The Effects of Anodization Conditions on TiO2 Nanotubes Features Obtained Using Aqueous Electrolytes with Xanthan Gum
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
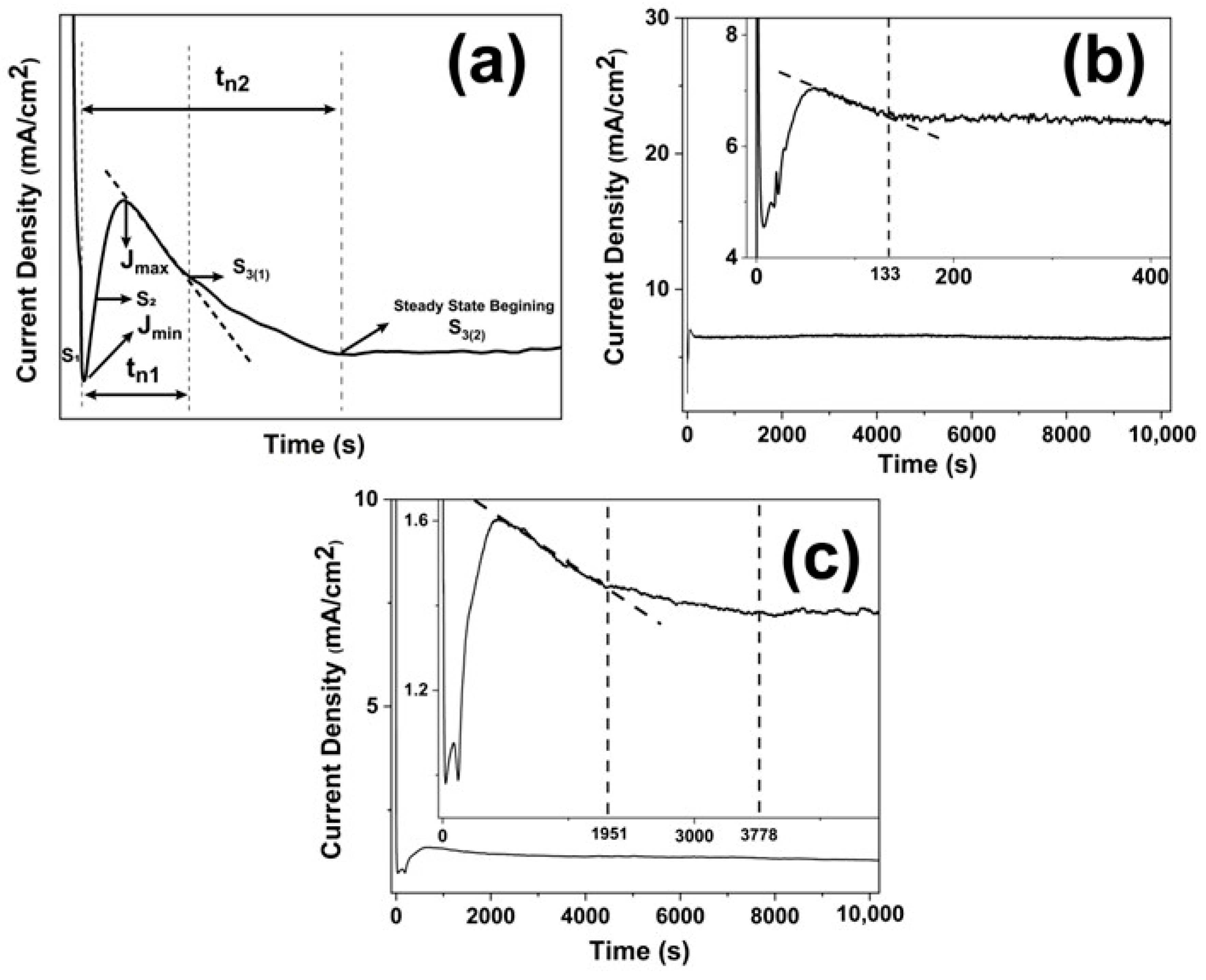
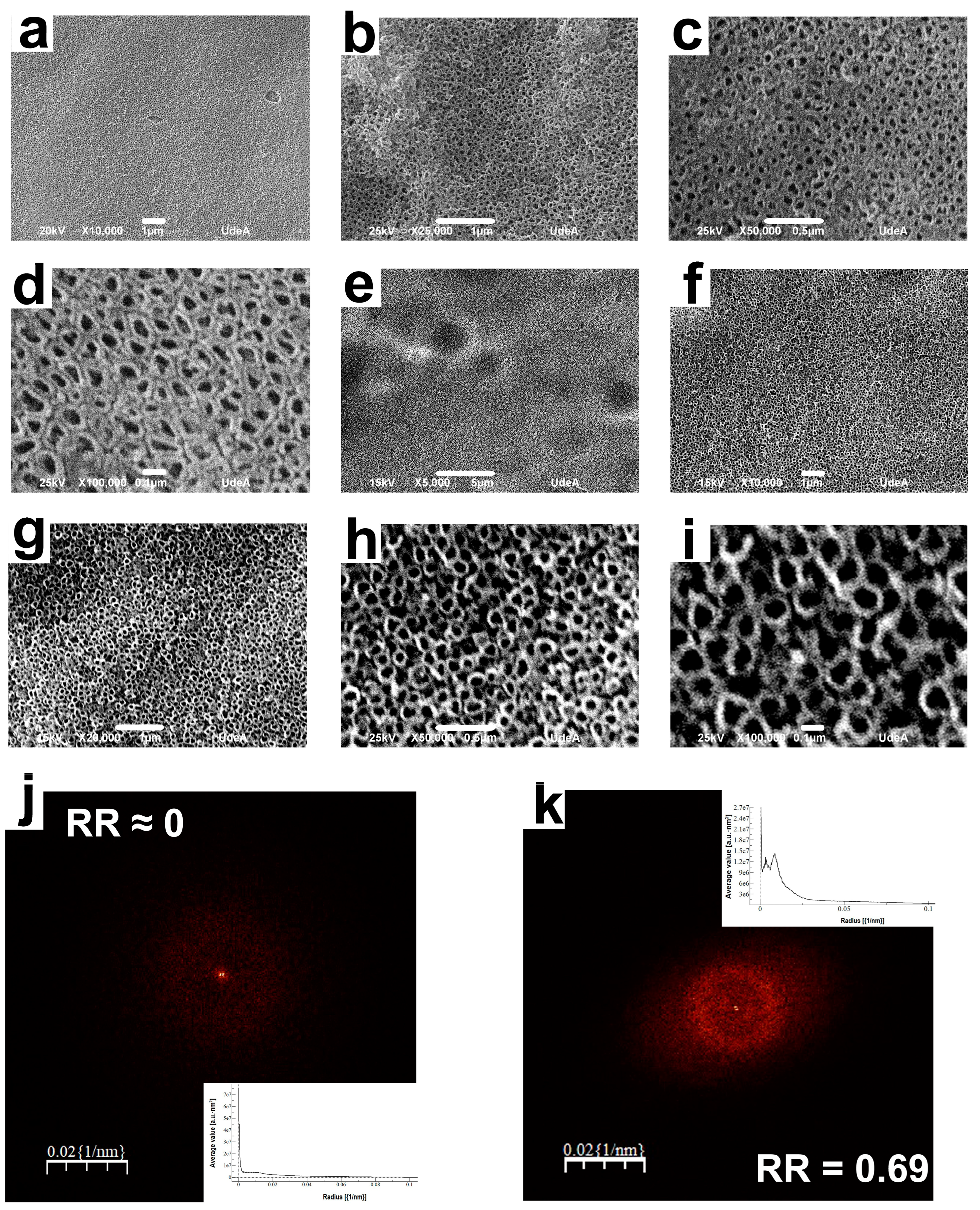
3.1. Current Density vs. Time Transients and Nanotube Organization
3.2. The Effect of the Anodization Time
3.3. The Effect of NaF Concentration
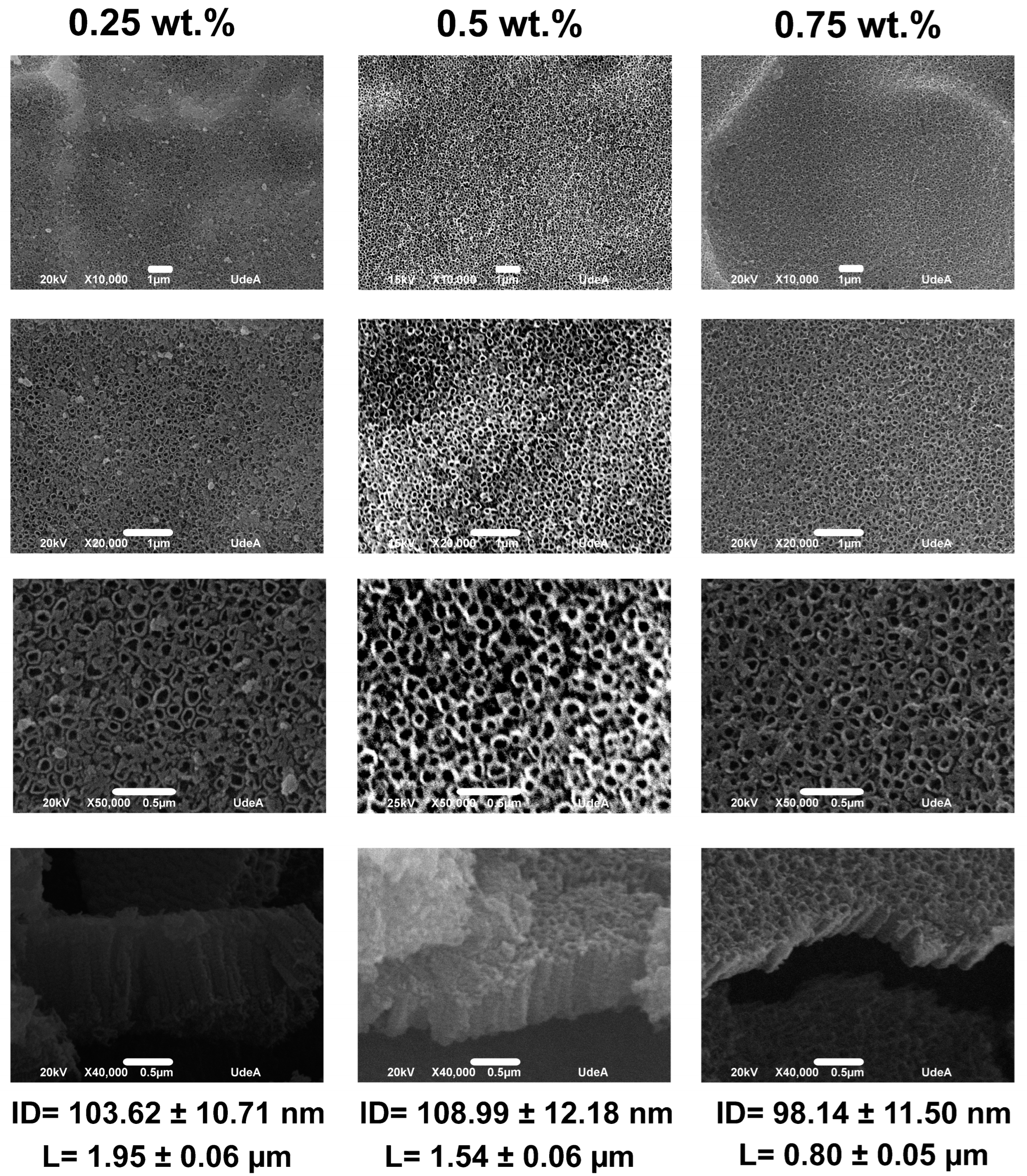
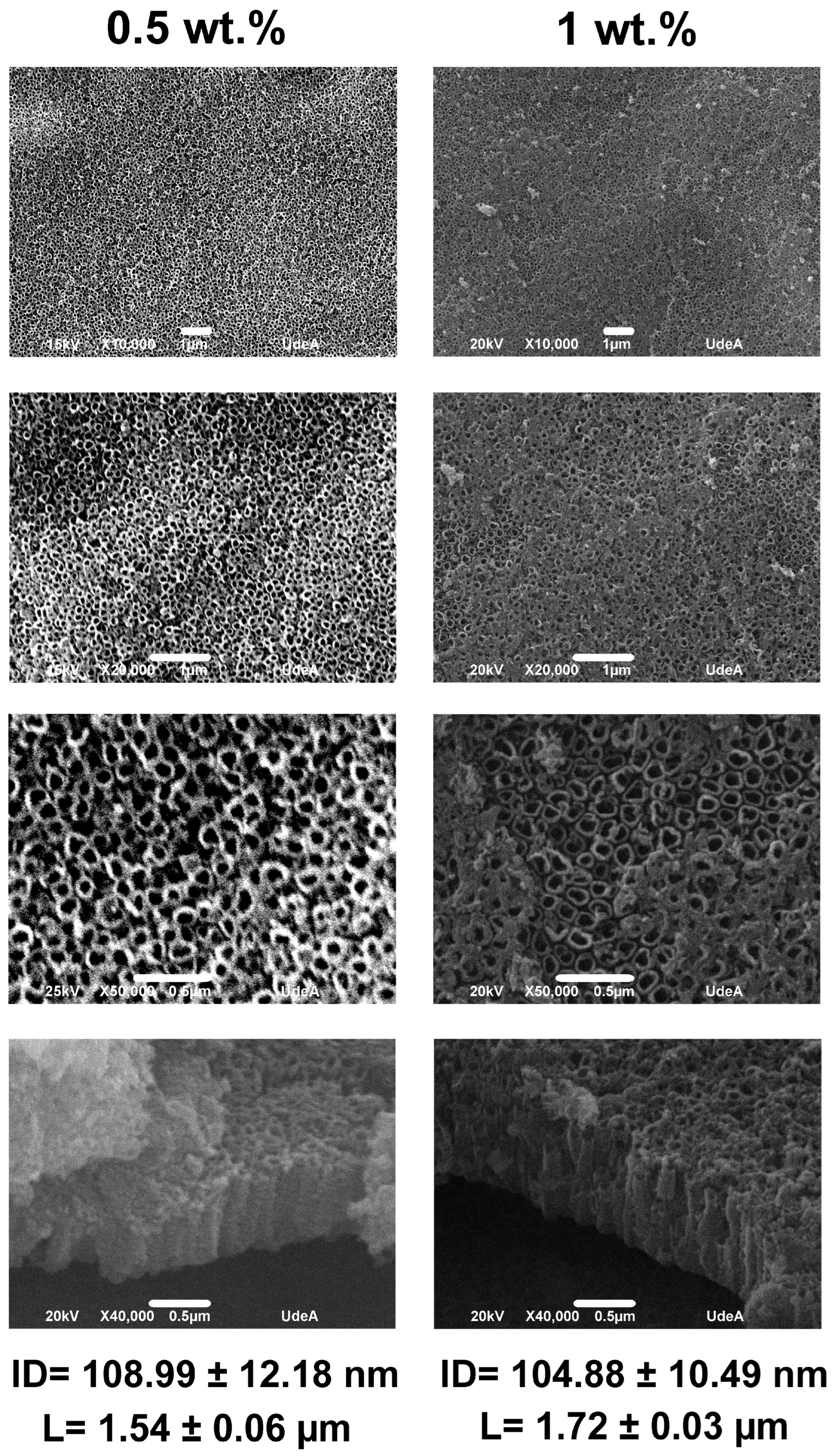
3.4. The Effect of XG Concentration
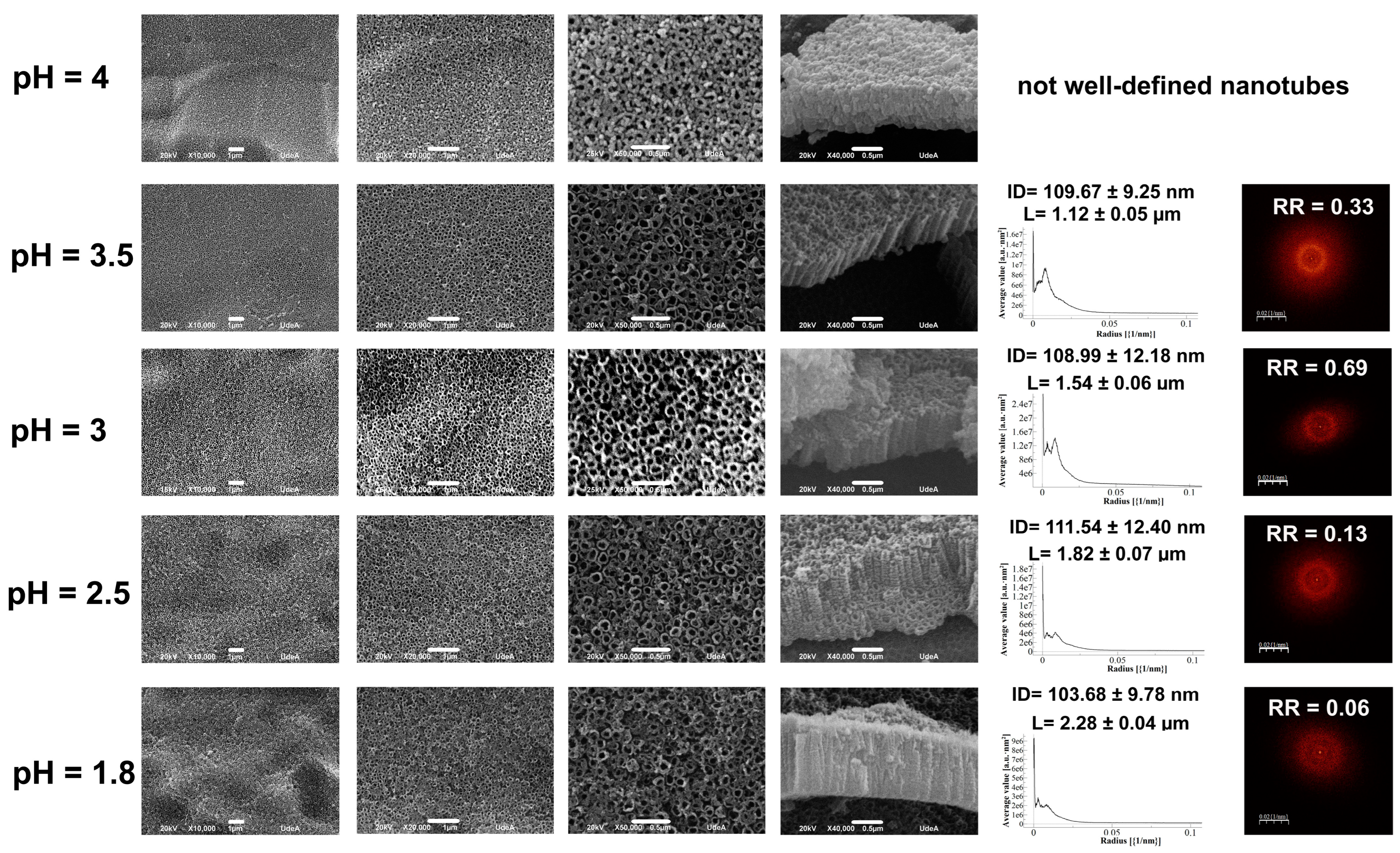
3.5. Electrolyte pH Effect
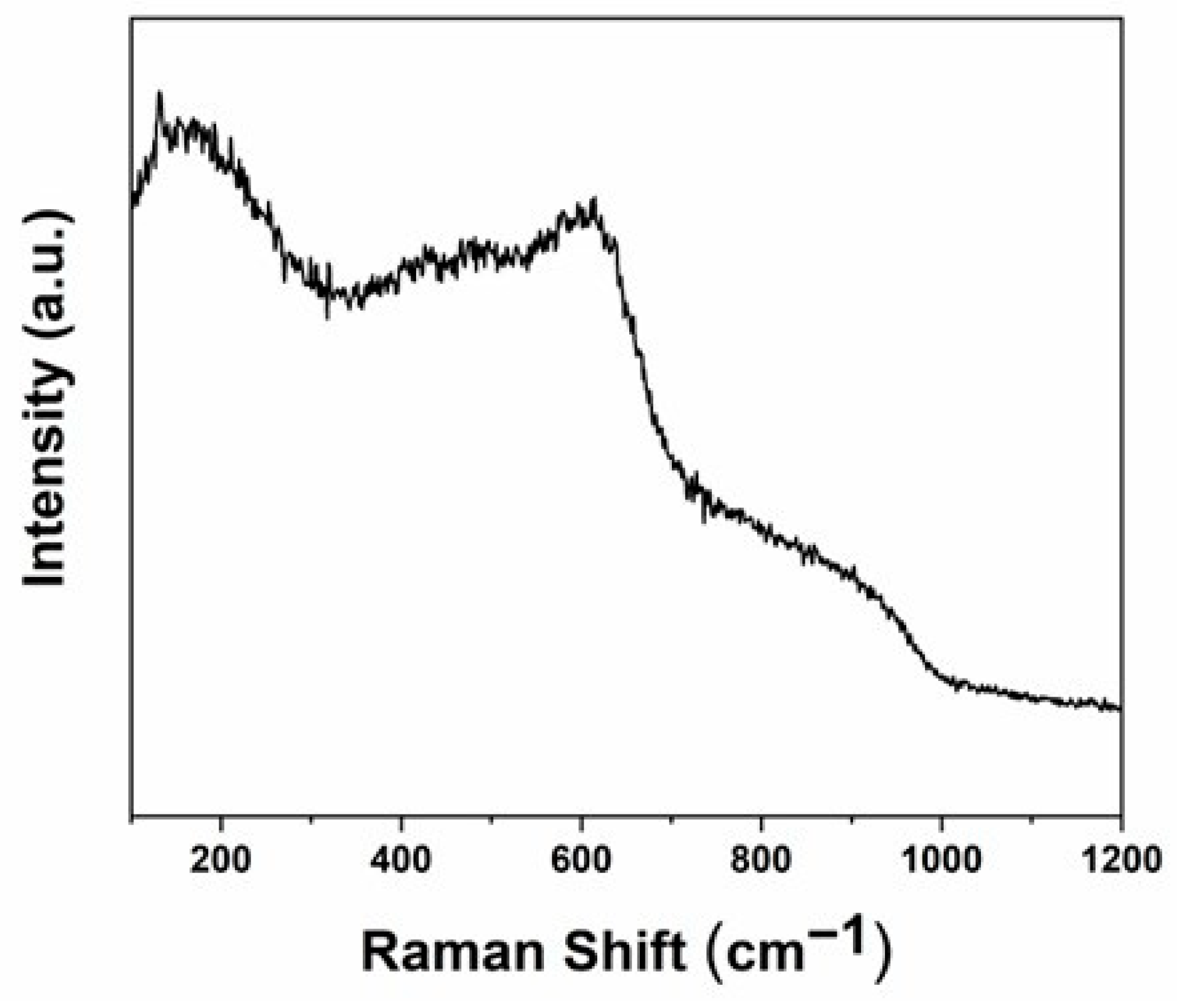
3.6. Raman Characterization
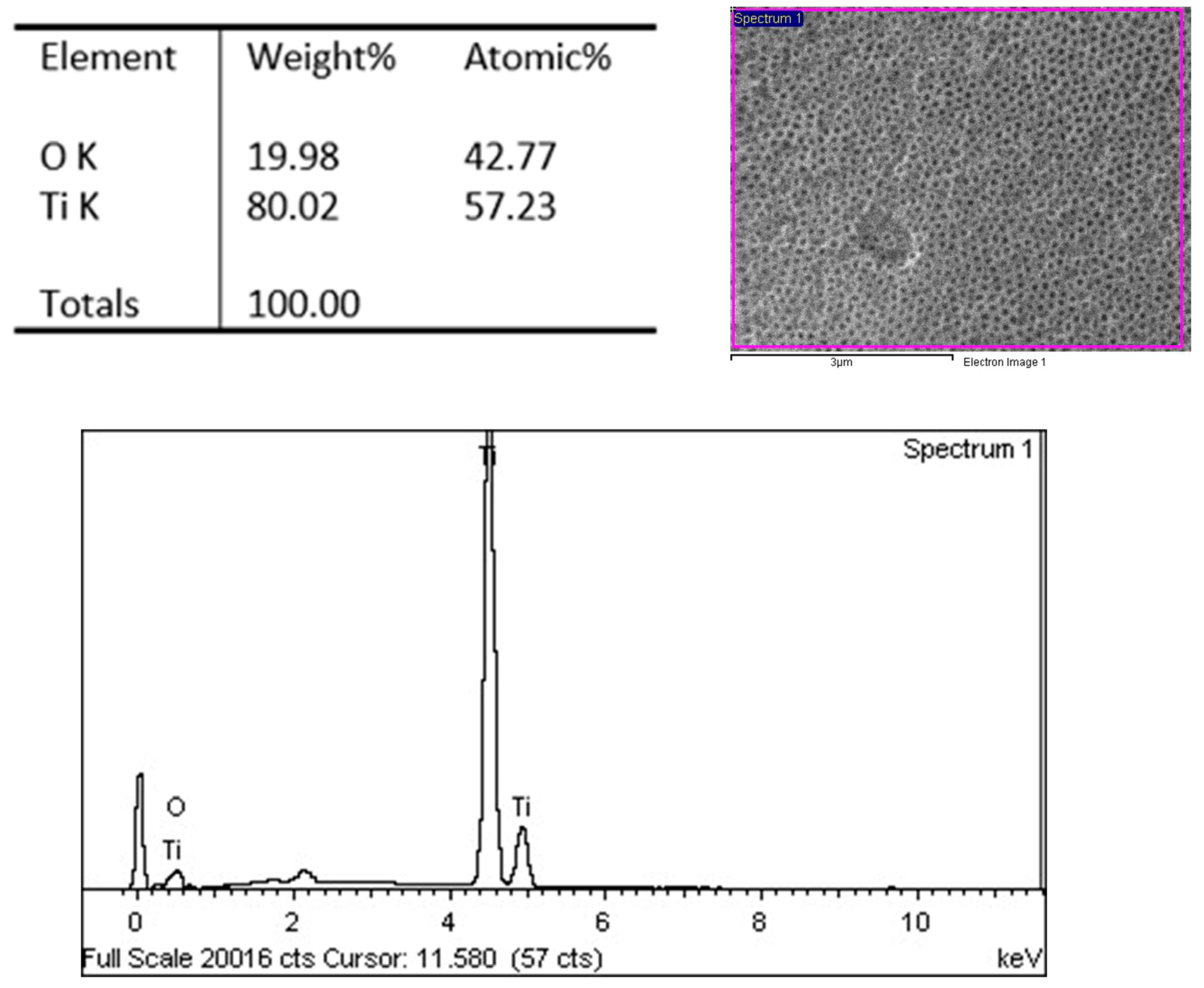
3.7. EDX Characterization
4. Conclusions
- Compared with the nanotubes formed in an aqueous electrolyte without XG, using XG in the aqueous electrolyte increased the nanotube organization; the value of the RR parameter of nanotubes obtained with and without adding XG to the anodizing electrolyte was 0.69 and 0.0, respectively. In addition, the inclusion of XG did not affect the packability of the coating.
- Anodization parameters like fluoride concentration, anodizing time, pH, and XG concentration did not affect the internal diameter values, but produced changes in the length of the nanotubes; the values obtained for the internal diameter in the various conditions evaluated were around 100 nm, whereas the nanotube lengths varied from about 0.80 to 2.28 µm.
- Based on the outcomes of this experimental study, organized nanotubular structures could be obtained using an aqueous electrolyte which is low-cost, environmentally friendly, and non-toxic. According to the present results, the more suitable conditions are 0.5 wt.% XG, 0.5 wt.% NaF, a pH of 3, and an anodizing time of 3 h.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Echeverry-Rendón, M.; Galvis, O.; Aguirre, R.; Robledo, S.; Castaño, J.G.; Echeverría, F. Modification of Titanium Alloys Surface Properties by Plasma Electrolytic Oxidation (PEO) and Influence on Biological Response. J. Mater. Sci. Mater. Med. 2017, 28, 169. [Google Scholar] [CrossRef]
- Kim, W.T.; Choi, W.Y. Anodic Growth Behavior of TiO2 Nanotube Arrays with Process Parameter Control. J. Nanomater. 2019, 2019, 8293427. [Google Scholar] [CrossRef]
- Aguirre, R.; Nicolás, O.; Ochoa, B.; Tamayo, J.A.; Botero, C. Formation of Highly Ordered TiO2 Nanotubes on Ti6Al4V Alloys Manufactured by Electron Beam Powder Bed Fusion (E-PBF). Int. J. Adv. Manuf. Technol. 2023, 128, 257–266. [Google Scholar] [CrossRef]
- Aguirre Ocampo, R.; Echeverría Echeverría, F. Effect of the Anodization Parameters on TiO2 Nanotubes Characteristics Produced in Aqueous Electrolytes with CMC. Appl. Surf. Sci. 2019, 469, 994–1006. [Google Scholar] [CrossRef]
- Ocampo, R.A.; Echeverría, F.E. Antibacterial and Biological Behavior of TiO2 Nanotubes Produced by Anodizing Technique. Crit. Rev. Biomed. Eng. 2021, 49, 51–65. [Google Scholar] [CrossRef]
- Aguirre Ocampo, R.; Echeverry-Rendón, M.; Robledo, S.; Echeverría Echeverría, F. Effect of TiO2 Nanotubes Size, Heat Treatment, and UV Irradiation on Osteoblast Behavior. Mater. Chem. Phys. 2022, 275, 125137. [Google Scholar] [CrossRef]
- Puga, M.L.; Venturini, J.; ten Caten, C.S.; Bergmann, C.P. Influencing Parameters in the Electrochemical Anodization of TiO2 Nanotubes: Systematic Review and Meta-Analysis. Ceram. Int. 2022, 48, 19513–19526. [Google Scholar] [CrossRef]
- Cao, W.; Chen, K.; Xue, D. Highly Ordered TiO2 Nanotube Arrays with Engineered Electrochemical Energy Storage Performances. Materials 2021, 14, 510. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Guo, F.; Liu, J.; Yang, F. Kinetic Analysis of the Anodic Growth of TiO2 Nanotubes: Effects of Voltage and Temperature. J. Mater. Chem. C 2019, 7, 14098–14108. [Google Scholar] [CrossRef]
- Aguirre Ocampo, R.; Echeverría Echeverría, F. TiO2 Nanotubes Produced on Curved Titanium Surfaces Using Aqueous Electrolytes with Carboxymethyl Cellulose. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 125, 114391. [Google Scholar] [CrossRef]
- Ocampo, R.A.; Echeverry-Rendón, M.; DeAlba-Montero, I.; Robledo, S.; Ruiz, F.; Echeverría Echeverría, F. Effect of Surface Characteristics on the Antibacterial Properties of TiO2 Nanotubes Produced in Aqueous Electrolytes with Carboxymethyl Cellulose. J. Biomed. Mater. Res. Part A 2021, 109, 104–121. [Google Scholar] [CrossRef]
- Asgari, V.; Noormohammadi, M.; Ramazani, A.; Kashi, M.A. A Facile Method to Form Highly-Ordered TiO2 nanotubes at a Stable Growth Rate of 1000 Nm Min-1under 60 v Using an Organic Electrolyte for Improved Photovoltaic Properties. J. Phys. D Appl. Phys. 2017, 50, 375501. [Google Scholar] [CrossRef]
- Nie, X.; Yin, S.; Duan, W.; Zhao, Z.; Li, L.; Zhang, Z. Recent Progress in Anodic Oxidation of TiO2 Nanotubes and Enhanced Photocatalytic Performance: A Short Review. Nano 2021, 16, 2130002. [Google Scholar] [CrossRef]
- Ghani, T.; Mujahid, M.; Mehmood, M.; Zhang, G. Fabrication of Self-Branched TiO2 Nanotubes by Anodization Method, Ordering and Crystallinity. J. Porous Mater. 2019, 26, 193–203. [Google Scholar] [CrossRef]
- Fraoucene, H.; Hatem, D.; Vacandio, F.; Pasquinelli, M. TiO2 Nanotubes with Nanograss Structure: The Effect of the Anodizing Voltage on the Formation Mechanism and Structure Properties. J. Electron. Mater. 2019, 48, 2046–2054. [Google Scholar] [CrossRef]
- Hu, N.; Wu, Y.; Xie, L.; Mohd, S.; Gao, N.; Starink, M.J.; Tong, L.; Chu, P.K.; Wang, H. Enhanced Interfacial Adhesion and Osseointegration of Anodic TiO2 Nanotube Arrays on Ultra-Fine-Grained Titanium and Underlying Mechanisms. Acta Biomater. 2020, 106, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Dev, P.R.; David, T.M.; Samuel Justin, S.J.; Wilson, P.; Palaniappan, A. A Plausible Impact on the Role of Pulses in Anodized TiO2 Nanotube Arrays Enhancing Ti3+ Defects. J. Nanoparticle Res. 2020, 22, 56. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, N.; Ji, X.; Laaksonen, A.; Lu, X.; Zhang, S. Excellent Trace Detection of Proteins on TiO2 Nanotube Substrates through Novel Topography Optimization. J. Phys. Chem. C 2020, 124, 27790–27800. [Google Scholar] [CrossRef]
- Alijani, M.; Sopha, H.; Ng, S.; Macak, J.M. High Aspect Ratio TiO2 Nanotube Layers Obtained in a Very Short Anodization Time. Electrochim. Acta 2021, 376, 138080. [Google Scholar] [CrossRef]
- Luan, N.H.; Chang, C. Investigation of the Aging Effect of Electrolyte on the Morphology and Photocatalytic Properties of TiO2 NTs Synthesized Using the Anodization Route. J. Mater. Sci. 2021, 56, 19106–19118. [Google Scholar] [CrossRef]
- Ribeiro, B.; Offoiach, R.; Rossetti, S.; Salatin, E.; Lekka, M.; Fedrizzi, L. On Growth and Morphology of TiO2 Nanotubes on CP-Ti by Anodic Oxidation in Ethylene Glycol Electrolyte: Influence of Electrolyte Aging and Anodization Parameters. Materials 2022, 15, 3338. [Google Scholar] [CrossRef]
- Ocampo, R.A.; Echeverría, F.E. TiO2 Nanotubes Produced on Thin Titanium Wires Using Aqueous Electrolytes. Mater. Manuf. Process. 2022, 1–7. [Google Scholar] [CrossRef]
- Mohiuddin, I.; Mohmad, S.; Ahmad, S.; Masoodi, F.A. Advances in Xanthan Gum Production, Modifications and Its Applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Use of Xanthan Gum for Whole Cell Immobilization and Its Impact in Bioremediation—A Review. Bioresour. Technol. 2022, 351, 126918. [Google Scholar] [CrossRef] [PubMed]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A Software for Scanning Probe Microscopy and a Tool for Nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Apolinário, A.; Sousa, C.T.; Ventura, J.; Costa, J.D.; Leitão, D.C.; Moreira, J.M.; Sousa, J.B.; Andrade, L.; Mendes, A.M.; Araújo, J.P. The Role of the Ti Surface Roughness in the Self-Ordering of TiO2 Nanotubes: A Detailed Study of the Growth Mechanism. J. Mater. Chem. A 2014, 2, 9067. [Google Scholar] [CrossRef]
- Gong, T.; Li, C.; Li, X.; Yue, H.; Zhu, X.; Zhao, Z.; Lv, R.; Zhu, J. Evidence of Oxygen Bubbles Forming Nanotube Embryos in Porous Anodic Oxides. Nanoscale Adv. 2021, 3, 4659–4668. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, J.; Gong, T.; Sun, M.; Zhao, Z.; Li, X.; Yu, H.; Zhu, X. Quantitative Analysis of the Volume Expansion of Nanotubes during Constant Voltage Anodization. Surf. Interfaces 2021, 26, 101419. [Google Scholar] [CrossRef]
- Xing-Ping, F. Controllable Preparation of TiO2 Nanotubes and Their Photocatalytic Performance. Mater. Res. Express 2022, 9, 065005. [Google Scholar] [CrossRef]
- Regonini, D.; Clemens, F.J. Anodized TiO2 Nanotubes: Effect of Anodizing Time on Film Length, Morphology and Photoelectrochemical Properties. Mater. Lett. 2015, 142, 97–101. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Y.; Guo, T. Effect of H2O Content in Electrolyte on Synthesis and Field Emission Property of Anodized TiO2 Nanotubes. Surf. Coat. Technol. 2014, 245, 28–33. [Google Scholar] [CrossRef]
- Aguirre, O.R.; Echeverría, E.F. Effects of Fluoride Source on the Characteristics of Titanium Dioxide Nanotubes. Appl. Surf. Sci. 2018, 445, 308–319. [Google Scholar] [CrossRef]
- Aguirre, R.; Echeverry-Rendón, M.; Quintero, D.; Castaño, J.G.; Harmsen, M.C.; Robledo, S.; Echeverría, E.F. Formation of Nanotubular TiO2 Structures with Varied Surface Characteristics for Biomaterial Applications. J. Biomed. Mater. Res. Part A 2018, 106, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Abdulmajeed, A.A.; Moon, J.; Shahramian, K.; Punkkinen, R.; Shimada, J.; Vallittu, P.K.; Lassila, L.V. The Effect of Ultraviolet Treatment on TiO2 Nanotubes: A Study of Surface Characteristics, Bacterial Adhesion, and Gingival Fibroblast Response. Metals 2022, 12, 80. [Google Scholar] [CrossRef]
- Kim, M.; Shin, N.; Lee, J.; Lee, K.; Kim, Y.T.; Choi, J. Photoelectrochemical Water Oxidation in Anodic TiO2 Nanotubes Array: Importance of Mass Transfer. Electrochem. Commun. 2021, 132, 107133. [Google Scholar] [CrossRef]
- Stȩpniowski, W.J.; Michalska-Domańska, M.; Norek, M.; Czujko, T. Fast Fourier Transform Based Arrangement Analysis of Poorly Organized Alumina Nanopores Formed via Self-Organized Anodization in Chromic Acid. Mater. Lett. 2014, 117, 69–73. [Google Scholar] [CrossRef]
- Farsinezhad, S.; Dalrymple, A.N.; Shankar, K. Toward Single-Step Anodic Fabrication of Monodisperse TiO2 Nanotube Arrays on Non-Native Substrates. Phys. Status Solidi Appl. Mater. Sci. 2014, 211, 1113–1121. [Google Scholar] [CrossRef]
- Zaraska, L.; St??pniowski, W.J.; Ciepiela, E.; Sulka, G.D. The Effect of Anodizing Temperature on Structural Features and Hexagonal Arrangement of Nanopores in Alumina Synthesized by Two-Step Anodizing in Oxalic Acid. Thin Solid Film. 2013, 534, 155–161. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Stępniowski, W.J.; Jaroszewicz, L.R. Characterization of Nanopores Arrangement of Anodic Alumina Layers Synthesized on Low-(AA1050) and High-Purity Aluminum by Two-Step Anodizing in Sulfuric Acid with Addition of Ethylene Glycol at Low Temperature. J. Porous Mater. 2016, 24, 779–786. [Google Scholar] [CrossRef]
- Jarosz, M.; Kapusta-Kołodziej, J.; Jaskuła, M.; Sulka, G.D. Effect of Different Polishing Methods on Anodic Titanium Dioxide Formation. J. Nanomater. 2015, 2015, 295126. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.; Oh, C.; Ryu, J.; Kim, H.; Park, E.; No, K.; Hong, S. Effects of NH4F and Distilled Water on Structure of Pores in TiO2 Nanotube Arrays. Sci. Rep. 2018, 8, 12487. [Google Scholar] [CrossRef]
- Tang, Y.; Tao, J.; Dong, Z.; Oh, J.T.; Chen, Z. The Formation of Micrometer-Long TiO2 Nanotube Arrays by Anodization of Titanium Film on Conducting Glass Substrate. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 045002. [Google Scholar] [CrossRef]
- Khudhair, D.; Bhatti, A.; Li, Y.; Hamedani, H.A.; Garmestani, H.; Hodgson, P.; Nahavandi, S. Anodization Parameters Influencing the Morphology and Electrical Properties of TiO2 Nanotubes for Living Cell Interfacing and Investigations. Mater. Sci. Eng. C 2016, 59, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gulati, K.; Wang, N.; Zhang, Z.; Ivanovski, S. Understanding and Augmenting the Stability of Therapeutic Nanotubes on Anodized Titanium Implants. Mater. Sci. Eng. C 2018, 88, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Song, H.; Huang, Y.; Su, R.; Besenbacher, F. Fluoride Concentration Controlled TiO2 Nanotubes: The Interplay of Microstructure and Photocatalytic Performance. RSC Adv. 2016, 6, 18333–18339. [Google Scholar] [CrossRef]
- Raji, H.A.; Moayed, R.Z.; Nozari, M.A. The Viscosity of Xanthan Gum Grout with Different PH and Ionic Strength. Int. J. Geotech. Geol. Eng. 2021, 15, 38–43. [Google Scholar]
- Sturgeon, M.R.; Lai, P.; Hu, M.Z. A Comparative Study of Anodized Titania Nanotube Architectures in Aqueous and Nonaqueous Solutions. J. Mater. Res. 2011, 26, 2612–2623. [Google Scholar] [CrossRef]
- Regonini, D.; Satka, a.; Jaroenworaluck, a.; Allsopp, D.W.E.; Bowen, C.R.; Stevens, R. Factors Influencing Surface Morphology of Anodized TiO2 Nanotubes. Electrochim. Acta 2012, 74, 244–253. [Google Scholar] [CrossRef]
- Sreekantan, S.; Lockman, Z.; Hazan, R.; Tasbihi, M.; Tong, L.K.; Mohamed, A.R. Influence of Electrolyte PH on TiO2 Nanotube Formation by Ti Anodization. J. Alloys Compd. 2009, 485, 478–483. [Google Scholar] [CrossRef]
- Lai, C.W.; Sreekantan, S. Photoelectrochemical Properties of TiO2 Nanotube Arrays: Effect of Electrolyte PH and Annealing Temperature. J. Exp. Nanosci. 2012, 9, 230–239. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio- Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Paulose, M.; Varghese, O.K.; Mor, G.K.; Grimes, C.A.; Ong, K.G. Unprecedented Ultra-High Hydrogen Gas Sensitivity in Undoped Titania Nanotubes. Nanotechnology 2006, 17, 398–402. [Google Scholar] [CrossRef]
- Brunchi, C.E.; Bercea, M.; Morariu, S.; Dascalu, M. Some Properties of Xanthan Gum in Aqueous Solutions: Effect of Temperature and PH. J. Polym. Res. 2016, 23, 123. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan Gum in Aqueous Solutions: Fundamentals and Applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Bejenariu, A.; Popa, M.; Picton, L.; Le Cerf, D. Effect of Concentration, PH and Temperature on Xanthan Conformation: A Preliminary Study before Crosslinking. Rev. Roum. Chim. 2010, 55, 147–152. [Google Scholar]
- Hardcastle, F. Raman Spectroscopy of Titania (TiO2) Nanotubular Water-Splitting Catalysts. J. Ark. Acad. Sci. 2011, 65, 43–48. [Google Scholar] [CrossRef]
- Albu, S.P.; Taccardi, N.; Paramasivam, I.; Hebert, K.R.; Schmuki, P. Oxide Growth Efficiencies and Self-Organization of TiO2 Nanotubes. J. Electrochem. Soc. 2012, 159, 697–703. [Google Scholar] [CrossRef][Green Version]
- Necula, M.G.; Mazare, A.; Ion, R.N.; Ozkan, S.; Park, J.; Schmuki, P.; Cimpean, A. Lateral Spacing of TiO2 Nanotubes Modulates Osteoblast Behavior. Materials 2019, 12, 2956. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirre Ocampo, R.; Echeverría Echeverría, F. The Effects of Anodization Conditions on TiO2 Nanotubes Features Obtained Using Aqueous Electrolytes with Xanthan Gum. Inventions 2023, 8, 109. https://doi.org/10.3390/inventions8050109
Aguirre Ocampo R, Echeverría Echeverría F. The Effects of Anodization Conditions on TiO2 Nanotubes Features Obtained Using Aqueous Electrolytes with Xanthan Gum. Inventions. 2023; 8(5):109. https://doi.org/10.3390/inventions8050109
Chicago/Turabian StyleAguirre Ocampo, Robinson, and Félix Echeverría Echeverría. 2023. "The Effects of Anodization Conditions on TiO2 Nanotubes Features Obtained Using Aqueous Electrolytes with Xanthan Gum" Inventions 8, no. 5: 109. https://doi.org/10.3390/inventions8050109
APA StyleAguirre Ocampo, R., & Echeverría Echeverría, F. (2023). The Effects of Anodization Conditions on TiO2 Nanotubes Features Obtained Using Aqueous Electrolytes with Xanthan Gum. Inventions, 8(5), 109. https://doi.org/10.3390/inventions8050109




