional nitrogen turbines used to utilize the physical heat of generator gas (source No. 14). Nitrogen from the air separation plant is superheated by the heat of the generator gas and sent to nitrogen gas turbines to generate electricity. However, at the turbine exhaust, the flow has a relatively high temperature, which is caused by the limitation on the exhaust pressure, which cannot be lower than 1 atm.
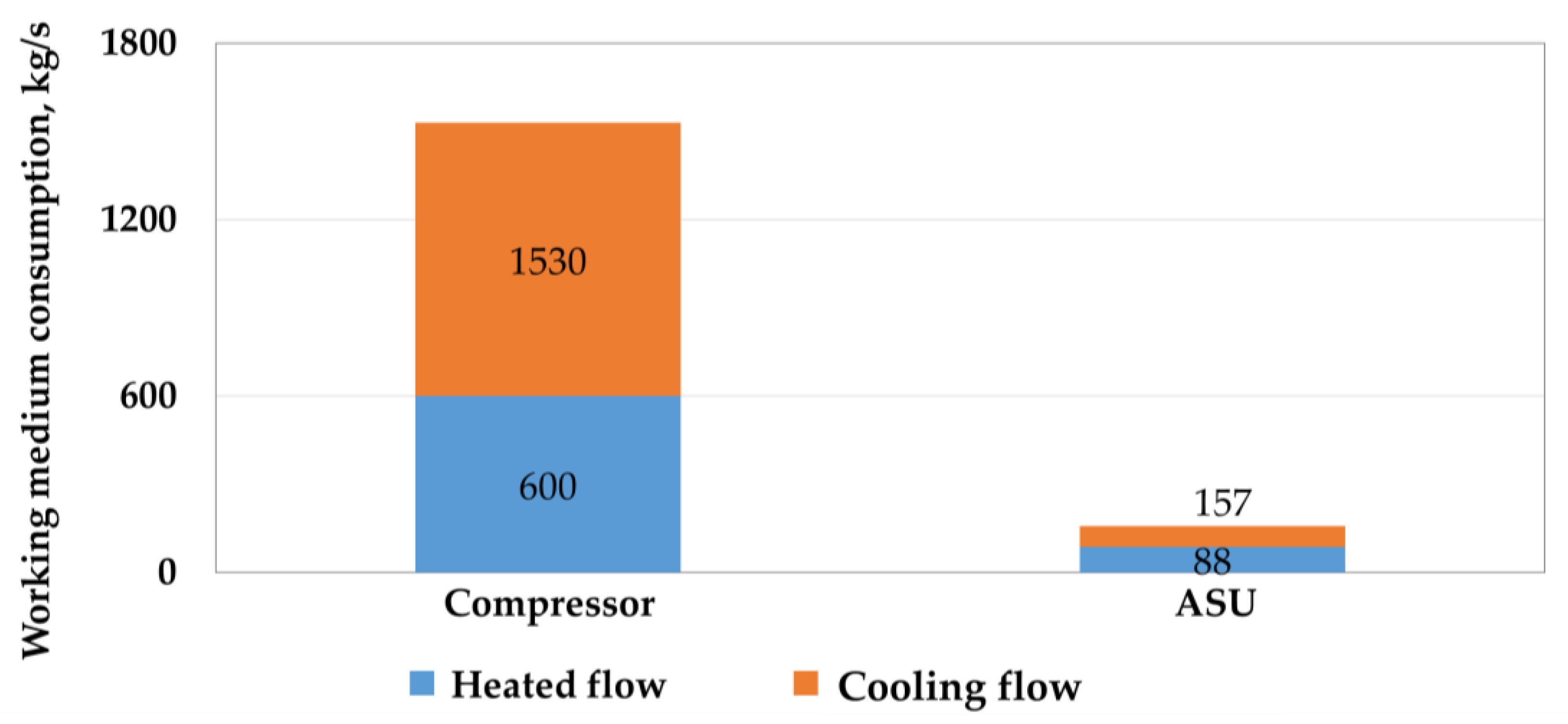
Utilization of the low-grade heat of the carbon dioxide compressor in the carbon dioxide Rankine cycle gives 2.1 MW of additional net power generation. However, due to the small temperature difference and the large amount of heat utilized from the Allam cycle, a large flow rate of the cooling flow is observed, which exceeds the flow rate of the working flow by 2.55 times (by 1530 kg/s). This, in turn, will lead to a large metal consumption for the designed thermal circuit in the case of low-potential cycles. Figure 5 shows a comparison of the flow rates of the working and cooling flow for a carbon dioxide compressor and an ASU.
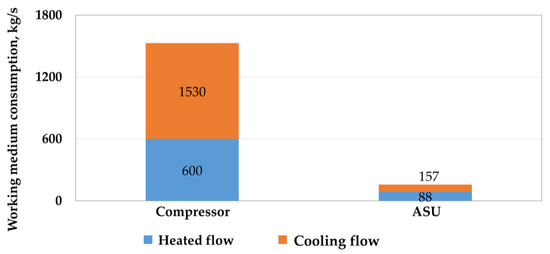
Figure 5.
Comparison of the flow rate of the working and cooling flow in a multistage compressor and an air separation unit.
3.2. Study of the Impact of Low-Grade Heat Utilization on Efficiency
The cycles shown in Figure 3 can be added to each LGH source separately, or one cycle can be used for both sources, which must first be combined.
In order to utilize low-grade heat from OFPP in a single cycle, a staged utilization scheme was developed. The low-boiling coolant flow through the heat exchangers receives heat from all LGH sources, while the order of installation of heat exchangers is formed according to the following principle: if two sources operate in the same temperature range, then they are connected in parallel, and if the temperatures are in different ranges, the heat exchangers are connected in series. The final scheme of staged utilization is shown in Figure 6, while the installation sequence and parameters of the heat exchangers are shown in Table 4.
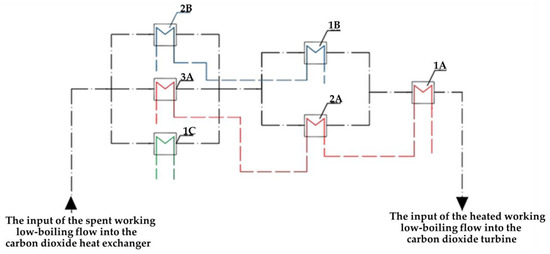
Figure 6.
Scheme of the Allam cycle LGH joint utilization with gasification and utilization of the generator gas heat in nitrogen gas turbines.

Table 4.
Input data for low-potential heat sources of OFPP.
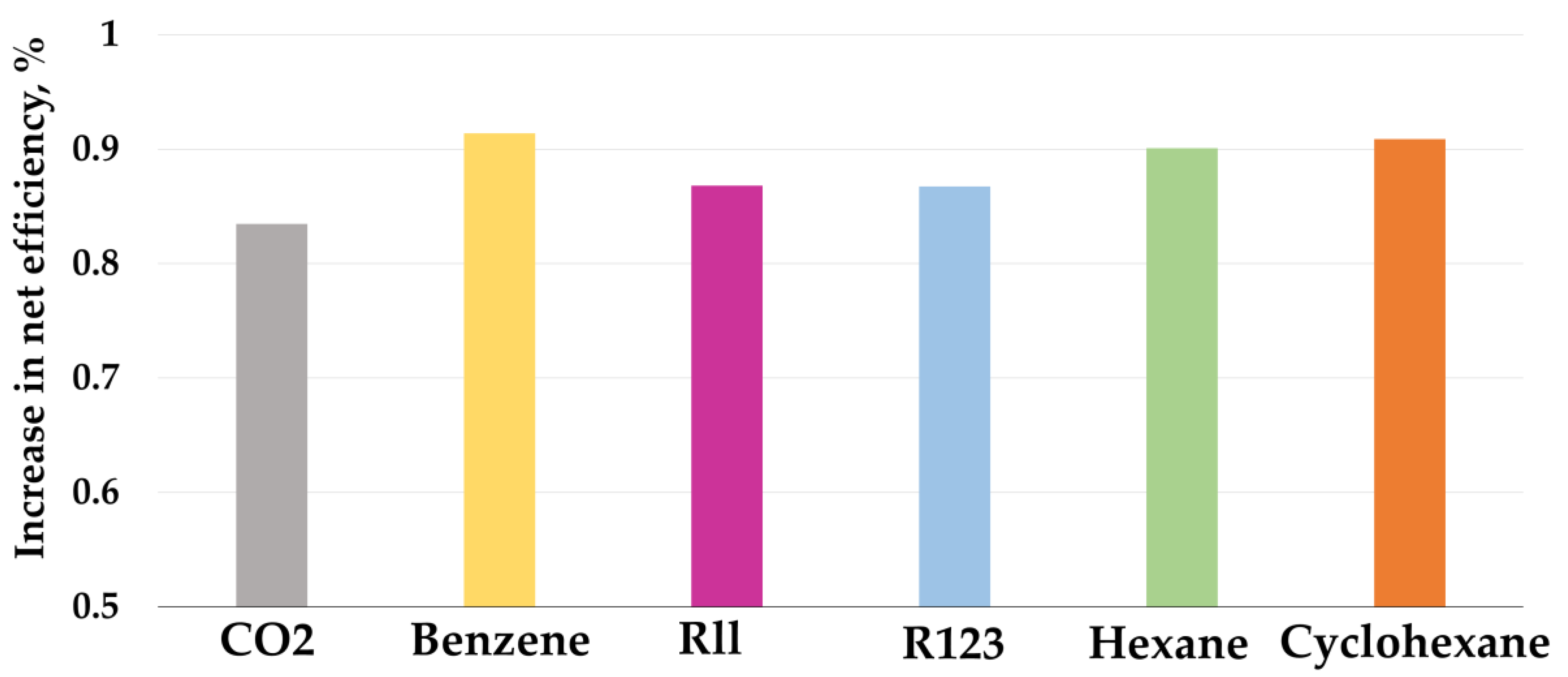
The thermodynamic efficiency of low-boiling coolant cycles is significantly affected by the type of working fluid, in connection with which a study was conducted to select the most energy-efficient low-boiling coolant, the results of which are shown in Figure 7. To study the effect of the refrigerant on the efficiency of low-grade heat utilization, the Rankine cycle was used, thanks to which, depending on the composition of the working flow, changes in thermodynamic properties were taken into account. At the outlet of the turbine, the minimum pressure changed depending on the saturation temperature, and the inlet pressure was optimized in increments of 0.1 MPa. As a result, due to changes in thermodynamic properties, the power of the turbine and the cost of compressing the working flow in the pump changed, which affected the increase in additional power.
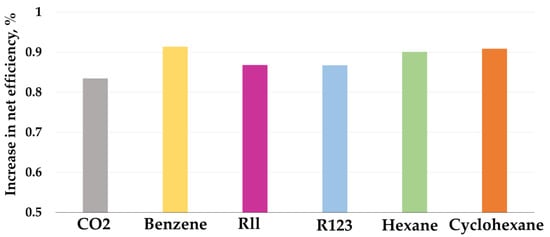
Figure 7.
Increase in net efficiency during the Allam cycle LGH utilization with gasification.
It was determined that, for the Allam cycle with gasification, the greatest increase in efficiency, equal to 0.914 (Table 5), is achieved using benzene as a working fluid. This is due to the fact that benzene has the lowest saturation pressure, equal to 15 kPa, while carbon dioxide has it equal to 7.1 MPa, due to which the turbine operates with a large pressure drop. Furthermore, when using benzene, there are minimal costs for compression in the pump, which is due to the high density of benzene in the liquid phase; indeed, at 30 °C, the benzene density is 868 kg/m3.

Table 5.
Calculation results for cycles using low-grade heat.
The use of benzene as a working fluid is not advisable due to its toxicity; its use is contrary to modern environmental requirements. At the same time, carbon dioxide is the working flow of the main cycle and a by-product of power generation and, therefore, its use as a coolant in the utilization cycle is more appropriate. Therefore, carbon dioxide will be used as the working fluid of the Brayton and Rankine utilization cycles.
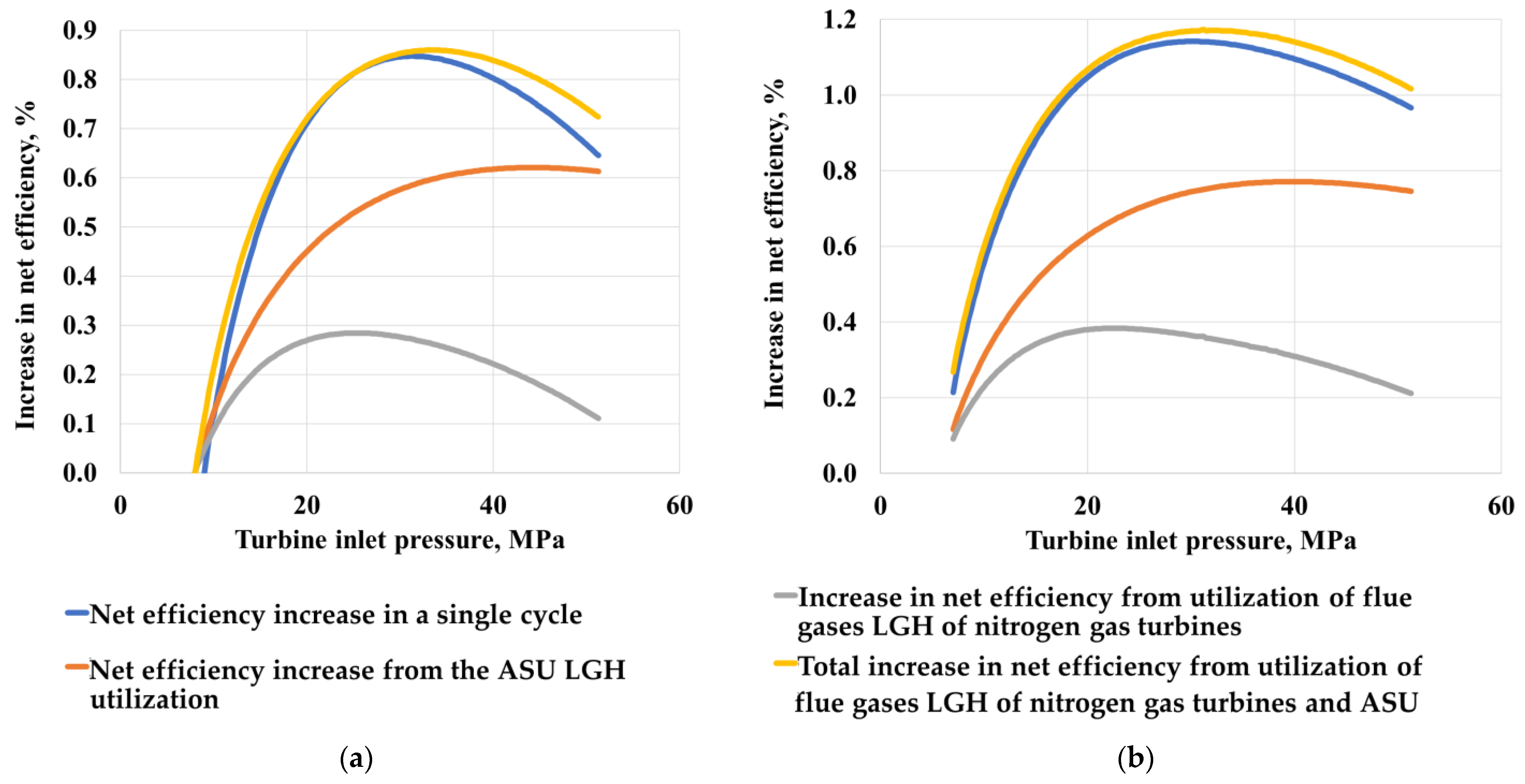
The thermodynamic optimization of the parameters of the LGH utilizing cycles (Figure 8) was carried out with varying pressure at the inlet and outlet of the turbine with a step of 1 bar from 10 MPa to 50 MPa. Initially, the pressure value at the turbine exhaust was fixed and the optimal value of the pressure at the inlet was determined. Then, the optimal initial pressure was fixed, and the pressure value at the turbine outlet was determined. At the same time, for the scheme of the organic Rankine cycle, the pressure at the outlet of the turbine was not optimized due to the limitation on the saturation pressure of the working flow.
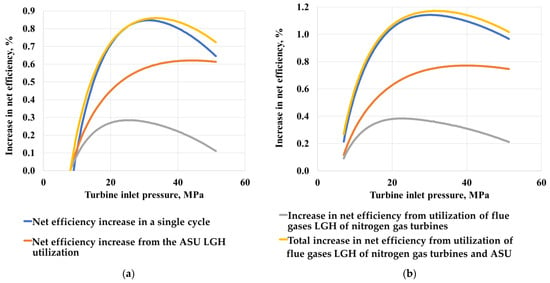
Figure 8.
The increase in the efficiency of the Allam cycle with coal gasification when using the Brayton cycle (a) and the Rankine cycle (b).
The results of a comparison of supercritical Rankine and Brayton cycles on carbon dioxide indicate that the greatest total increase in the efficiency of the Allam cycle is achieved when using the Rankine carbon dioxide cycle as a low-potential heat utilizer. This is due to the fact that when using the Brayton cycle, the temperature of the cooling flow at the inlet to the heat exchanger increases, which causes the temperature of the heating flow to rise at the outlet of the heat exchanger, which leads to an increase in the cost of compression in the compressor, due to a decrease in the density of the working flow. The Rankine cycle allows for deeper cooling to 30 °C, which significantly reduces the cost of cooling CO2 pumping.
The study determined that when the utilizing cycle is operating at low temperatures in the Brayton cycle, the use of a regenerator is impossible, since the temperature at the inlet to the utilizing heat exchanger rises, which reduces the amount of waste heat in the cycle. The absence of a regenerator drastically reduces the thermodynamic efficiency of the cycle, so the use of the Rankine cycle to use low-grade heat gives a higher power gain.
It is also worth noting that the maximum increase in energy efficiency occurs when the utilization cycle is separately connected to each source of low-grade heat rather than when using a single cycle with the combination of all sources. The energy efficiency of a separate LGH utilization is due to the fact that the use of a separate LGH utilization allows the low-boiling cycle to operate at the high temperatures of the working flow at the outlet of the carbon dioxide heat exchanger, due to which the net efficiency of the cycle with a low-boiling coolant increases, but the installation of several cycles will lead to large capital costs for equipment.
4. Conclusions
- This paper proposes new circuit solutions for oxygen–fuel energy complexes operating on the Allam cycle on coal fuel. The main sources of low-potential heat losses are determined and a unified scheme for its utilization is created;
- Several heat sources have the greatest potential for utilization, namely the compressed air flow in front of the ASU and the exhaust gases of nitrogen gas turbines, the total capacity of which is 40 MW. The coolers of the working flow in the carbon dioxide compressor have a high thermal power equal to 154.2 MW. However, these sources operate at low temperatures, due to which a large consumption of a low-boiling flow in the Rankine carbon dioxide cycle (1530 kg/s) is observed with a slight increase in power (2.1 MW);
- It has been established that benzene, due to its thermophysical properties, namely a saturation pressure of 15 kPa and density at 30 °C equal to 868 kg/m3, is the most effective coolant for organic Brayton and Rankine cycles; however, due to its toxicity, its use is contrary to the modern environmental requirements set for humanity. At the same time, carbon dioxide, which is the working heat of the main cycle and a by-product of the energy complex, is the most promising coolant for recycling cycles;
- It was determined that the use of the Rankine carbon dioxide cycle for the utilization of low-grade heat in the main cycle makes it possible to increase the efficiency of the power plant by 1.198%, and the use of the Brayton carbon dioxide cycle increases efficiency by 0.87%. In this regard, we can conclude that it is more expedient to use the organic Rankine cycle as a cycle for the utilization of low-grade heat.
Author Contributions
Conceptualization, V.K. and I.K.; methodology, V.K. and I.K.; software, D.K.; validation, D.P. and D.L.; formal analysis, D.L.; investigation, D.K., D.L. and D.P.; resources, D.P.; data curation, D.K.; writing—original draft preparation, D.P.; writing—review and editing, V.K.; visualization, D.K.; supervision, V.K.; project administration, I.K.; funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study conducted by the Moscow Power Engineering Institute was financially supported by the Ministry of Science and Higher Education of the Russian Federation (project no. FSWF-2020-0020).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Savaresi, A. The Paris Agreement: A new beginning? J. Energy Nat. Resour. Law 2016, 34, 16–26. [Google Scholar] [CrossRef]
- Tamme, E.; Beck, L.L. European Carbon Dioxide Removal Policy: Current Status and Future Opportunities. Front. Clim. 2021, 3, 682882. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, Y.; Yuan, J.; Wu, M.; Li, D.; Zhou, Y.; Kang, J. ESG and Corporate Financial Performance: Empirical Evidence from China’s Listed Power Generation Companies. Sustainability 2018, 10, 2607. [Google Scholar] [CrossRef]
- Walther, M. Sustainable Electric Power from a Responsible Investing Perspective. In Sustainable Electricity II; Springer: Cham, Switzerland, 2019; pp. 57–74. [Google Scholar]
- Chen, W.; Zhang, G.; Li, B.; Liu, M.; Liu, J. Simulation study on 660 MW coal-fired power plant coupled with a steam ejector to ensure NOx reduction ability. Appl. Therm. Eng. 2017, 111, 550–561. [Google Scholar] [CrossRef]
- Sohn, J.; Hwang, I.S.; Hwang, J. Improvement of ammonia mixing in an industrial scale selective catalytic reduction De-NOx system of a coal-fired power plant: A numerical analysis. Process. Saf. Environ. Prot. 2021, 147, 334–345. [Google Scholar] [CrossRef]
- Ma, T.; Takeuchi, K. Technology choice for reducing NOx emissions: An empirical study of Chinese power plants. Energy Policy 2017, 102, 362–376. [Google Scholar] [CrossRef]
- Dutta, R.; Nord, L.O.; Bolland, O. Selection and design of post-combustion CO2 capture process for 600 MW natural gas fueled thermal power plant based on operability. Energy 2017, 121, 643–656. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Emrouznejad, A.; Khosroshahi, H.; Khashei, M.; Rajabi, P. Performance evaluation of thermal power plants considering CO2 emission: A multistage PCA, clustering, game theory and data envelopment analysis. J. Clean. Prod. 2019, 223, 641–650. [Google Scholar] [CrossRef]
- Kindra, V.O.; Milukov, I.A.; Shevchenko, I.V.; Shabalova, S.I.; Kovalev, D.S. Thermodynamic analysis of cycle arrangements of the coal-fired thermal power plants with carbon capture. Arch. Thermodyn. 2021, 42, 103–121. [Google Scholar]
- Kanniche, M.; Le Moullec, Y.; Authier, O.; Hagi, H.; Bontemps, D.; Neveux, T.; Louis-Louisy, M. Up-to-date CO2 Capture in Thermal Power Plants. Energy Procedia 2017, 114, 95–103. [Google Scholar] [CrossRef]
- Peng, J.; Yu, B.-Y.; Liao, H.; Wei, Y.-M. Marginal abatement costs of CO2 emissions in the thermal power sector: A regional empirical analysis from China. J. Clean. Prod. 2018, 171, 163–174. [Google Scholar] [CrossRef]
- Hong, J.; Chaudhry, G.; Brisson, J.; Field, R.; Gazzino, M.; Ghoniem, A.F. Analysis of oxy-fuel combustion power cycle utilizing a pressurized coal combustor. Energy 2009, 34, 1332–1340. [Google Scholar] [CrossRef]
- Kindra, V.; Rogalev, A.; Zlyvko, O.; Sokolov, V.; Milukov, I. Research and development of a high-performance oxy-fuel combustion power cycle with coal gasification. Arch. Thermodyn. 2021, 42, 155–168. [Google Scholar]
- Kindra, V.; Osipov, S.; Zlyvko, O.; Shcherbatov, I.; Sokolov, V. Thermodynamic analysis of an innovative steam turbine power plant with oxy-methane combustors. Arch. Thermodyn. 2021, 42, 123–140. [Google Scholar]
- Allam, R.; Martin, S.; Forrest, B.; Fetvedt, J.; Lu, X.; Freed, D.; Brown, G.W., Jr.; Sasaki, T.; Itoh, M.; Manning, J. Demonstration of the Allam Cycle: An Update on the Development Status of a High Efficiency Supercritical Carbon Dioxide Power Process Employing Full Carbon Capture. Energy Procedia 2017, 114, 5948–5966. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Wu, J.; Zhang, S.; Zheng, S. A modified Allam cycle without compressors realizing efficient power generation with peak load shifting and CO2 capture. Energy 2019, 174, 478–487. [Google Scholar] [CrossRef]
- Thomas, C.E. Petroleum and coal proven reserves: The case for coal and the demise of OPEC. In Stopping Climate Change: The Case for Hydrogen and Coal; Springer: Cham, Switzerland, 2017; pp. 35–40. [Google Scholar]
- Maddahi, L.; Hossainpour, S. Thermo- economic evaluation of 300 MW coal based oxy-fuel power plant integrated with organic Rankine cycle. Int. J. Greenh. Gas Control 2019, 88, 383–392. [Google Scholar] [CrossRef]
- Rogalev, A.; Rogalev, N.; Kindra, V.; Zlyvko, O.; Vegera, A. A Study of Low-Potential Heat Utilization Methods for Oxy-Fuel Combustion Power Cycles. Energies 2021, 14, 3364. [Google Scholar] [CrossRef]
- Mitchell, C.; Avagyan, V.; Chalmers, H.; Lucquiaud, M. An initial assessment of the value of Allam Cycle power plants with liquid oxygen storage in future GB electricity system. Int. J. Greenh. Gas Control 2019, 87, 1–18. [Google Scholar] [CrossRef]
- Allam, R.J.; Fetvedt, J.E.; Forrest, B.A.; Freed, D.A. The Oxy-Fuel, Supercritical CO2 Allam Cycle: New Cycle Developments to Produce Even Lower-Cost Electricity From Fossil Fuels Without Atmospheric Emissions. In Volume 3B: Oil and Gas Applications; Organic Rankine Cycle Power Systems; Supercritical CO2 Power Cycles; Wind Energy; American Society of Mechanical Engineers: Düsseldorf, Germany, 2014; p. V03BT36A016. [Google Scholar]
- Hung, T.; Wang, S.; Kuo, C.; Pei, B.; Tsai, K. A study of organic working fluids on system efficiency of an ORC using low-grade energy sources. Energy 2010, 35, 1403–1411. [Google Scholar] [CrossRef]
- Drescher, U.; Brüggemann, D. Fluid selection for the Organic Rankine Cycle (ORC) in biomass power and heat plants. Appl. Therm. Eng. 2007, 27, 223–228. [Google Scholar] [CrossRef]
- Branchini, L.; De Pascale, A.; Peretto, A. Systematic comparison of ORC configurations by means of comprehensive performance indexes. Appl. Therm. Eng. 2013, 61, 129–140. [Google Scholar] [CrossRef]
- Ho, C.K.; Carlson, M.; Garg, P.; Kumar, P. Technoeconomic Analysis of Alternative Solarized s-CO2 Brayton Cycle Configurations. J. Sol. Energy Eng. 2016, 138, 051008. [Google Scholar] [CrossRef]
- Wang, L.; Pan, L.-M.; Wang, J.; Chen, D.; Huang, Y.; Hu, L. Investigation on the temperature sensitivity of the S-CO2 Brayton cycle efficiency. Energy 2019, 178, 739–750. [Google Scholar] [CrossRef]
- Kindra, V.; Rogalev, A.; Zlyvko, O.V.; Zonov, A.; Smirnov, M.; Kaplanovich, I. Research on oxy-fuel combustion power cycle using nitrogen for turbine cooling. Arch. Thermodyn. 2020, 41, 191–202. [Google Scholar]
- Shevchenko, I.V.; Rogalev, N.D.; Kindra, V.O.; Osipov, S.K.; Rostova, D.M. Numerical analysis of the influence of turbulators constructive features on heat transfer in gas turbine blade cooling channels. Int. J. Appl. Eng. Res. 2017, 12, 6853–6861. [Google Scholar]
- Chan, W.; Lei, X.; Chang, F.; Li, H. Thermodynamic analysis and optimization of Allam cycle with a reheating configuration. Energy Convers. Manag. 2020, 224, 113382. [Google Scholar] [CrossRef]
- Cormos, C.C. Integrated assessment of IGCC power generation technology with carbon capture and storage (CCS). Energy 2012, 42, 434–445. [Google Scholar] [CrossRef]
- Matuszewska, D. Molecular Complexity of Working Fluids Dedicated to Organic Rankine Cycle (ORC). In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 946, p. 012008. [Google Scholar]
- Liu, Z.; Yang, X.; Liu, X.; Yu, Z.; Chen, Y. Performance assessment of a novel combined heating and power system based on transcritical CO2 power and heat pump cycles using geothermal energy. Energy Convers. Manag. 2020, 224, 113355. [Google Scholar] [CrossRef]
- Ahn, Y.; Bae, S.J.; Kim, M.; Cho, S.K.; Baik, S.; Lee, J.I.; Cha, J.E. Review of supercritical CO2 power cycle technology and current status of research and development. Nucl. Eng. Technol. 2015, 47, 647–661. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Huang, D. Supercritical CO2 Brayton cycle: A state-of-the-art review. Energy 2019, 189, 115900. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Meratizaman, M.; Reyhani, H.A.; Pourali, O.; Amidpour, M. Energetic, exergetic and economic assessment of oxygen production from two columns cryogenic air separation unit. Energy 2015, 90, 1298–1316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).