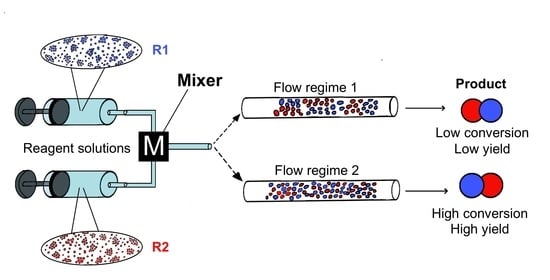
Mixer Design and Flow Rate as Critical Variables in Flow Chemistry Affecting the Outcome of a Chemical Reaction: A Review
Abstract
:1. Introduction
- Flow chemistry facilitates the optimization of reactions at a much faster rate, resulting in a reduction in costs and time. Reaction time, temperature, ratio of reagents, concentration, and reagents themselves can all be rapidly modified. One reaction can be followed by another, separated by solvent, increasing throughput. Automated flow chemistry allows for rapid variation of reaction conditions on a very small scale (≤100 μL).
- Performing reactions in flow can be safer than in batch reactors, since only a small portion of reactive or hazardous material is transformed to product at any particular moment.
- The use of flow chemistry has the advantage of enhanced heat and mass transfer, which can result in faster reaction rates and higher yields.
- Flow reactors provide excellent reaction selectivity and allow for preparation of products that are not accessible under batch conditions.
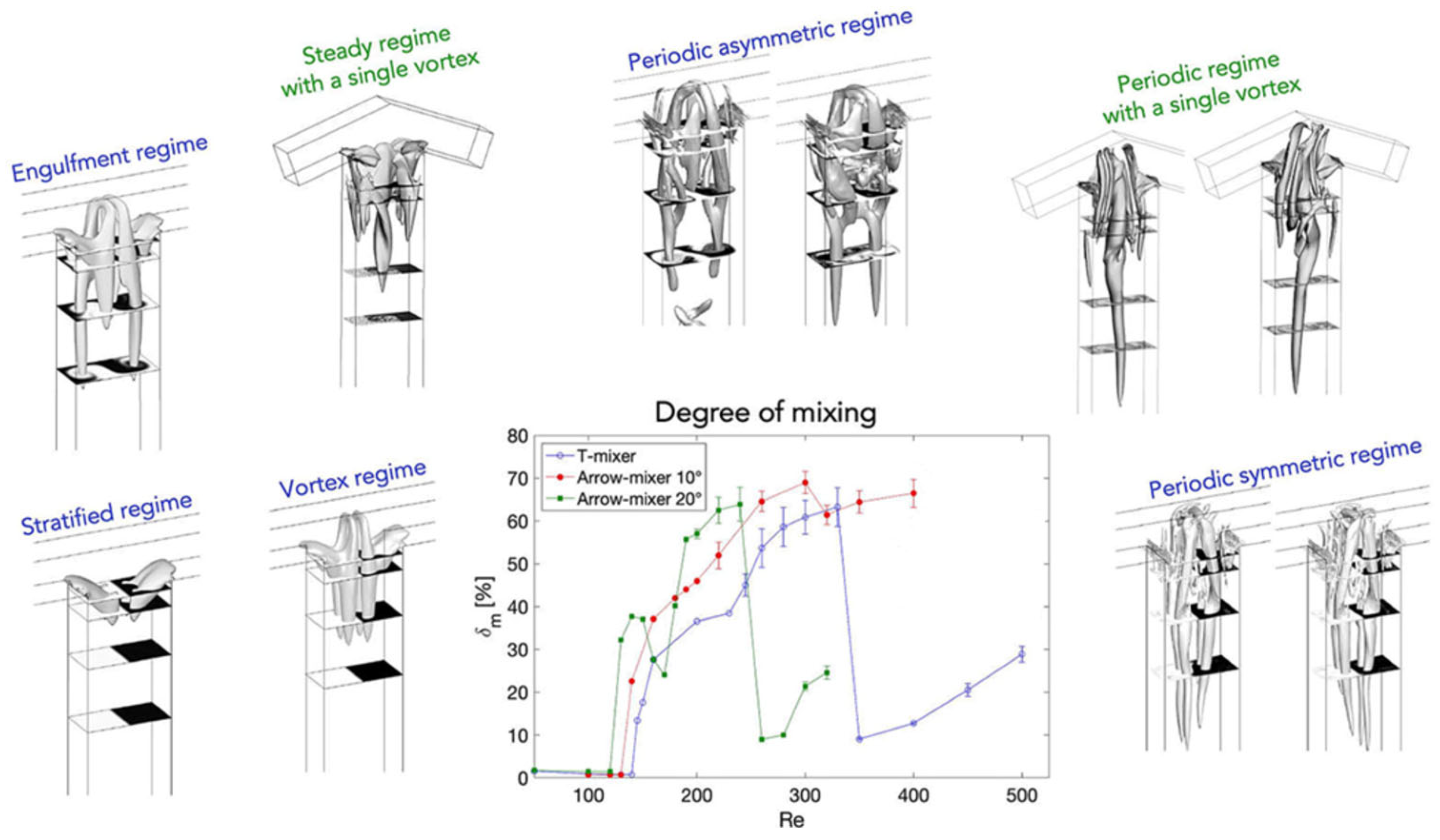
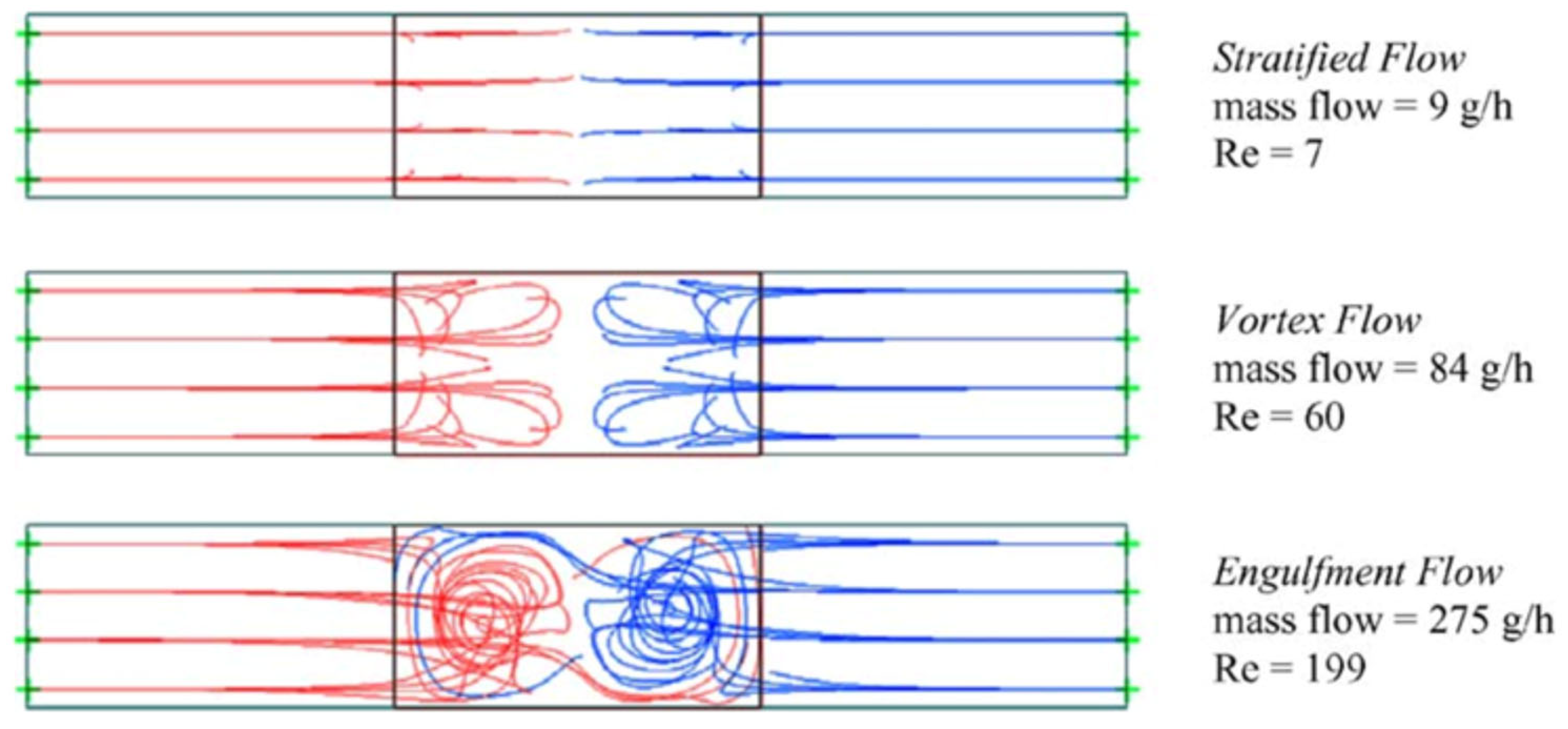
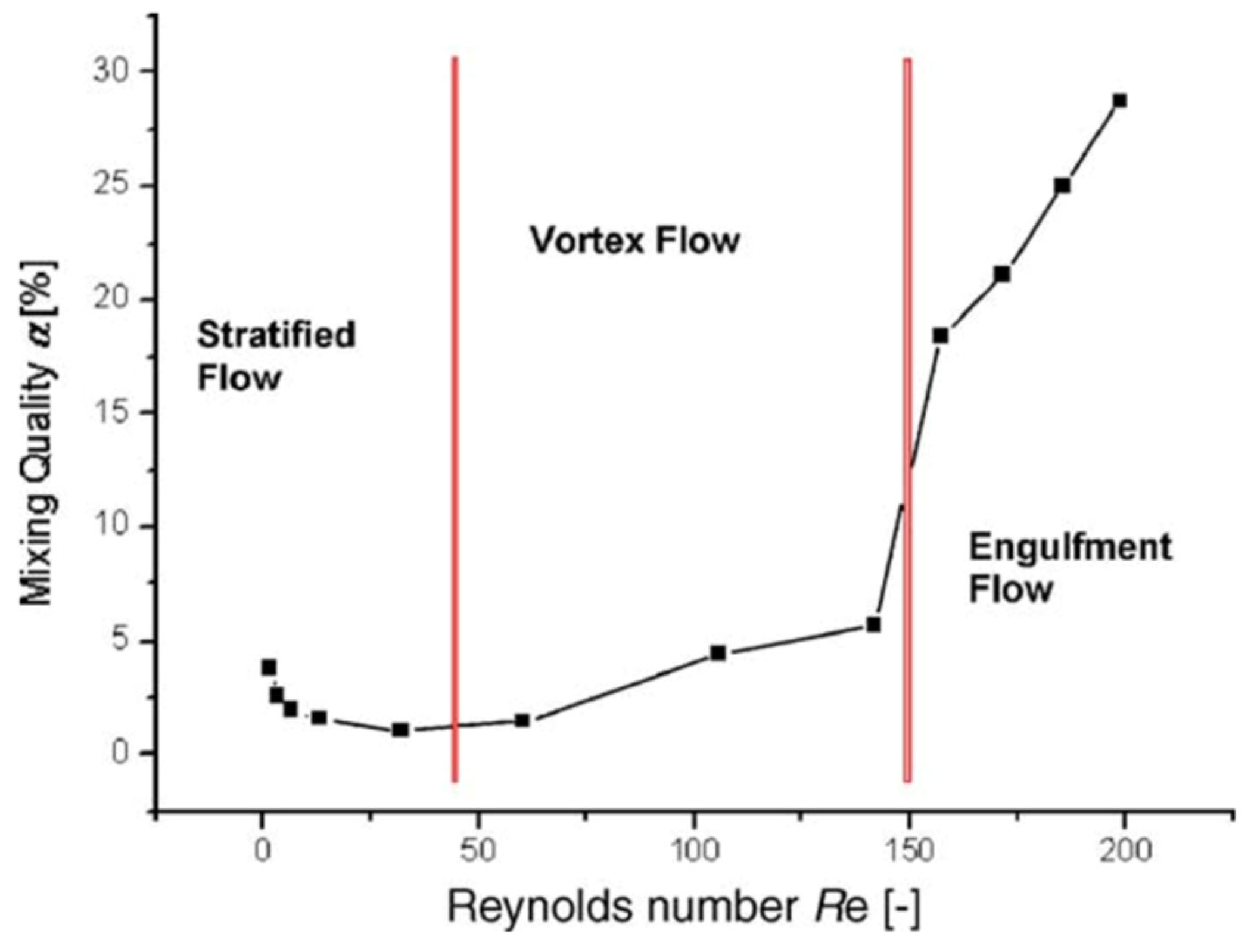
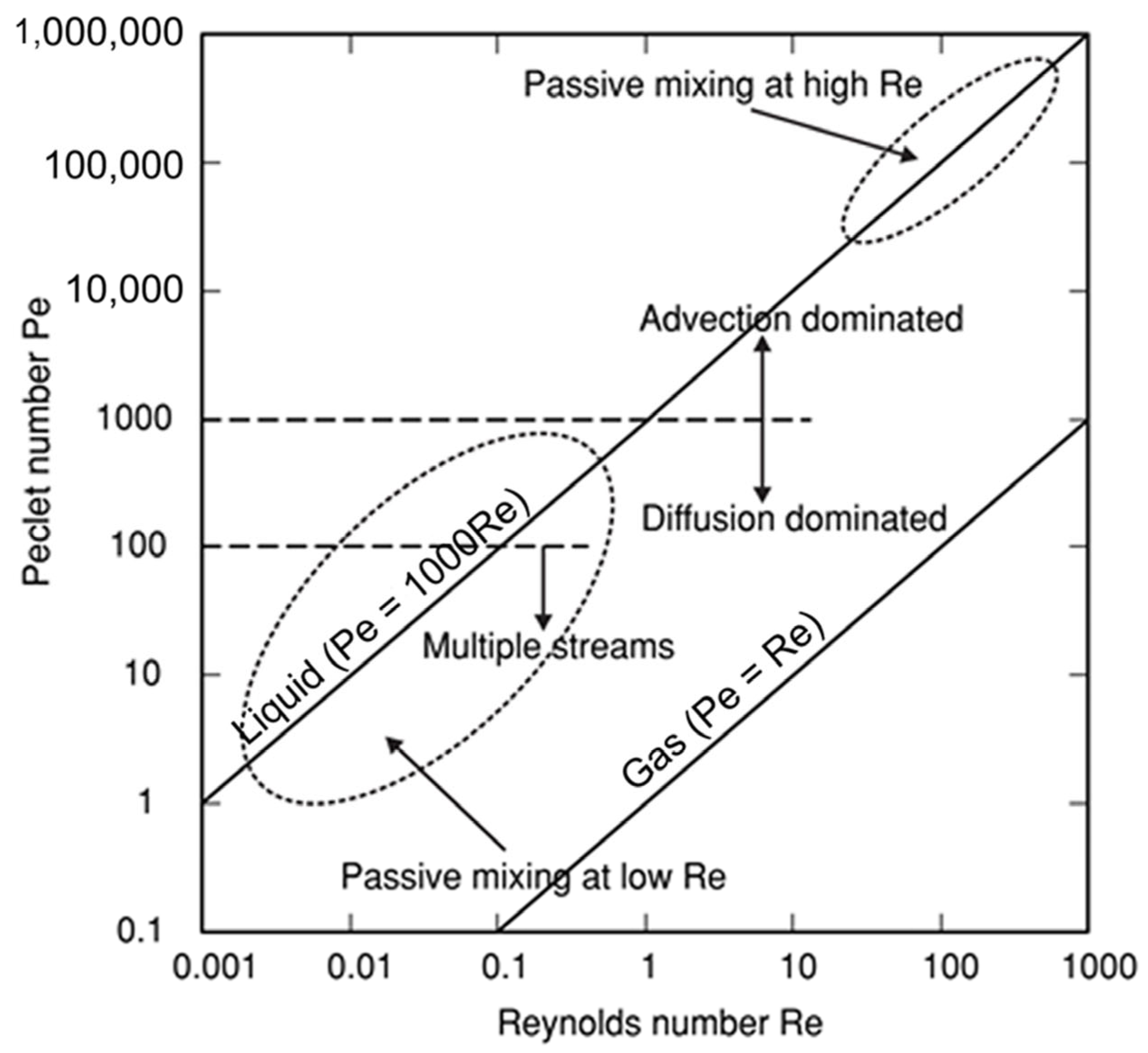
2. Mixing Performance
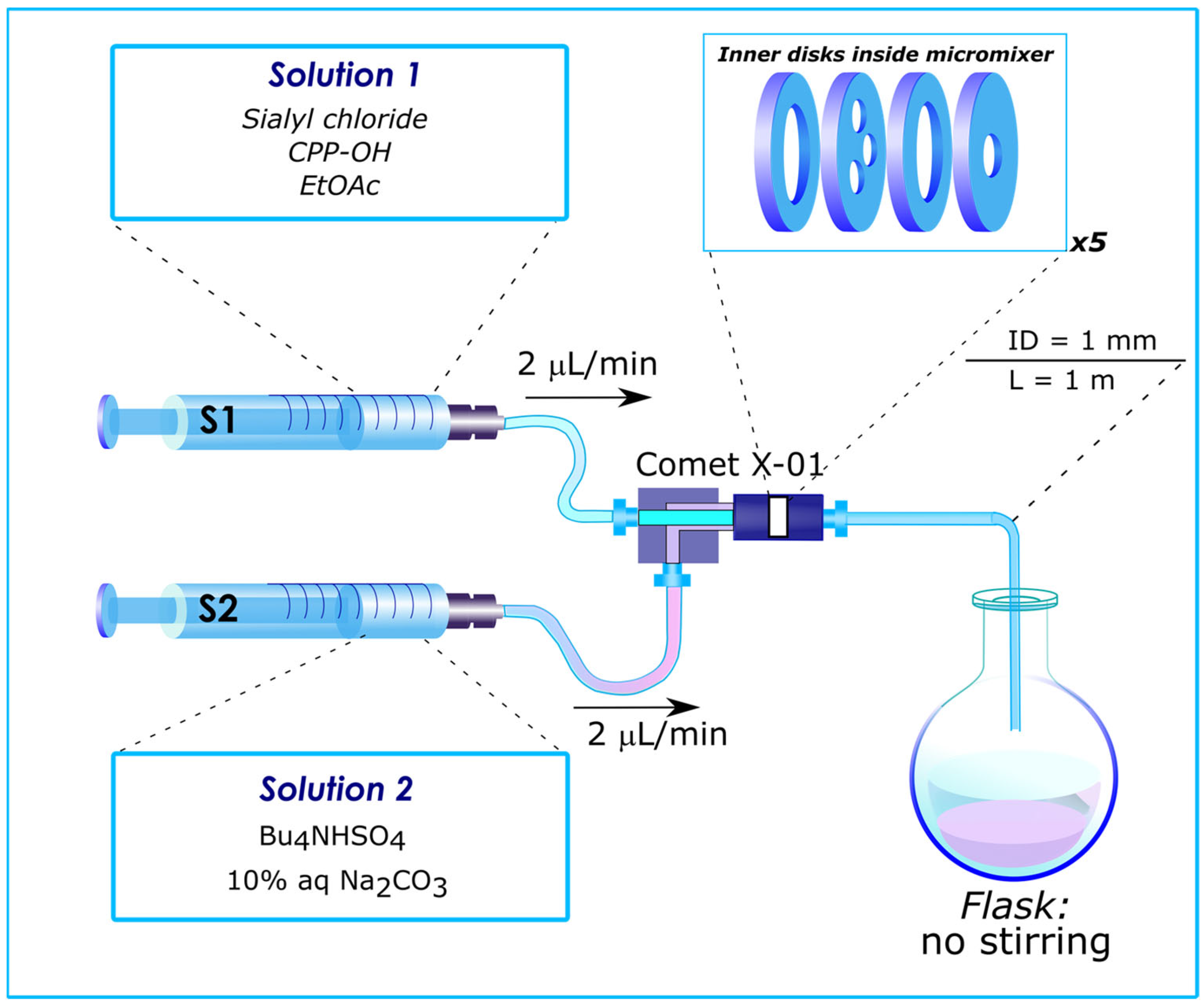
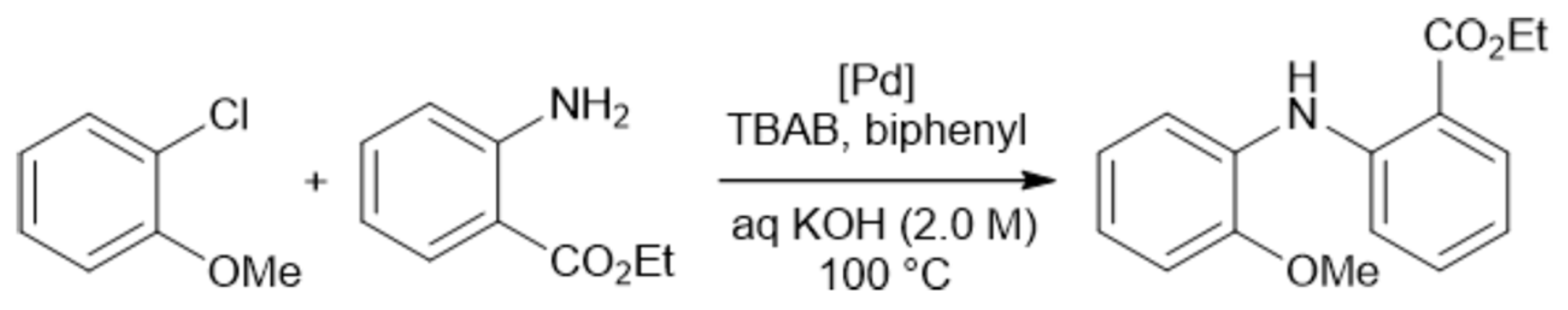
3. Influence of Mixer Design and Flow Rate on Reaction Outcome
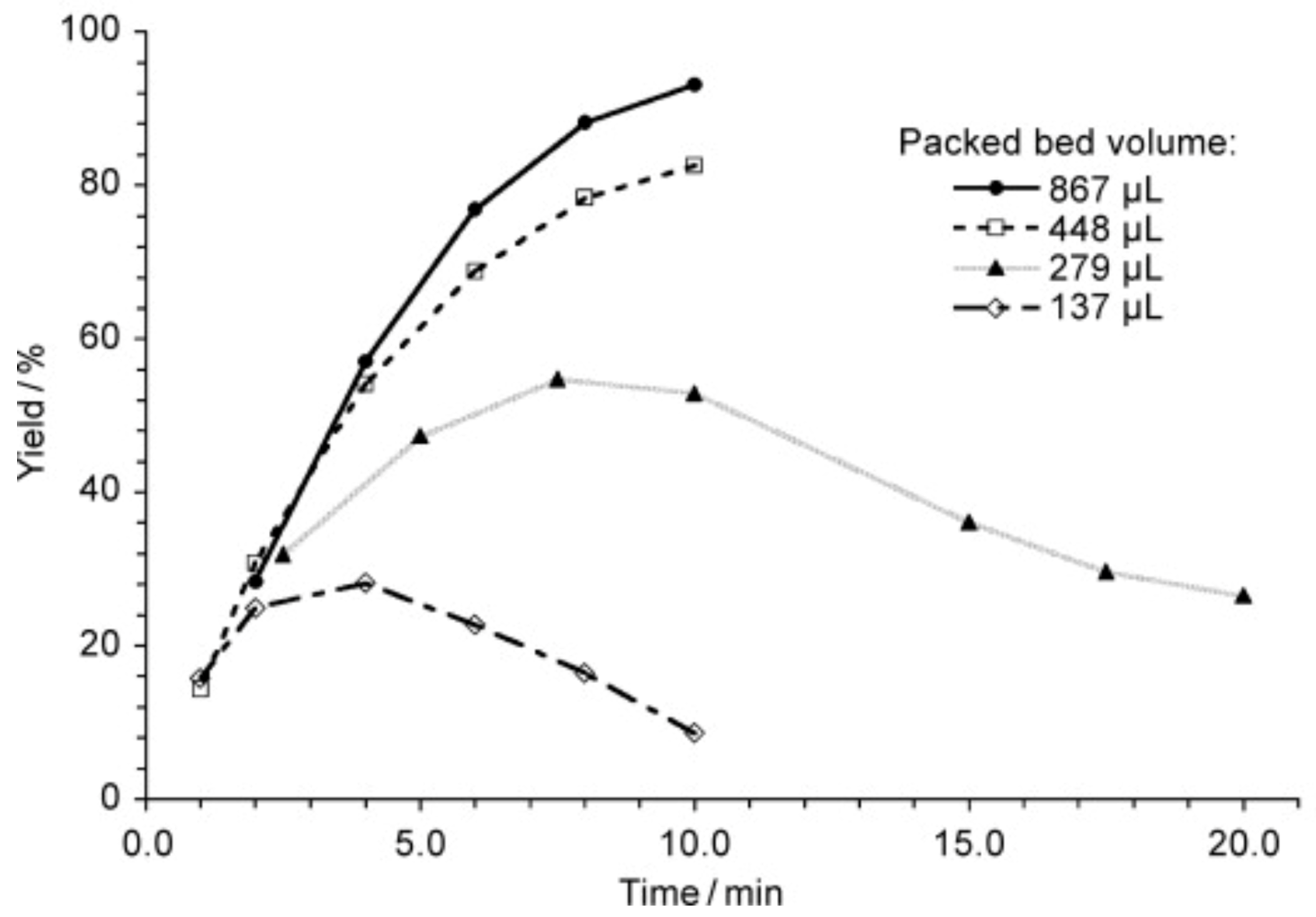
4. Influence of Residence Time on the Reaction Outcome
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jahnisch, K.; Hessel, V.; Löwe, H.; Baerns, M. Chemistry in microstructured reactors. Angew. Chem. Int. Ed. 2004, 43, 406. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- deMello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Fukase, K. Acid-mediated reactions under microfluidic conditions: A new strategy for practical synthesis of biofunctional natural products. Beilstein J. Org. Chem. 2009, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Fukase, K. Renaissance of Traditional Organic Reactions under Microfluidic Conditions: A New Paradigm for Natural Products Synthesis. Org. Process Res. Dev. 2009, 13, 983–990. [Google Scholar] [CrossRef]
- Wegner, J.; Ceylan, S.; Kirschning, A. Ten key issues in modern flow chemistry. Chem. Commun. 2011, 47, 4583–4592. [Google Scholar] [CrossRef] [PubMed]
- Hessel, V.; Kralisch, D.; Kockmann, N.; Noël, T.; Wang, Q. Novel Process Windows for Enabling, Accelerating, and Uplifting Flow Chemistry. ChemSusChem 2013, 6, 746–789. [Google Scholar] [CrossRef] [PubMed]
- Elvira, K.S.; Casadevall i Solvas, X.; Wootton, R.C.R.; demello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Fukase, K.; Shimoyama, A.; Manabe, Y. Effective Synthesis of Oligosaccharide under Microfiuidic Conditions. J. Synth. Org. Chem. Jpn. 2015, 73, 452–459. [Google Scholar] [CrossRef]
- Fukase, K.; Tanaka, K.; Fujimoto, Y.; Shimoyama, A.; Manabe, Y. Sugar synthesis by microfluidic techniques. In Glycochemical Synthesis; Hung, S.-C., Zulueta, M.M.L., Eds.; Wiley Online Books; Wiley: Hoboken, NJ, USA, 2016; pp. 205–219. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Sevim, S.; Sorrenti, A.; Franco, C.; Furukawa, S.; Pané, S.; deMello, A.J.; Puigmartí-Luis, J. Self-assembled materials and supramolecular chemistry within microfluidic environments: From common thermodynamic states to non-equilibrium structures. Chem. Soc. Rev. 2018, 47, 3788–3803. [Google Scholar] [CrossRef] [PubMed]
- Guidi, M.; Seeberger, P.H.; Gilmore, K. How to approach flow chemistry. Chem. Soc. Rev. 2020, 49, 8910–8932. [Google Scholar] [CrossRef]
- Hone, C.A.; Kappe, C.O. Towards the Standardization of Flow Chemistry Protocols for Organic Reactions. Chem. Methods 2021, 1, 454–467. [Google Scholar] [CrossRef]
- Leslie, A.; Joseph, A.M.; Baumann, M. Functional Group Interconversion Reactions in Continuous Flow Reactors. Curr. Org. Chem. 2021, 25, 2217–2231. [Google Scholar] [CrossRef]
- Paratore, F.; Bacheva, V.; Bercovici, M.; Kaigala, G.V. Reconfigurable microfluidics. Nat. Rev. Chem. 2021, 6, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Burange, A.S.; Osman, S.M.; Luque, R. Understanding flow chemistry for the production of active pharmaceutical ingredients. iScience 2022, 25, 103892. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, L.; Wen, Z.; Noel, T. A field guide to flow chemistry for synthetic organic chemists. Chem. Sci. 2023, 14, 4230–4247. [Google Scholar] [CrossRef] [PubMed]
- Monbaliu, J.-C.M.; Legros, J. Will the next generation of chemical plants be in miniaturized flow reactors? Lab Chip 2023, 23, 1349–1357. [Google Scholar] [CrossRef]
- Numata, M.; Kanzaki, C. Supramolecular Chemistry of a Moving Solution: Flow Drives New Non-covalent Bond Formation. Chem. Lett. 2023, 52, 602–610. [Google Scholar] [CrossRef]
- Ehrfeld, W.; Hessel, V.; Löwe, H. (Eds.) Micromixers. In Microreactors; Wiley: Hoboken, NJ, USA, 2000; pp. 41–85. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Bojang, A.A.; Wu, H.-S. Design, fundamental principles of fabrication and applications of microreactors. Processes 2020, 8, 891. [Google Scholar] [CrossRef]
- Dong, Z.; Wen, Z.; Zhao, F.; Kuhn, S.; Noel, T. Scale-up of micro- and milli-reactors: An overview of strategies, design principles and applications. Chem. Eng. Sci. X 2021, 10, 100097. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Ananikov, V.P. Development of 3D + G printing for the design of customizable flow reactors. Chem. Eng. J. 2022, 430, 132670. [Google Scholar] [CrossRef]
- Whitesides, G.M. Whitesides’ Group: Writing a Paper. Adv. Mater. 2004, 16, 1375–1377. [Google Scholar] [CrossRef]
- Kononov, L.O. Chemical reactivity and solution structure: On the way to a paradigm shift? RSC Adv. 2015, 5, 46718–46734. [Google Scholar] [CrossRef]
- Reynolds, O. XXIX. An experimental investigation of the circumstances which determine whether the motion of water shall be direct or sinuous, and of the law of resistance in parallel channels. Philos. Trans. R. Soc. Lond. 1883, 174, 935–982. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wu, Z. Micromixers—A review. J. Micromech. Microeng. 2005, 15, R1–R16. [Google Scholar] [CrossRef]
- Hisamoto, H.; Saito, T.; Tokeshi, M.; Hibara, A.; Kitamori, T. Fast and high conversion phase-transfer synthesis exploiting the liquid–liquid interface formed in a microchannel chip. Chem. Commun. 2001, 2662–2663. [Google Scholar] [CrossRef]
- Ottino, J.M. Mixing, Chaotic Advection, and Turbulence. Annu. Rev. Fluid Mech. 1990, 22, 207–254. [Google Scholar] [CrossRef]
- Ottino, J.M. Mixing and chemical reactions a tutorial. Chem. Eng. Sci. 1994, 49, 4005–4027. [Google Scholar] [CrossRef]
- Bałdyga, J.; Bourne, J.R. Turbulent Mixing and Chemical Reactions; Wiley: Chichester, UK, 1999; p. 896. [Google Scholar]
- Bourne, J.R. Mixing and the Selectivity of Chemical Reactions. Org. Process Res. Dev. 2003, 7, 471–508. [Google Scholar] [CrossRef]
- Hessel, V.; Löwe, H.; Schönfeld, F. Micromixers—A review on passive and active mixing principles. Chem. Eng. Sci. 2005, 60, 2479–2501. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chang, C.-L.; Wang, Y.-N.; Fu, L.-M. Microfluidic Mixing: A Review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.; Lemenand, T.; Della Valle, D.; Peerhossaini, H. Static mixers: Mechanisms, applications, and characterization methods—A review. Chem. Eng. Res. Des. 2014, 92, 205–228. [Google Scholar] [CrossRef]
- Arimond, J.; Erwin, L. A simulation of a motionless mixer. Chem. Eng. Commun. 1985, 37, 105–126. [Google Scholar] [CrossRef]
- Ehrfeld, W.; Golbig, K.; Hessel, V.; Löwe, H.; Richter, T. Characterization of Mixing in Micromixers by a Test Reaction: Single Mixing Units and Mixer Arrays. Ind. Eng. Chem. Res. 1999, 38, 1075–1082. [Google Scholar] [CrossRef]
- Wörz, O.; Jäckel, K.P.; Richter, T.; Wolf, A. Microreactors—A New Efficient Tool for Reactor Development. Chem. Eng. Technol. 2001, 24, 138–142. [Google Scholar] [CrossRef]
- Johnson, T.J.; Ross, D.; Locascio, L.E. Rapid Microfluidic Mixing. Anal. Chem. 2002, 74, 45–51. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic Mixer for Microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Engler, M.; Kockmann, N.; Kiefer, T.; Woias, P. Numerical and experimental investigations on liquid mixing in static micromixers. Chem. Eng. J. 2004, 101, 315–322. [Google Scholar] [CrossRef]
- Kockmann, N.; Engler, M.; Woias, P. Theoretische und experimentelle Untersuchungen der Mischvorgänge in T-förmigen Mikroreaktoren—Teil 3: Konvektives Mischen und chemische Reaktionen. Chem. Ing. Tech. 2004, 76, 1777–1783. [Google Scholar] [CrossRef]
- Mae, K.; Maki, T.; Hasegawa, I.; Eto, U.; Mizutani, Y.; Honda, N. Development of a new micromixer based on split/recombination for mass production and its application to soap free emulsifier. Chem. Eng. J. 2004, 101, 31–38. [Google Scholar] [CrossRef]
- Bothe, D.; Stemich, C.; Warnecke, H.-J. Fluid mixing in a T-shaped micro-mixer. Chem. Eng. Sci. 2006, 61, 2950–2958. [Google Scholar] [CrossRef]
- Hardt, S.; Pennemann, H.; Schönfeld, F. Theoretical and experimental characterization of a low-Reynolds number split-and-recombine mixer. Microfluid. Nanofluid. 2006, 2, 237–248. [Google Scholar] [CrossRef]
- Kockmann, N.; Kiefer, T.; Engler, M.; Woias, P. Convective mixing and chemical reactions in microchannels with high flow rates. Sens. Actuators B 2006, 117, 495–508. [Google Scholar] [CrossRef]
- Kockmann, N.; Dreher, S.; Woias, P. Unsteady Laminar Flow Regimes and Mixing in T-Shaped Micromixers. In Proceedings of the ASME 2007 5th International Conference on Nanochannels, Microchannels, and Minichannels, Puebla, Mexico, 18–20 June 2007; ASME: Puebla, Mexico, 2007; pp. 671–678. [Google Scholar] [CrossRef]
- Kockmann, N. Transport Phenomena in Micro Process Engineering, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008; p. 365. [Google Scholar] [CrossRef]
- Fang, W.-F.; Yang, J.-T. A novel microreactor with 3D rotating flow to boost fluid reaction and mixing of viscous fluids. Sens. Actuators B 2009, 140, 629–642. [Google Scholar] [CrossRef]
- Singh, M.K.; Anderson, P.D.; Meijer, H.E.H. Understanding and Optimizing the SMX Static Mixer. Macromol. Rapid Commun. 2009, 30, 362–376. [Google Scholar] [CrossRef]
- Dreher, S.; Engler, M.; Kockmann, N.; Woias, P. Theoretical and Experimental Investigations of Convective Micromixers and Microreactors for Chemical Reactions. In Micro and Macro Mixing: Analysis, Simulation and Numerical Calculation; Bockhorn, H., Mewes, D., Peukert, W., Warnecke, H.-J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 325–346. [Google Scholar] [CrossRef]
- Suh, Y.K.; Kang, S. A Review on Mixing in Microfluidics. Micromachines 2010, 1, 82–111. [Google Scholar] [CrossRef]
- Kockmann, N.; Roberge, D.M. Scale-up concept for modular microstructured reactors based on mixing, heat transfer, and reactor safety. Chem. Eng. Process. 2011, 50, 1017–1026. [Google Scholar] [CrossRef]
- Zhdanov, V.; Chorny, A. Development of macro- and micromixing in confined flows of reactive fluids. Int. J. Heat Mass Transf. 2011, 54, 3245–3255. [Google Scholar] [CrossRef]
- Schwolow, S.; Hollmann, J.; Schenkel, B.; Roeder, T. Application-Oriented Analysis of Mixing Performance in Microreactors. Org. Process Res. Dev. 2012, 16, 1513–1522. [Google Scholar] [CrossRef]
- Orsi, G.; Roudgar, M.; Brunazzi, E.; Galletti, C.; Mauri, R. Water-ethanol mixing in T-shaped microdevices. Chem. Eng. Sci. 2013, 95, 174–183. [Google Scholar] [CrossRef]
- Wunderlich, B.; Nettels, D.; Benke, S.; Clark, J.; Weidner, S.; Hofmann, H.; Pfeil, S.H.; Schuler, B. Microfluidic mixer designed for performing single-molecule kinetics with confocal detection on timescales from milliseconds to minutes. Nat. Protoc. 2013, 8, 1459–1474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, L.; Huang, T.; Wen, L.; Chen, J. Experimental and CFD studies on the intensified micromixing performance of micro-impinging stream reactors built from commercial T-junctions. Chem. Eng. Sci. 2014, 119, 124–133. [Google Scholar] [CrossRef]
- Woitalka, A.; Kuhn, S.; Jensen, K.F. Scalability of mass transfer in liquid–liquid flow. Chem. Eng. Sci. 2014, 116, 1–8. [Google Scholar] [CrossRef]
- Ghotli, R.A.; Abdul Aziz, A.R.; Ibrahim, S. Liquid-liquid mass transfer studies in various stirred vessel designs. Rev. Chem. Eng. 2015, 31, 329–343. [Google Scholar] [CrossRef]
- Jasińska, M. Test Reactions to Study Efficiency of Mixing. Chem. Eng. Process. 2015, 36, 171–208. [Google Scholar] [CrossRef]
- Wu, K.-J.; Nappo, V.; Kuhn, S. Hydrodynamic Study of Single- and Two-Phase Flow in an Advanced-Flow Reactor. Ind. Eng. Chem. Res. 2015, 54, 7554–7564. [Google Scholar] [CrossRef]
- Luo, J.-Z.; Luo, Y.; Chu, G.-W.; Arowo, M.; Xiang, Y.; Sun, B.-C.; Chen, J.-F. Micromixing efficiency of a novel helical tube reactor: CFD prediction and experimental characterization. Chem. Eng. Sci. 2016, 155, 386–396. [Google Scholar] [CrossRef]
- Plouffe, P.; Bittel, M.; Sieber, J.; Roberge, D.M.; Macchi, A. On the scale-up of micro-reactors for liquid–liquid reactions. Chem. Eng. Sci. 2016, 143, 216–225. [Google Scholar] [CrossRef]
- Gobert, S.R.L.; Kuhn, S.; Braeken, L.; Thomassen, L.C.J. Characterization of Milli- and Microflow Reactors: Mixing Efficiency and Residence Time Distribution. Org. Process Res. Dev. 2017, 21, 531–542. [Google Scholar] [CrossRef]
- Kockmann, N. Transport Phenomena and Chemical Reactions in Modular Microstructured Devices. Heat Transf. Eng. 2017, 38, 1316–1330. [Google Scholar] [CrossRef]
- Rahimi, M.; Azimi, N.; Parsamogadam, M.A.; Rahimi, A.; Masahy, M.M. Mixing performance of T, Y, and oriented Y-micromixers with spatially arranged outlet channel: Evaluation with Villermaux/Dushman test reaction. Microsyst. Technol. 2017, 23, 3117–3130. [Google Scholar] [CrossRef]
- Reckamp, J.M.; Bindels, A.; Duffield, S.; Liu, Y.C.; Bradford, E.; Ricci, E.; Susanne, F.; Rutter, A. Mixing Performance Evaluation for Commercially Available Micromixers Using Villermaux-Dushman Reaction Scheme with the Interaction by Exchange with the Mean Model. Org. Process Res. Dev. 2017, 21, 816–820. [Google Scholar] [CrossRef]
- Zhou, M.; Bai, D.; Zong, Y.; Zhao, L.; Thornock, J.N. Numerical investigation of turbulent reactive mixing in a novel coaxial jet static mixer. Chem. Eng. Process. 2017, 122, 190–203. [Google Scholar] [CrossRef]
- Ansari, M.A.; Kim, K.-Y.; Kim, S.M. Numerical and Experimental Study on Mixing Performances of Simple and Vortex Micro T-Mixers. Micromachines 2018, 9, 204. [Google Scholar] [CrossRef]
- Levesque, F.; Rogus, N.J.; Spencer, G.; Grigorov, P.; McMullen, J.P.; Thaisrivongs, D.A.; Davies, I.W.; Naber, J.R. Advancing Flow Chemistry Portability: A Simplified Approach to Scaling Up Flow Chemistry. Org. Process Res. Dev. 2018, 22, 1015–1021. [Google Scholar] [CrossRef]
- Mariotti, A.; Galletti, C.; Mauri, R.; Salvetti, M.V.; Brunazzi, E. Steady and unsteady regimes in a T-shaped micro-mixer: Synergic experimental and numerical investigation. Chem. Eng. J. 2018, 341, 414–431. [Google Scholar] [CrossRef]
- Rossetti, I. Continuous flow (micro-)reactors for heterogeneously catalyzed reactions: Main design and modelling issues. Catal. Today 2018, 308, 20–31. [Google Scholar] [CrossRef]
- Su, Y.; Song, Y.; Xiang, L. Continuous-Flow Microreactors for Polymer Synthesis: Engineering Principles and Applications. Top. Curr. Chem. 2018, 376, 44. [Google Scholar] [CrossRef]
- Li, W.; Xia, F.; Qin, H.; Zhang, M.; Li, W.; Zhang, J. Numerical and experimental investigations of micromixing performance and efficiency in a pore-array intensified tube-in-tube microchannel reactor. Chem. Eng. J. 2019, 370, 1350–1365. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, N.; Chen, J.; Rodgers, V.G.J.; Brisk, P.; Grover, W.H. Finding the optimal design of a passive microfluidic mixer. Lab Chip 2019, 19, 3618–3627. [Google Scholar] [CrossRef] [PubMed]
- Camarri, S.; Mariotti, A.; Galletti, C.; Brunazzi, E.; Mauri, R.; Salvetti, M.V. An Overview of Flow Features and Mixing in Micro T and Arrow Mixers. Ind. Eng. Chem. Res. 2020, 59, 3669–3686. [Google Scholar] [CrossRef]
- Cheng, D.; Chen, F.-E. Experimental and Numerical Studies of the Phase-Transfer-Catalyzed Wittig Reaction in Liquid-Liquid Slug-Flow Microchannels. Ind. Eng. Chem. Res. 2020, 59, 4397–4410. [Google Scholar] [CrossRef]
- Desir, P.; Chen, T.-Y.; Bracconi, M.; Saha, B.; Maestri, M.; Vlachos, D.G. Experiments and computations of microfluidic liquid-liquid flow patterns. React. Chem. Eng. 2020, 5, 39–50. [Google Scholar] [CrossRef]
- Hosseini, F.; Rahimi, M. Computational fluid dynamics and experimental investigations on liquid-liquid mass transfer in T-type microchannels with different mixing channel barrier shapes. Sep. Sci. Technol. 2020, 55, 3502–3516. [Google Scholar] [CrossRef]
- Mariotti, A.; Antognoli, M.; Galletti, C.; Mauri, R.; Salvetti, M.V.; Brunazzi, E. The role of flow features and chemical kinetics on the reaction yield in a T-shaped micro-reactor. Chem. Eng. J. 2020, 396, 125223. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Li, F.; Li, L.; Ge, X.; Zhang, S.; Qiu, T. Scale-up of microreactor: Effects of hydrodynamic diameter on liquid-liquid flow and mass transfer. Chem. Eng. Sci. 2020, 226, 115838. [Google Scholar] [CrossRef]
- Zhang, H.; Kopfmüller, T.; Achermann, R.; Zhang, J.; Teixeira, A.; Shen, Y.; Jensen, K.F. Accessing multidimensional mixing via 3D printing and showerhead micromixer design. AIChE J. 2020, 66, e16873. [Google Scholar] [CrossRef]
- Gill, K.K.; Gibson, R.; Yiu, K.H.C.; Hester, P.; Reis, N.M. Microcapillary film reactor outperforms single-bore mesocapillary reactors in continuous flow chemical reactions. Chem. Eng. J. 2021, 408, 127860. [Google Scholar] [CrossRef]
- Manzano Martínez, A.N.; Jansen, R.; Walker, K.; Assirelli, M.; van der Schaaf, J. Experimental and modeling study on meso- and micromixing in the rotor–stator spinning disk reactor. Chem. Eng. Res. Des. 2021, 173, 279–288. [Google Scholar] [CrossRef]
- Kravtsova, A.Y.; Ianko, P.E.; Kashkarova, M.V.; Bilsky, A.V.; Kravtsov, Y.V.; Naumov, I.V. Conditions of the Effective Mixing in a T-Type Micromixer at Low Reynolds Numbers. In Processes in GeoMedia—Volume V; Chaplina, T., Ed.; Springer International Publishing: Cham, Switzerland, 2022; Volume 5, pp. 35–41. [Google Scholar] [CrossRef]
- Trabzon, L.; Karimian, G.; Khosroshahi, A.R.; Gül, B.; Bakhshayesh, A.G.; Kocak, A.F.; Akyıldız, D.; Aldi, Y.E. High-throughput nanoscale liposome formation via electrohydrodynamic-based micromixer. Phys. Fluids 2022, 34, 102011. [Google Scholar] [CrossRef]
- Jafarpour, A.M.; Khosroshahi, A.R.; Hanifi, M.; Moghanlou, F.S. Experimental study on the performance of a mini-scale Y-type mixer with two liquid metal-enabled pumps. Phys. Fluids 2022, 34, 112110. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, M.; Zheng, C.; Li, H. Numerical Simulation of Mixing Characteristics in a Split-and-Recombine Microchannel. Heat Transf. Eng. 2023, 44, 1563–1577. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, N.; Liu, H.; Tang, J.; Wang, C.; Wang, R.; Yang, X. Modification of Meso-Micromixing Interaction Reaction Model in Continuous Reactors. Processes 2023, 11, 1576. [Google Scholar] [CrossRef]
- Falk, L.; Commenge, J.M. Performance comparison of micromixers. Chem. Eng. Sci. 2010, 65, 405–411. [Google Scholar] [CrossRef]
- Rehage, H.; Kind, M. The first Damköhler number and its importance for characterizing the influence of mixing on competitive chemical reactions. Chem. Eng. Sci. 2021, 229, 116007. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, K.H.; Fang, W.F.; Tsai, S.H.; Fang, J.M.; Yang, J.T. Flash synthesis of carbohydrate derivatives in chaotic microreactors. Chem. Eng. J. 2011, 174, 421–424. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Fang, W.-F.; Liu, Y.-C.; Yang, J.-T. Analysis of chaos and FRET reaction in split-and-recombine microreactors. Microfluid. Nanofluid. 2011, 11, 339–352. [Google Scholar] [CrossRef]
- Tanaka, K.; Motomatsu, S.; Koyama, K.; Tanaka, S.I.; Fukase, K. Large-scale synthesis of immunoactivating natural product, pristane, by continuous microfluidic dehydration as the key step. Org. Lett. 2007, 9, 299–302. [Google Scholar] [CrossRef]
- Tanaka, K.; Mori, Y.; Fukase, K. Practical Synthesis of a Manβ(1-4)GlcNTroc Fragment via Microfluidic β-Mannosylation. J. Carbohydr. Chem. 2009, 28, 1–11. [Google Scholar] [CrossRef]
- Uchinashi, Y.; Nagasaki, M.; Zhou, J.; Tanaka, K.; Fukase, K. Reinvestigation of the C5-acetamide sialic acid donor for α-selective sialylation: Practical procedure under microfluidic conditions. Org. Biomol. Chem. 2011, 9, 7243–7248. [Google Scholar] [CrossRef] [PubMed]
- Uchinashi, Y.; Tanaka, K.; Manabe, Y.; Fujimoto, Y.; Fukase, K. Practical and Efficient Method for α-Sialylation with an Azide Sialyl Donor Using a Microreactor. J. Carbohydr. Chem. 2014, 33, 55–67. [Google Scholar] [CrossRef]
- Audiger, L.; Watts, K.; Elmore, S.C.; Robinson, R.I.; Wirth, T. Ritter Reactions in Flow. ChemSusChem 2012, 5, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Graz, M.; Saunders, R.; Evans, G.J.S.; Kaul, S.; Wirth, T. Flow Synthesis of Symmetrical Di- and Trisulfides Using Phase-Transfer Catalysis. J. Flow Chem. 2013, 3, 118–121. [Google Scholar] [CrossRef]
- Pradipta, A.R.; Tsutsui, A.; Ogura, A.; Hanashima, S.; Yamaguchi, Y.; Kurbangalieva, A.; Tanaka, K. Microfluidic Mixing of Polyamine with Acrolein Enables the Detection of the [4 + 4] Polymerization of Intermediary Unsaturated Imines: The Properties of a Cytotoxic 1,5-Diazacyclooctane Hydrogel. Synlett 2014, 25, 2442–2446. [Google Scholar] [CrossRef]
- Umezu, S.; Yoshiiwa, T.; Tokeshi, M.; Shindo, M. Generation of ynolates via reductive lithiation using flow microreactors. Tetrahedron Lett. 2014, 55, 1822–1825. [Google Scholar] [CrossRef]
- Matthew, H.; Thomas, W. Safe use of nitromethane for aldol reactions in flow. J. Flow Chem. 2016, 6, 202–205. [Google Scholar] [CrossRef]
- Nagasaki, M.; Manabe, Y.; Minamoto, N.; Tanaka, K.; Silipo, A.; Molinaro, A.; Fukase, K. Chemical synthesis of a complex-type N-glycan containing a core fucose. J. Org. Chem. 2016, 81, 10600–10616. [Google Scholar] [CrossRef]
- Ogasawara, S.; Hayashi, Y. Multistep Continuous-Flow Synthesis of (–)-Oseltamivir. Synthesis 2017, 49, 424–428. [Google Scholar] [CrossRef]
- Ikawa, T.; Masuda, S.; Akai, S. Microflow Fluorinations of Benzynes: Efficient Synthesis of Fluoroaromatic Compounds. Chem. Pharm. Bull. 2018, 66, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Koge, T.; Katsuragi, S.; Akai, S.; Oishi, T. Flow Synthesis of (R)- and (S)-(E)-1-Iodohexa-1,5-dien-3-ol: Chiral Building Blocks for Natural Product Synthesis. Chem. Lett. 2018, 47, 1116–1118. [Google Scholar] [CrossRef]
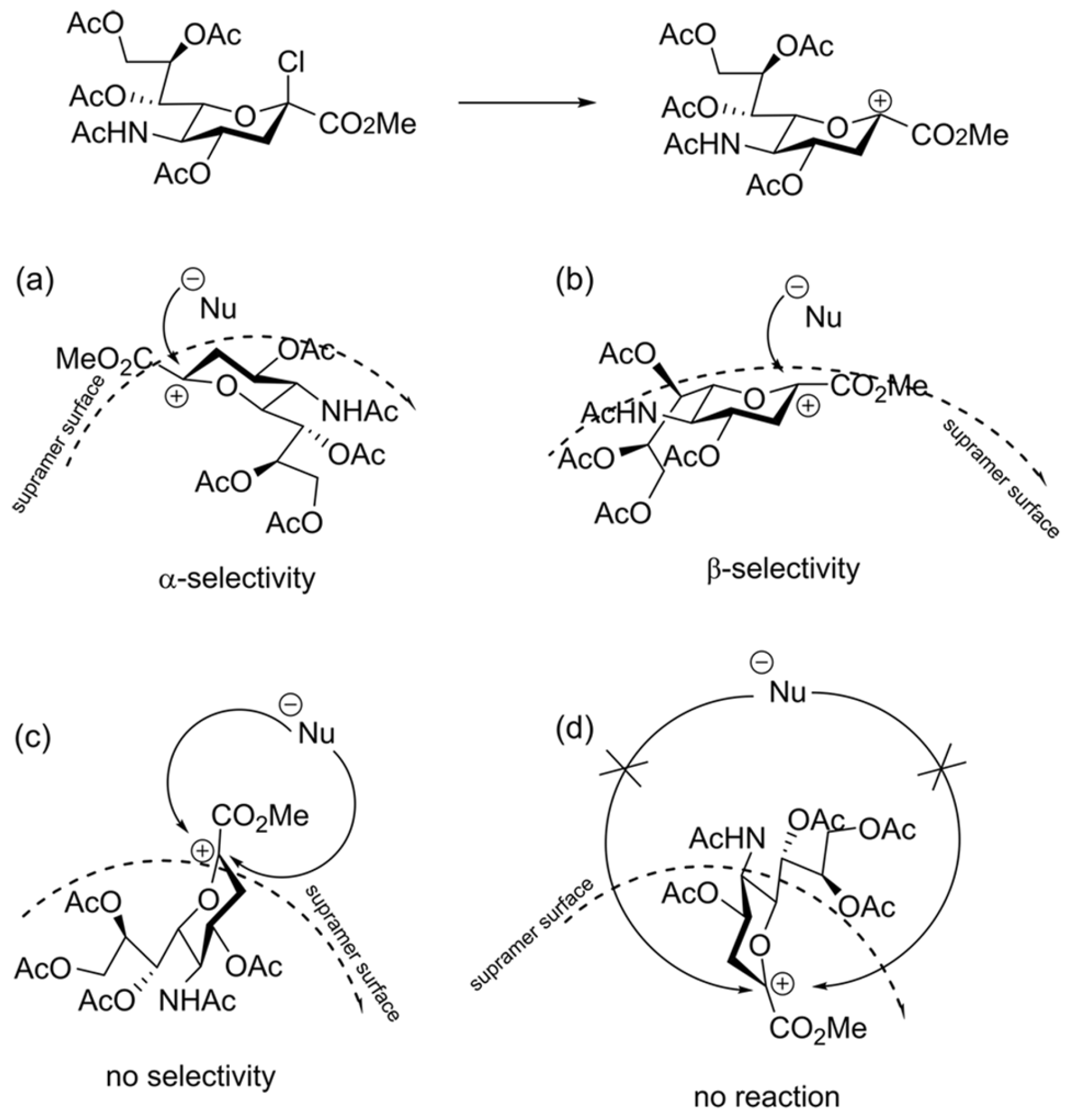
- Myachin, I.V.; Orlova, A.V.; Kononov, L.O. Glycosylation in flow: Effect of the flow rate and type of the mixer. Russ. Chem. Bull. 2019, 68, 2126–2129. [Google Scholar] [CrossRef]
- Kondo, M.; Wathsala, H.D.P.; Sako, M.; Hanatani, Y.; Ishikawa, K.; Hara, S.; Takaai, T.; Washio, T.; Takizawa, S.; Sasai, H. Exploration of flow reaction conditions using machine-learning for enantioselective organocatalyzed Rauhut–Currier and [3 + 2] annulation sequence. Chem. Commun. 2020, 56, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Fukumoto, S.; Iwase, T.; Mizota, I.; Shimizu, M.; Hachiya, I. Umpolung Reactions of α-Tosyloximino Esters in a Flow System. Synlett 2020, 31, 1930–1936. [Google Scholar] [CrossRef]
- Myachin, I.V.; Mamirgova, Z.Z.; Stepanova, E.V.; Zinin, A.I.; Chizhov, A.O.; Kononov, L.O. Black swan in phase transfer catalysis: Influence of mixing mode on the stereoselectivity of glycosylation. Eur. J. Org. Chem. 2022, 2022, e202101377. [Google Scholar] [CrossRef]
- Myachin, I.V.; Kononov, L.O. Phase-transfer catalyzed microfluidic glycosylation: A small change in concentration results in a dramatic increase in stereoselectivity. Catalysts 2023, 13, 313. [Google Scholar] [CrossRef]
- Miller, P.W.; Long, N.J.; De Mello, A.J.; Vilar, R.; Passchier, J.; Gee, A. Rapid formation of amides via carbonylative coupling reactions using a microfluidic device. Chem. Commun. 2006, 546–548. [Google Scholar] [CrossRef]
- Webb, D.; Jamison, T.F. Diisobutylaluminum Hydride Reductions Revitalized: A Fast, Robust, and Selective Continuous Flow System for Aldehyde Synthesis. Org. Lett. 2012, 14, 568–571. [Google Scholar] [CrossRef]
- Ganiek, M.A.; Becker, M.R.; Ketels, M.; Knochel, P. Continuous Flow Magnesiation or Zincation of Acrylonitriles, Acrylates, and Nitroolefins. Application to the Synthesis of Butenolides. Org. Lett. 2016, 18, 828–831. [Google Scholar] [CrossRef]
- Hafner, A.; Meisenbach, M.; Sedelmeier, J. Flow Chemistry on Multigram Scale: Continuous Synthesis of Boronic Acids within 1 s. Org. Lett. 2016, 18, 3630–3633. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, A.; Tomita, R.; Kurimoto, K.; Yamamura, H. Selective deprotection of trityl group on carbohydrate by microflow reaction inhibiting migration of acetyl group. Synth. Commun. 2016, 46, 556–562. [Google Scholar] [CrossRef]
- Naber, J.R.; Buchwald, S.L. Packed-Bed Reactors for Continuous-Flow C–N Cross-Coupling. Angew. Chem. Int. Ed. 2010, 49, 9469–9474. [Google Scholar] [CrossRef] [PubMed]
- Haroun, S.; Sanei, Z.; Jivan, S.; Schaffer, P.; Ruth, T.J.; Li, P.C.H. Continuous-flow synthesis of [11C]raclopride, a positron emission tomography radiotracer, on a microfluidic chip. Can. J. Chem. 2013, 91, 326–332. [Google Scholar] [CrossRef]
- Park, C.P.; Kim, D.-P. Dual-Channel Microreactor for Gas–Liquid Syntheses. J. Am. Chem. Soc. 2010, 132, 10102–10106. [Google Scholar] [CrossRef] [PubMed]
- Nagaki, A.; Togai, M.; Suga, S.; Aoki, N.; Mae, K.; Yoshida, J.I. Control of extremely fast competitive consecutive reactions using micromixing. Selective Friedel-Crafts aminoalkylation. J. Am. Chem. Soc. 2005, 127, 11666–11675. [Google Scholar] [CrossRef] [PubMed]
- Rys, P. Disguised chemical selectivities. Acc. Chem. Res. 1976, 9, 345–351. [Google Scholar] [CrossRef]
- Roy, R.; Tropper, F.D.; Cao, S.; Kim, J.M. Anomeric Group Transformations Under Phase-Transfer Catalysis. In Phase-Transfer Catalysis; Halpern, M., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997; Volume 659, pp. 163–180. [Google Scholar] [CrossRef]
- Boons, G.J.; Demchenko, A.V. Recent advances in O-sialylation. Chem. Rev. 2000, 100, 4539–4566. [Google Scholar] [CrossRef]
- De Meo, C.; Jones, B.T. Chapter two—Chemical synthesis of glycosides of N-acetylneuraminic acid. Adv. Carbohydr. Chem. Biochem. 2018, 75, 215–316. [Google Scholar] [CrossRef]
- De Meo, C.; Goeckner, N. 2.07—Synthesis of glycosides of sialic acid. In Comprehensive Glycoscience, 2nd ed.; Barchi, J.J., Vidal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 228–266. [Google Scholar] [CrossRef]
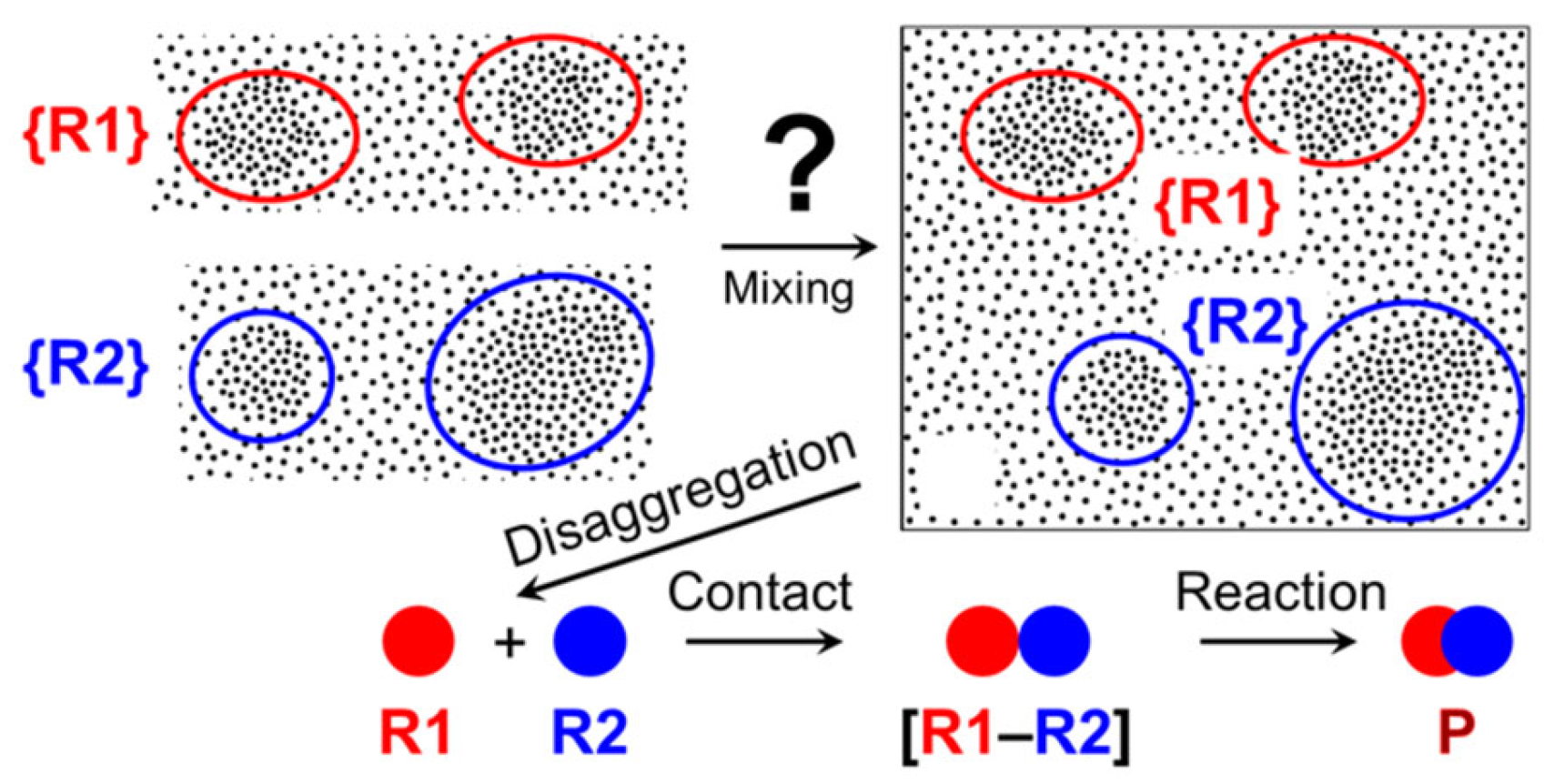
- Sedlák, M. Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: I. Light scattering characterization. J. Phys. Chem. B 2006, 110, 4329–4338. [Google Scholar] [CrossRef]
- Sedlák, M. Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: II. Kinetics of the formation and long-time stability. J. Phys. Chem. B 2006, 110, 4339–4345. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, M. Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids. III. Correlation with molecular properties and interactions. J. Phys. Chem. B 2006, 110, 13976–13984. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, M.; Rak, D. Large-Scale Inhomogeneities in Solutions of Low Molar Mass Compounds and Mixtures of Liquids: Supramolecular Structures or Nanobubbles? J. Phys. Chem. B 2013, 117, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, M.; Rak, D. On the origin of mesoscale structures in aqueous solutions of tertiary butyl alcohol: The mystery resolved. J. Phys. Chem. B 2014, 118, 2726–2737. [Google Scholar] [CrossRef] [PubMed]
- Rak, D.; Ovadová, M.; Sedlák, M. (Non)existence of bulk nanobubbles: The role of ultrasonic cavitation and organic solutes in water. J. Phys. Chem. Lett. 2019, 10, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Rak, D.; Sedlák, M. On the mesoscale solubility in liquid solutions and mixtures. J. Phys. Chem. B 2019, 123, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Rak, D.; Sedlák, M. Solvophobicity-Driven Mesoscale Structures: Stabilizer-Free Nanodispersions. Langmuir 2023, 39, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, D.; Anisimov, M.A. Phase behavior and mesoscale solubilization in aqueous solutions of hydrotropes. Fluid Phase Equilib. 2014, 362, 170–176. [Google Scholar] [CrossRef]
- Zemb, T.; Kunz, W. Weak aggregation: State of the art, expectations and open questions. Curr. Opin. Colloid Interface Sci. 2016, 22, 113–119. [Google Scholar] [CrossRef]
- Orlova, A.V.; Laptinskaya, T.V.; Malysheva, N.N.; Kononov, L.O. Light scattering in non-aqueous solutions of low-molecular-mass compounds: Application for supramer analysis of reaction solutions. J. Solution Chem. 2020, 49, 629–644. [Google Scholar] [CrossRef]
- Orlova, A.V.; Kononov, L.O. Polarimetry as a method for studying the structure of aqueous carbohydrate solutions: Correlation with other methods. RENSIT 2020, 12, 95–106. [Google Scholar] [CrossRef]
- Orlova, A.V.; Ahiadorme, D.A.; Laptinskaya, T.V.; Kononov, L.O. Supramer analysis of 2,3,5-tri-O-benzoyl-α-D-arabinofuranosyl bromide solutions in different solvents: Supramolecular aggregation of solute molecules in 1,2-dichloroethane mediated by halogen bonds. Russ. Chem. Bull. 2021, 70, 2214–2219. [Google Scholar] [CrossRef]
- Jelley, E.E. Spectral Absorption and Fluorescence of Dyes in the Molecular State. Nature 1936, 138, 1009–1010. [Google Scholar] [CrossRef]
- Jelley, E.E. Molecular, Nematic and Crystal States of I: I-Diethyl-Cyanine Chloride. Nature 1937, 139, 631. [Google Scholar] [CrossRef]
- Scheibe, G. Über die Veränderlichkeit der Absorptionsspektren in Lösungen und die Nebenvalenzen als ihre Ursache. Angew. Chem. 1937, 50, 212–219. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.; Ankri, R. Theoretical Models, Preparation, Characterization and Applications of Cyanine J-Aggregates: A Minireview. ChemistryOpen 2022, 11, e202200103. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Shuai, Z. Molecular mechanism of aggregation-induced emission. Aggregate 2021, 2, e91. [Google Scholar] [CrossRef]
- Ribo, J.M.; El-Hachemi, Z.; Arteaga, O.; Canillas, A.; Crusats, J. Hydrodynamic Effects in Soft-matter Self-assembly: The Case of J-Aggregates of Amphiphilic Porphyrins. Chem. Rec. 2017, 17, 713–724. [Google Scholar] [CrossRef]
- Maeda, T.; Mori, T.; Ikeshita, M.; Ma, S.C.; Muller, G.; Ariga, K.; Naota, T. Vortex Flow-controlled Circularly Polarized Luminescence of Achiral Pt(II) Complex Aggregates Assembled at the Air-Water Interface. Small Methods 2022, 6, 2200936. [Google Scholar] [CrossRef]
- Adero, P.O.; Amarasekara, H.; Wen, P.; Bohé, L.; Crich, D. The experimental evidence in support of glycosylation mechanisms at the SN1–SN2 interface. Chem. Rev. 2018, 118, 8242–8284. [Google Scholar] [CrossRef] [PubMed]
- Crich, D. En route to the transformation of glycoscience: A chemist’s perspective on internal and external crossroads in glycochemistry. J. Am. Chem. Soc. 2021, 143, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Ötvös, S.B.; Mándity, I.M.; Fülöp, F. Highly efficient 1,4-addition of aldehydes to nitroolefins: Organocatalysis in continuous flow by solid-supported peptidic catalysts. ChemSusChem 2012, 5, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.; Jamison, T.F. A Continuous Homologation of Esters: An Efficient Telescoped Reduction-Olefination Sequence. Org. Lett. 2012, 14, 2465–2467. [Google Scholar] [CrossRef] [PubMed]
- Andreana, P.R.; Crich, D. Guidelines for O-glycoside formation from first principles. ACS Cent. Sci. 2021, 7, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Ribó, J.M.; Crusats, J.; Sagués, F.; Claret, J.; Rubires, R. Chiral Sign Induction by Vortices During the Formation of Mesophases in Stirred Solutions. Science 2001, 292, 2063–2066. [Google Scholar] [CrossRef] [PubMed]
- Crusats, J.; El-Hachemi, Z.; Ribó, J.M. Hydrodynamic effects on chiral induction. Chem. Soc. Rev. 2010, 39, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Yang, D.; Duan, P.; Liu, M. Towards homochiral supramolecular entities from achiral molecules by vortex mixing-accompanied self-assembly. Chem. Sci. 2019, 10, 2718–2724. [Google Scholar] [CrossRef]
- Nagornaya, M.O.; Orlova, A.V.; Stepanova, E.V.; Zinin, A.I.; Laptinskaya, T.V.; Kononov, L.O. The use of the novel glycosyl acceptor and supramer analysis in the synthesis of sialyl-α(2–3)-galactose building block. Carbohydr. Res. 2018, 470, 27–35. [Google Scholar] [CrossRef]
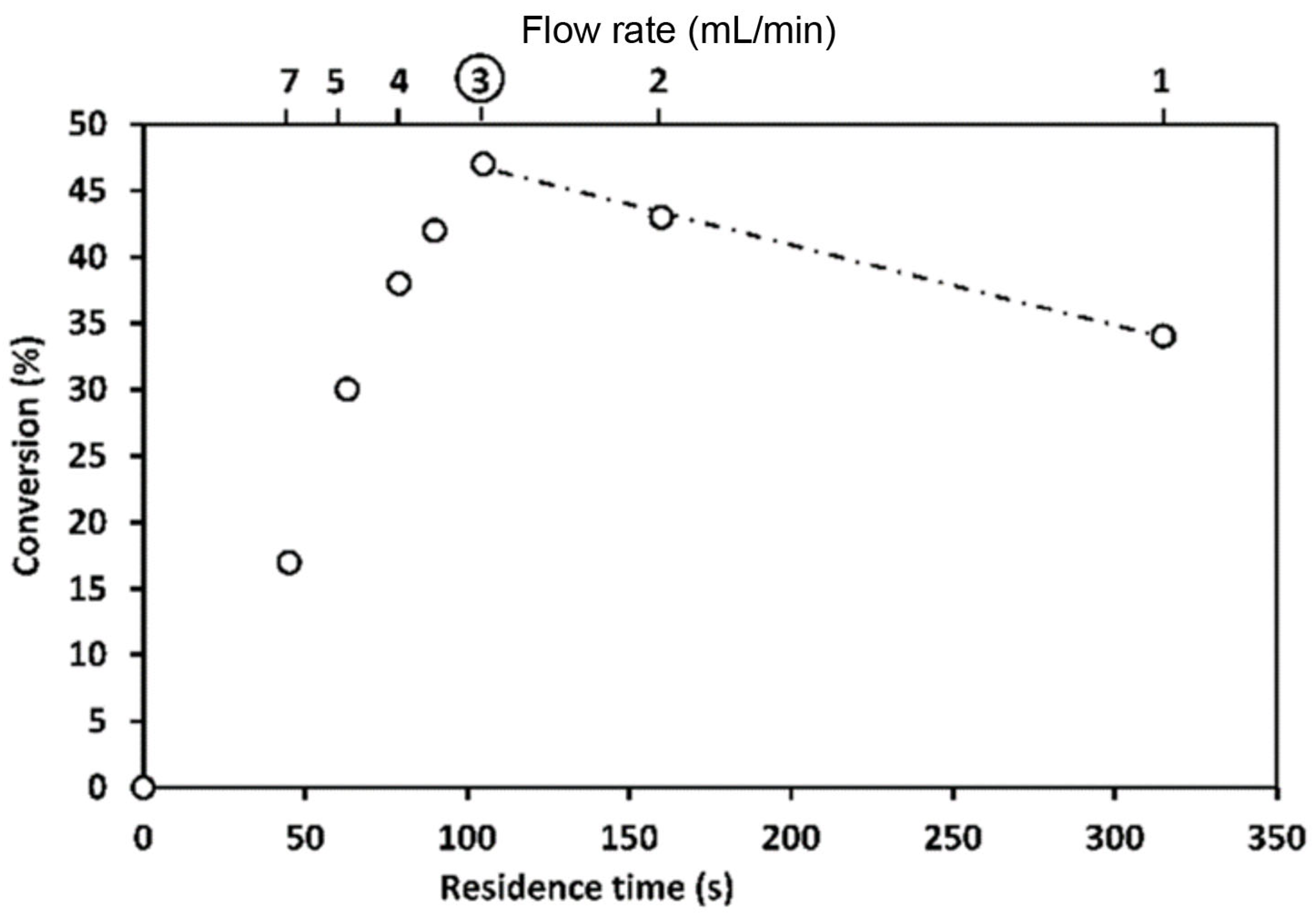
- Penverne, C.; Hazard, B.; Rolando, C.; Penhoat, M. Scale-up Study of Benzoic Acid Alkylation in Flow: From Microflow Capillary Reactor to a Milliflow Reactor. Org. Process Res. Dev. 2017, 21, 1864–1868. [Google Scholar] [CrossRef]
- Nagaki, A.; Kenmoku, A.; Moriwaki, Y.; Hayashi, A.; Yoshida, J.-i. Cross-Coupling in a Flow Microreactor: Space Integration of Lithiation and Murahashi Coupling. Angew. Chem. Int. Ed. 2010, 49, 7543–7547. [Google Scholar] [CrossRef]
- Tomida, Y.; Nagaki, A.; Yoshida, J.-i. Asymmetric Carbolithiation of Conjugated Enynes: A Flow Microreactor Enables the Use of Configurationally Unstable Intermediates before They Epimerize. J. Am. Chem. Soc. 2011, 133, 3744–3747. [Google Scholar] [CrossRef]
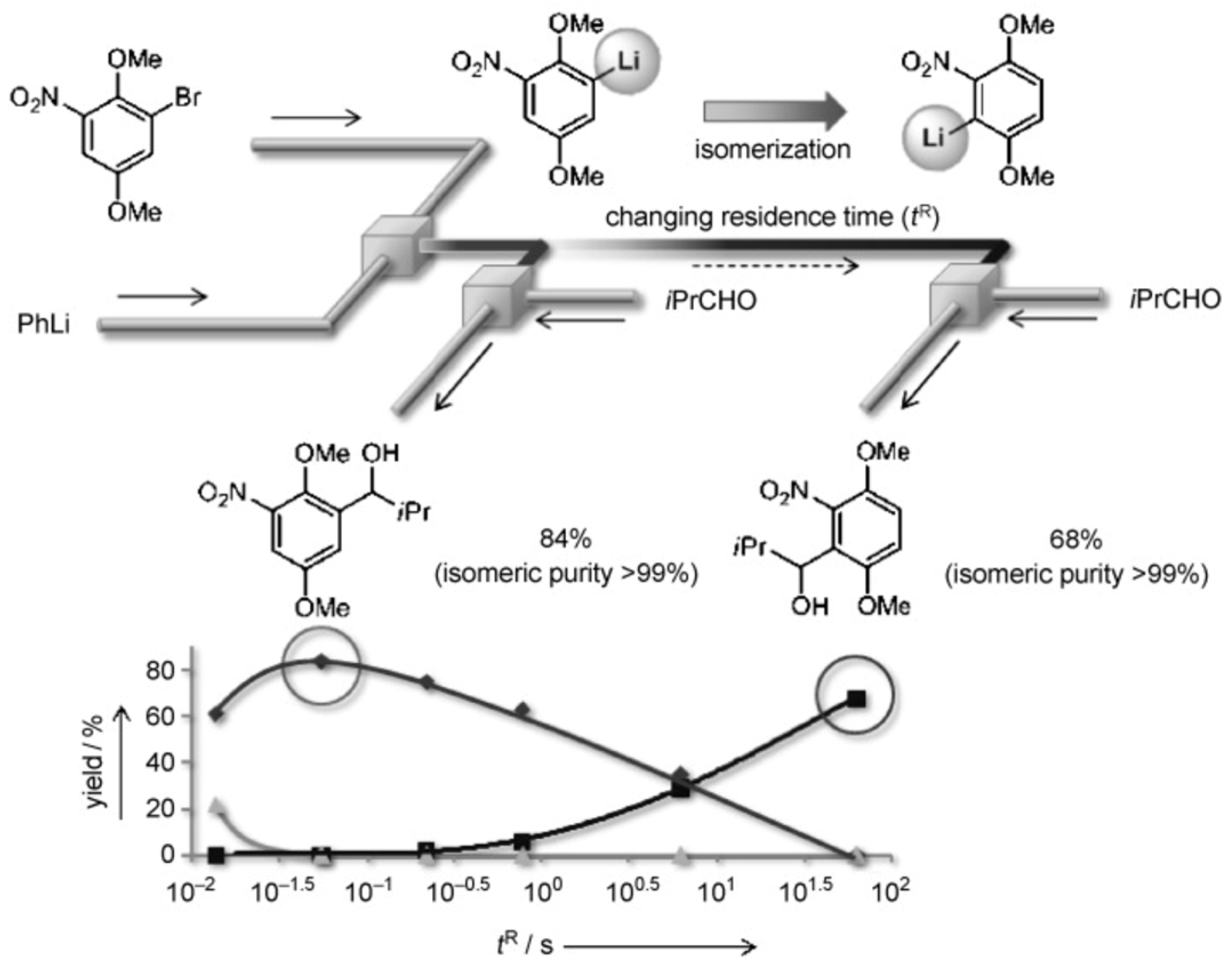
- Nagaki, A.; Kim, H.; Yoshida, J.-i. Nitro-Substituted Aryl Lithium Compounds in Microreactor Synthesis: Switch between Kinetic and Thermodynamic Control. Angew. Chem. Int. Ed. 2009, 48, 8063–8065. [Google Scholar] [CrossRef]


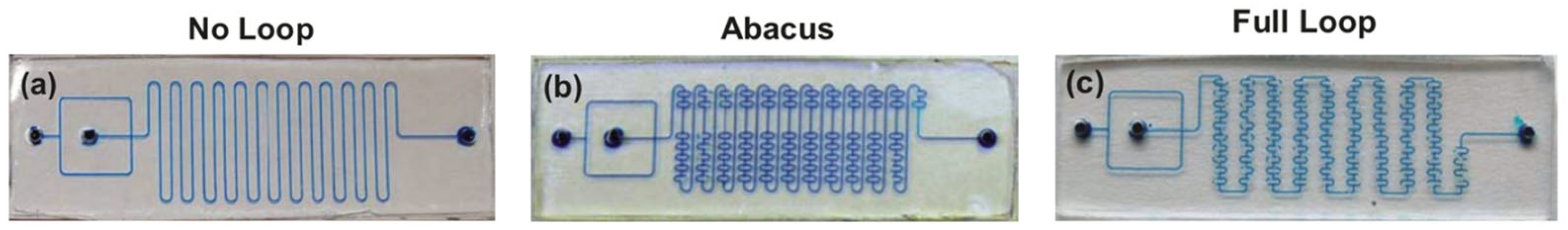
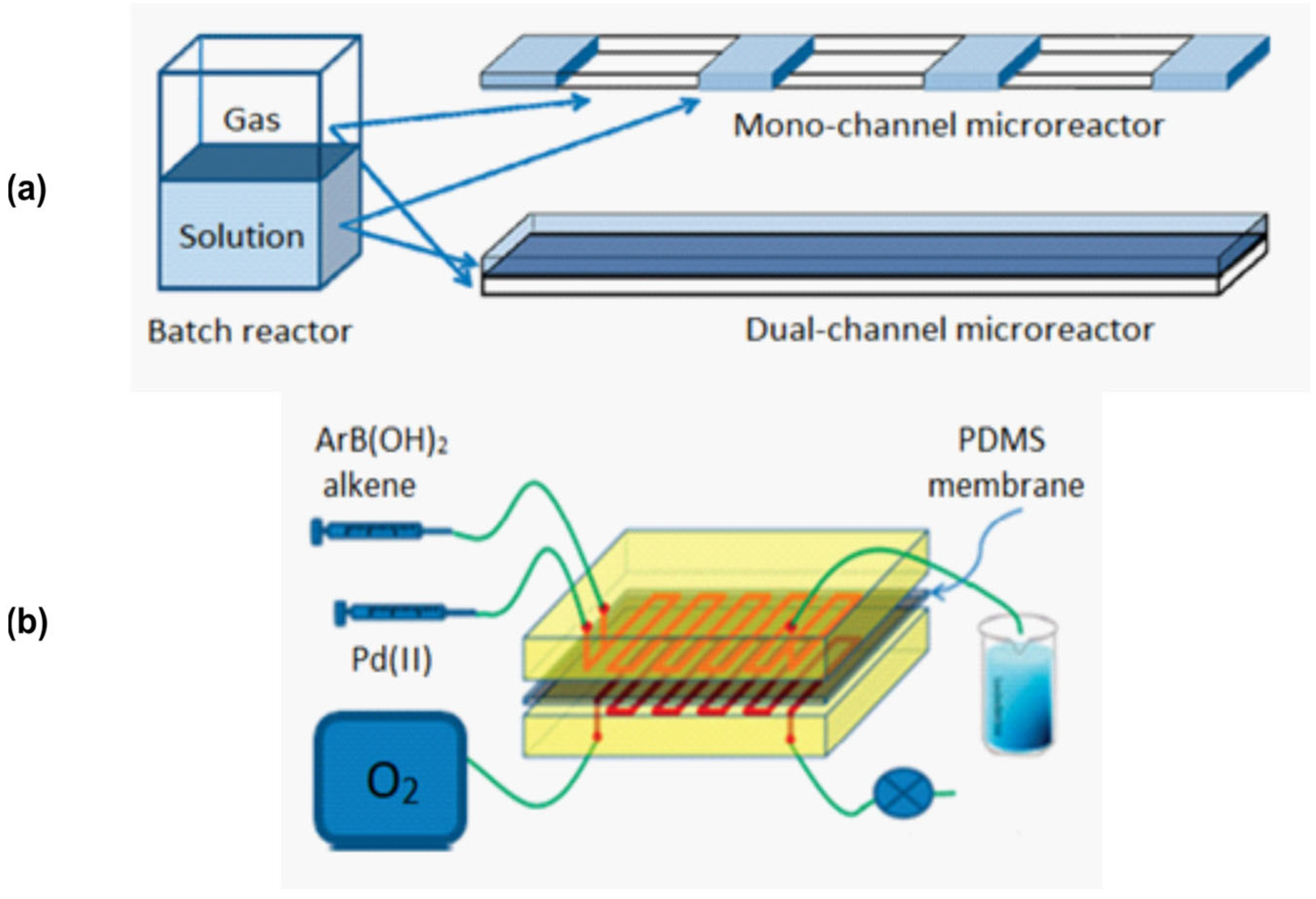

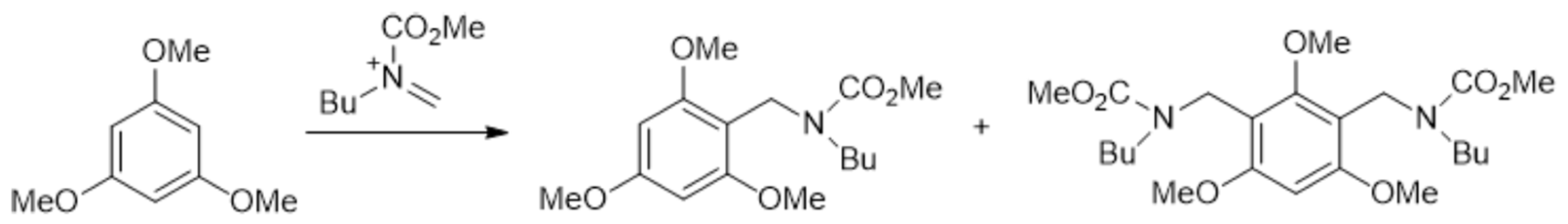
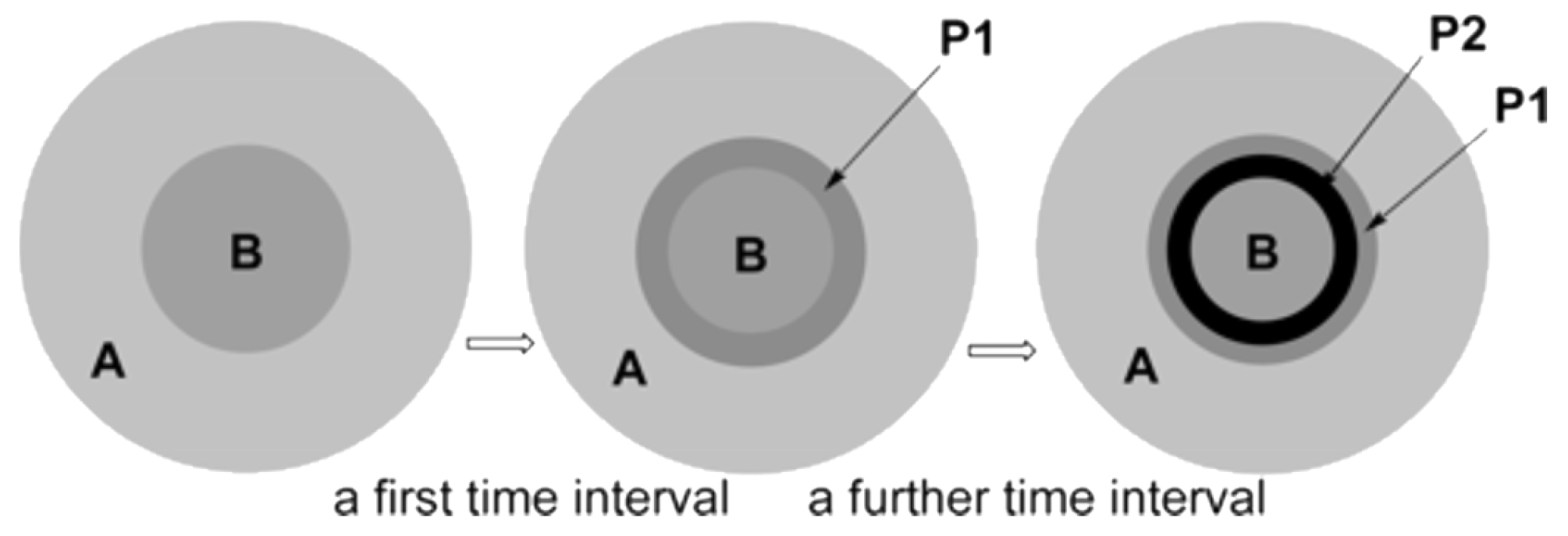




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myachin, I.V.; Kononov, L.O. Mixer Design and Flow Rate as Critical Variables in Flow Chemistry Affecting the Outcome of a Chemical Reaction: A Review. Inventions 2023, 8, 128. https://doi.org/10.3390/inventions8050128
Myachin IV, Kononov LO. Mixer Design and Flow Rate as Critical Variables in Flow Chemistry Affecting the Outcome of a Chemical Reaction: A Review. Inventions. 2023; 8(5):128. https://doi.org/10.3390/inventions8050128
Chicago/Turabian StyleMyachin, Ilya V., and Leonid O. Kononov. 2023. "Mixer Design and Flow Rate as Critical Variables in Flow Chemistry Affecting the Outcome of a Chemical Reaction: A Review" Inventions 8, no. 5: 128. https://doi.org/10.3390/inventions8050128
APA StyleMyachin, I. V., & Kononov, L. O. (2023). Mixer Design and Flow Rate as Critical Variables in Flow Chemistry Affecting the Outcome of a Chemical Reaction: A Review. Inventions, 8(5), 128. https://doi.org/10.3390/inventions8050128