1. Introduction
The Palmaris longus muscle (PLM) is described as probably the most variable muscle in the human body. Not only in terms of absence but also in variability of origin and insertion and morphology [
1]. The PLM can be absent unilateral and bilateral in about 22.4% of human beings (Caucasians) with a range of 3.0%–63.9%, depending on the ethnic background of a population [
2]. A great diversity is reported between different populations in the prevalence of absence of the PLM [
3].
Stecco
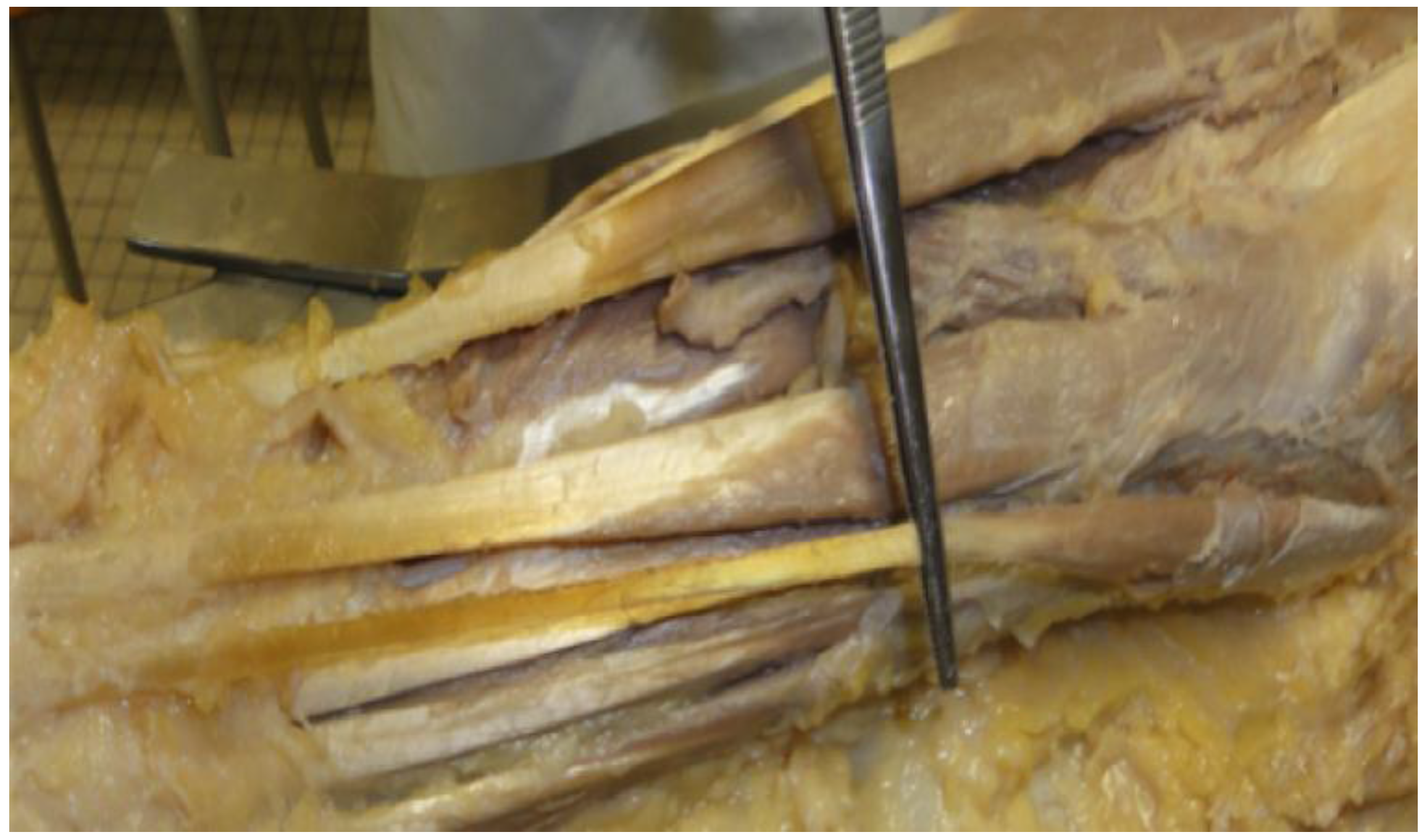
et al. considers the PLM as a slender fusiform muscle with a short proximal belly and a long distal tendon (
Figure 1). It is located in the most superficial layer of the anterior compartment of the forearm, between the radial and the ulnar flexors of the wrist [
4]. In most cases, the distal tendon of the PLM shows a lateral slip that attaches to the superficial surface of the abductor pollicis brevis muscle that may contribute to thumb abduction [
5]. In the proximal part of the forearm, the PLM is situated deep to the antebrachial fascia, while in the lower third of the forearm its tendon perforated the antebrachial fascia [
4]. The origin of the PLM is at the epicondylus medialis of the humerus and the insertion is on the aponeurosis Palmaris [
6]. The PLM shares its origin with different muscles such as the flexor digitorum superficialis muscle, the flexor carpi radialis muscle, and the flexor carpi ulnaris muscle [
4].
Anatomical variations of the muscle belly have been described by numerous reports. The many anatomical variants of the PLM are probably related to the human evolution [
7]. The PLM can be hypertrophic, reversed, centrally placed, digastric, duplicated, or bifid. When the PLM is located deep to the ligamentum carpi transversum, it is also called a palmaris profundus muscle. This muscle can be categorized as a duplicated PLM [
1]. A palmaris accesorius muscle can also occur and is recognized as a group of anomalies in origin and insertion and particular accessory insertion slips [
7]. The PLM is vascularized by a small branch of the anterior ulnar recurrent artery and its innervation is derived from branches of the median nerve, which contains fibers from all five roots (C5-T1). The median nerve is derived from the medial and lateral cords of the brachial plexus [
8].
Literature suggests that when the PLM is present, it may function as a weak wrist and elbow flexor [
9]. Other functions of the PLM are assistance in carpo-metacarpal flexion and cupping of the palm [
2], abduction of the thumb, stabilization of the superficial structures in the palm [
10], and a tensor of the aponeurosis palmaris [
6]. In subjects with an absent PLM, these functions also exist; therefore, it can be assumed that the presence of the PLM is of little overall value to hand function.
A lack of agreement is present in literature about the function of the PLM. This is why it is difficult to determine whether the PLM provides an advantage to handgrip in sport.
Fowlie
et al. [
11] determined the influence of the presence of the PLM in sports that require different sorts of handgrip. Characteristics of grips used for each of the participants’ sport were compared. Tennis, badminton, and squash were defined as sustained dominant-sided cylindrical grip sports, while rowing and canoeing were considered as sustained two-handed cylindrical grip sports. In other sustained grip sports such as hockey and cricket, in which the stroke play is intermittent, the implement used is held throughout play. Golf was defined to be an intermittent grip sport because of the implement being released after stroke play. Fowlie
et al. [
11] reported that in both elite and non-elite athletes, the performance of the PLM was significantly higher in those who participated in sustained grip sports compared to intermittent grip sports. In elite athletes participating in dominant-handed and two-handed cylindrical grip sports, the presence of the PLM was greater on their dominant side compared with non-elite athletes participating in the same sports. Bilateral occurrence of the PLM was also higher in elite athletes [
11].
Sebastin
et al. [
12] tested handgrip and pinch strength comparing subjects with and without PLM. These authors concluded that there was no significant difference in hand strength between the two groups. This was also determined in a pediatric population by Cetin
et al [
13]. As for grip strength, they found that the presence of the PLM did not create a difference in general. As for pinch strength, they concluded that the PLM increased pinch strength in the fourth and fifth finger of the hand, especially in the right hand. Based on these data, they suggested that the PLM could impact the opposition movement of the fingers [
13].
Fleckenstein
et al. [
14] measured the contribution of the PLM to wrist flexion using magnetic resonance imaging before and after flexion exercises to determine muscle recruitment of wrist flexor muscle groups. Variations were determined by comparing the right and left forearm and the effect of slight (15°) pronation and supination of the wrist. Imaging after exercise showed focal regions of increased signal intensity, indicating relatively strong recruitment, most often in the whole muscle, although occasionally only in subvolumes of the muscles. Relatively strong recruitment of the flexor carpi radialis muscle was seen during pronation and/or use of the non-dominant side. They concluded that only seven of the eleven subjects recruited their PLM during isolated resisted wrist flexion with a weight of two kg [
14].
The term proprioception, originally introduced by Sherrington, describes the sense of limb position and movements subserved by deep receptors in muscles, tendons, and joints [
15], including capsular and ligamentous structures. The sensory end organs include the Pacinian receptors, Ruffini end receptors, Golgi tendon receptors, and free nerve endings. These aspects of afferent inputs are being increasingly studied in an attempt to describe and understand impairments, to improve rehabilitation effectiveness following trauma or surgery, and to prevent recurrent injury [
16]. Different testing techniques have been developed to measure the three submodalities of proprioception: Joint position sense (JPS), kinesthesia (KIN), and sense of resistance [
17]. The sources of proprioceptive information potentially include joint, muscle [
18], and cutaneous mechanoreceptors [
19]. Visual and auditory signals can provide additional cues to proprioception [
20]. Each of these sensory receptors have been demonstrated to contribute to the sense of position or motion of a body part in space [
21].
The methodologies used to gain information differ. In previous studies the modalities of proprioception have been measured by using dynamometers [
22], electrogoniometers, potentiometers [
23], electromagnetic sensors [
21], and video digitization/analysis [
24]. In this research we use the Flock of Birds tracking system, which captures the 3D position and orientation of a receiver with respect to a transmitter in six degrees of freedom, using pulsed magnetic fields.
In the present study further research has been conducted to determine whether presence of the PLM provides an advantage to handgrip in a sport such as tennis between elite tennis players and recreational athletes. Another aim of this study is to investigate the relationship between hand dominance and the performance of the PLM. The relation between the PLM and gender is also determined. The difference between presence or absence of the PLM and maximal grip strength and fatigue resistance is also a goal in this research. Furthermore maximal grip strength, fatigue resistance, and proprioceptive function of the hand and wrist between elite athletes with and without a PLM, is studied. Finally, this study tries to find out whether there is a difference between elite athletes and recreational athletes in the presence of the PLM, maximal grip strength, fatigue resistance, and proprioceptive function of the hand.
2. Materials and Methods
2.1. Study Participants
A total of 60 people participated in this study. The elite athletes group consisted of 30 healthy tennis players among which 23 (76.7%) were male and seven (23.3%) were female subjects. The elite athletes group included men with ranking A, B-15/4, B-15/2, or B-15/1, and women with ranking A, B-15/4, and B-15/2. Ranking is based on the tennis match results of certain competitions where they can deserve points. Tennisplayers are considered as an A player if they have an Association of Tennis Professionals (ATP)/Women’s Tennis Association (WTA) ranking in the top 1000 (the first 5% of men, the first 10% of women). Ranking B-15/4 consists of tennisplayers with an ATP/WTA ranking above number 1000, by men this means the following 10% of the tennisplayers, by women the following 15% of the players. B-15/2 players are being classified as the following 20% of these players by men and the following 20% by women. B-15/1 are the following 30% by men, and the following 25% by women. These athletes may also have reached this level in the past, up to a maximum ten years ago. The control group included 30 (23 male and 7 female) healthy recreational athletes who do not practice sports in which grip function is important such as tennis, badminton, squash, hockey, table tennis, etc.
Participants having a background of an injury or a surgical operation of the elbow or hand or having any pathology with a possible negative influence on the function of the hand, like carpal tunnel syndrome, diabetes, neurological disorder, or a self-reported history of inflammatory joint disease were excluded from the study.
The Human Ethics Committee of the Brussels Academic Hospital (UZ Brussel) approved the study on June 18, 2014 in accordance with the declaration of Helsinki of 1975. The project identification code of this study was B.U.N. 143201419488. Before participation in this research, all participants provided written informed consent. If the participant was younger than 18 years old, the informed consent was signed by the parents. The elite tennis players were recruited based on the club where they play tennis or through random contacts or friends. The control group was selected at random. Gender and age matching was attempted between elite sports and recreational athletes.
The measurements were organized at the tennis club of the players, at the researchers’ place or at the Experimental Anatomy lab of the Vrije Universiteit Brussel (VUB) campus Jette. Measurements were performed from June to August 2014.
At first, personal data such as name, gender, age, hand dominance, sports, and ranking were registered. Then, the presence of the PLM, maximal grip strength, fatigue endurance, and proprioception were evaluated.
2.2. Palmaris Longus Muscle
Sebastin
et al. [
25] described six different techniques for examining the presence of the PLM
in vivo (
Figure 2). Initially, the subject was asked to perform the standard test on both sides. This was the most commonly used technique. If the palmaris longus tendon was not visualized or palpable, the second test was performed, and so on, until the last test. When the six techniques were performed without observation of a palmaris longus tendon, the presence of PLM was excluded. Tests were performed in the following order:
Standard test (Schaeffer’s test): opposition of the tip of the thumb to the tip of the little finger and then flexion of the wrist [
26].
Thompson’s test: fist is made, then flexed, finally the thumb is opposed and flexed over the fingers [
27].
Mishra’s test I: passive hyperextension of the metacarpophalangeal joints of all fingers, then the subject is asked to actively flex the wrist [
28].
Mishra’s test II: abduction of the thumb against resistance with the wrist in slight palmar flexion [
28].
Pushpakumar’s “two-finger sign” method: fully extension of the index and middle finger, flexion of the wrist and other fingers, finally the thumb is fully opposed and flexed [
29].
Gangata’s test: manual isometric resistance against wrist flexion onto the palmar surface at the tips of the second to fifth metacarpals and, simultaneously, manual isometric resistance is applied against thumb abduction onto the lateral surface (where the palmar skin of the hand meets the dorsal skin) of the first metacarpophalangeal joint [
5].
2.3. Maximal Grip Strength and Fatigue Resistance
Maximal grip strength and fatigue resistance were measured in both hands with an electronic hand dynamometer (CAMRY, model EH101, Zhongshan, China) (
Figure 3) while the subject was seated on a chair. This device allowed continuous monitoring and recording of the pressure. The tests were executed with the shoulder in slight abduction and neutral rotation, the elbow flexed at 90° and neutral position of the forearm and wrist. The upper arm and forearm could not touch the trunk. Then, the subject was asked to squeeze the handles towards each other as hard as possible. To ensure that maximal effort would be reached, participants were instructed to perform three sets with 20 s of rest between the sets, alternating the right and left hand, starting with the dominant hand. The highest of three attempts was described as the maximal grip strength. All pressure measurements were expressed in kilograms (kg).
After this, the subject was again instructed to squeeze the handles towards each other as hard as possible and to maintain this maximal pressure until exhaustion. The time (in seconds) during which grip strength dropped to 50% of its maximum was noted as fatigue resistance [
30].
2.4. Proprioception
Proprioception was defined as the cumulative neural input to the central nervous system from mechanoreceptors, which were located in the joint capsules, ligaments, muscles, tendons, and skin [
31]. The ability to control the position of a joint was considered to be an aspect of proprioceptive functioning. Kinematics of the hand were registered by means of the Flock of Birds electromagnetic tracking system (Ascension Technologies, Shelburne, VT, USA) (
Figure 4). This system was able to measure 3D positional and orientation data from multiple sensors. All ferromagnetic materials were removed from the evaluation area to avoid interference [
32,
33]. Therefore, the tests were executed on a wooden chair and table.
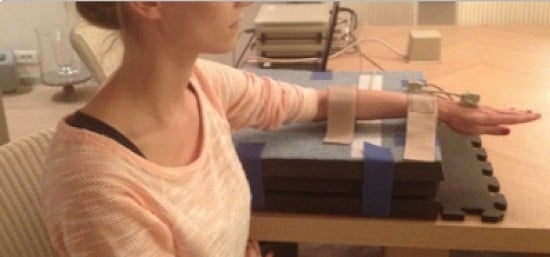
Each subject was seated in a chair with alternately one forearm placed in the arm supports. The subject’s arm was positioned in forearm pronation and neutral wrist flexion/extension. A construction was made to ensure the forearm was fixated with a strap proximal of the processus styloideus radii and ulnae, and a strap distal of the olecranon, while solitary hand movements were performed. One sensor was attached proximal and in line with the dorsal aspect of the metacarpo–phalangeal III joint, in the middle between the distal end of metacarpo–phalangeal III and a transverse line between processus styloideus radii and ulnae. The other sensor was mounted on a stabile support on a hard foam mat as shown in
Figure 5. The electromagnetic sensors were fixed with adhesive tape and velcro and registered angular displacement.
First, the JPS—the accuracy in which subjects can replicate a certain joint angle—was measured [
17]. After starting in neutral position (fingers in line with the forearm), the subject was requested to move to palmar and dorsal flexion with the hand three times and, finally, try to replicate the initial neutral position. The joint position error between the initial position and the repositioning position was calculated. To eliminate visual cues the subject’s view of the test joint was blocked.
In the second test kinesthesia (KIN)—the conscious awareness of position and movement in the joint, resulting from proprioceptive input to the central nervous system—was measured by performing a trajectory movement using a U-formed hard foam (
Figure 6) [
31]. The subject had to start in neutral position again in the U-formed hard foam, then move to palmar flexion and dorsal flexion until the fingers reached the foam while keeping the fingers in line with the forearm. This movement was repeated ten times with foam. Afterwards, the foam was removed and the subject had to perform the same movements three times, trying to reproduce, as accurately as possible, the previous range. In this test, the subject was also not allowed to look at the hands.
The third test consisted of movements, with and without feedback of the computer screen, which is called joint motion sense (JMS) in our study.
The participant had to start in neutral position, then move three times, first, 20 degrees to palmar flexion, and then 20 degrees to dorsal flexion, with feedback of the computer screen. Thereafter the same movement had to be performed three times, looking at the computer screen, but having the eyes closed without feedback.
2.5. Data Analysis
Data were collected in Microsoft Excel spreadsheets and kinematic processing for the proprioception components was conducted in a MathCAD 14 mathematical software routine. The range of motion was calculated and displayed in three axes (X, Y, Z). The Y coordinate presented the palmar and dorsal flexion of the hand, which could be seen as the main motion component. Other coupled motion components (X and Z) were excluded from the analysis. JPS was calculated by comparing the position of the Y coordinate with respect to the starting position, which was zero. KIN was measured by comparing the range of motion of the Y coordinate of the movements with and without the foam.
Calculation of the JMS was conducted by comparing the range of motion of the Y coordinate without feedback of the computer minus forty (20° palmar and 20° dorsal flexion). All of these outcomes were displayed in absolute values. Kinematic data were statistically analyzed using SPSS version 19 (IBM Corporation, Armonk, NY, USA), the statistical software program. Normality of data distribution was checked using the Kolmogorov–Smirnov test (p < 0.05).
The relationship between hand dominance and the performance of the PLM was determined by a Chi-square test. This test was also applied to analyze the correlation between the occurrence of the PLM and elite tennis players or recreational athletes.
Differences between subjects, controls, and subgroups (presence of the PLM, maximal grip strength, fatigue resistance, and proprioceptive function of the hand and wrist) were investigated using the analysis of covariance (ANCOVA). A value of p < 0.05 was considered statistically significant.
4. Discussion
The PLM agenesis has been variously reported to be from 3.0% in black people to 63.9% in Turkish people. The prevalence of absence of the PLM in the Caucasian group was found to be 22.4%, including unilateral and bilateral agenesis [
2]. It is important to be aware of the variability in prevalence of this muscle related to the population or ethnic group.
The prevalence of agenesis was found to be significantly more common on the left side [
3,
34]. This corresponds to our study, in which four subjects had a unilateral PLM on the right side while only two subjects showed a unilateral PLM on the left side.
The incidence of left-handedness is less common than right hand dominance. About 8%–15% of the general population is left-handed [
35]. In this study, 16.6% of the participants were left-handed, which corresponds to the general population. Eric
et al. [
36] reported in his study that a right-sided absence was more common in left-handed persons while the left-sided absence was more common in right-handed persons. Unilateral tendon absence was more common in the non-dominant hand. This study included 542 subjects. Due to the size of the study population, these results were very reliable. Our study (
Table 1) also showed that in the right-handed participants left-sided absence was more common. In left-handed dominance, however, the absence of the PLM was the same on both sides. Although in agreement with Eric
et al., due to the small size of the study population, this conclusion is not significant and cannot be generalized [
36].
In general, most people have a bilateral PLM, second comes bilateral absence, unilateral absence on the right is next, and unilateral absence on the left is less common. This was the case in our study and was also reported by Eric
et al. [
36].
Other studies however reported another descending order: bilateral present, bilateral agenesis, left agenesis, and right agenesis [
1,
3,
26,
37]. Finally Soltani
et al. found that bilateral presence of the PLM occurred the most, followed by unilaterally absence of the PLM while bilaterally absence of the PLM occurred less [
34].
Thompson
et al. reported that men were more prone to have a bilateral and unilateral absence of the PLM, but this was not statistically significant [
27]; other authors reported the incidence of agenesis to be higher in women [
1,
26,
34].
The study of Kapoor
et al. [
3] also described that men were more common to have a unilateral agenesis and women to have a bilateral agenesis. This difference was statistically significant. This is in contrast with our findings in which women were more likely to have a unilateral PLM while men were more likely to have no PLM at all, but there was, however, no significant difference. According to Eric
et al., the middle-age and young group showed a lower rate of the PLM presence as compared to the old group, although this difference was not significant [
36,
38]. In comparison, Venter
et al. reported no obvious trend in the PLM in the various age groups that would indicate phylogenetic degeneration of the PLM [
39].
The frequent use of the PLM in surgery begged the question whether the removal of this muscle would affect the hand function [
13]. The PLM is often used for reconstructive surgery because it fulfilles the criteria of length, thickness and availability without producing a deformity [
40]. Verdan [
41] suggested that a divided PLM did not need to be repaired because of its small importance, while Sebastin
et al. [
12] believed that repair of a divided PLM should always be done, even if it were the only divided tendon, as a repaired PLM was considered to protect the median nerve, which is necessary, especially in patients with self-mutilation. Repair of the PLM provided recovery of the function of this muscle, and saved it as a tendon graft for later use [
12].
The presence of the PLM, on the other hand, was correlated with a significant increase in risk of developing Dupuytren’s disease [
42], a common acquired pathological condition with fibrous degeneration of the superficial longitudinal layer of the aponeurosis and the retinacula cutis. The deep transverse layer seems to be only involved in late stages of the disease [
43].
Fowlie
et al. suggested that the presence of the PLM could provide a favorable contribution to sustained grip sports and cylindrical grip sports that require a higher level of skill [
11]. In athletes who participate in a sustained or cylindrical grip sport, the PLM may provide a larger pool of muscle fibers that can be recruited for strength and endurance, or a greater pool of proprioceptors that can contribute to superior grip precision. To maintain a stable grip during sustained grip sports, a submaximal isometric co-contraction of carpo-metacarpal flexors and finger flexors is required. To stabilize the wrist in a specific position, co-contraction of wrist flexors and extensors is also likely to occur [
11]. Chow
et al. and Wei
et al. supported the theory that there is electromyographic activity of wrist flexors and extensors during backhand and forehand volleys in tennis [
44,
45]. Chow
et al. [
44] studied the muscle activation characteristics of a tennis volley under varying ball speed and location conditions. This was determined by surface electromyography techniques which could not differentiate between individual muscles but only activation of the flexors and extensors in general. In all subjects, muscle activity increased when the ball speed was higher. This suggests that the grip force and wrist stiffness increased as ball speed increased. Assuming that a quicker racket movement is required in fast speed trials, an expansion in forearm muscle activity may be required as the speed of racket movement expanses [
44], which suggests that elite tennis players require more muscle activity because of higher ball speed.
Some authors have found that absence of the PLM was not significantly correlated with decreased grip or pinch strength measurements [
12,
13]. Cetin
et al. [
13] on the other hand, reported that having a PLM increased the pinch strength in the fourth and fifth finger of the hand, especially in the right hand. For the pinch strength of the second finger, no significant difference was found. The third finger of the right hand showed only in girls of 6–7 years old a significant correlation with the absence of the PLM, but no relation was found for the left hand. In addition, this muscle may impact the opposition movement of the fingers [
13]. These findings suggest that the PLM may not be as redundant as current opinion indicates, although we have to keep in mind that this study only included girls and boys with ages of 6–11 years old.
Petersen
et al. described a difference in grip strength between the dominant and non-dominant hand in left-handed subjects ranging from 0 to 5%, while in right-handed subjects the dominant hand is generally accepted to be 10% stronger than the non-dominant hand [
46]. In our study this was not always the case, as a lot of variations occurred between grip strength of the dominant and non-dominant hand.
The grip strength could also be influenced by the occupation of the subjects. It is logical that manual workers have a greater grip strength compared to sedentary workers and that people who use their hand function a lot may have a better grip strength. Another thing that has to be kept in mind is that we did not consider differences in body mass of our participants.
Unfortunately, kinesthesia and proprioception are used interchangeably in literature, which leads to confusion of these terms. Proprioception refers to the general central nervous system, which describes afferent information arising from internal peripheral areas of the body that have a contribution to postural control, joint stability and several conscious sensations [
47]. The advantage of registering active movement with the Flock of Birds electromagnetic tracking system is that subjects have free, unrestricted movement, unlike in the proprioception testing device, where they are limited to one degree of freedom. This is important for the wrist, because natural movement patterns involve multiplanar motions [
17].
As far as we know, this is the first study that measured the wrist proprioception in relation with the presence of the PLM and in relation with an elite sports population.
In the present study there is no significant correlation between the proprioceptive function and subgroups. The only significant relation is between kinesthesia on the non-dominant side and age, and between joint motion sense on the non-dominant side and gender, but this could be due to coincidence. No previous studies were found about the relationship between the proprioceptive function and gender, age, the PLM, or elite and non-elite athletes. This topic requires more research with a larger population to increase the validity.
The advantage of this measurement system for wrist joint proprioception allows the researcher to decrease extraneous influences that may affect joint position sense awareness and, therefore, improve the knowledge of the mechanisms underlying kinesthesia and proprioception. Gay
et al. [
16] approved, with an experimental setup similar to the one used in our study, that the measurements were repeatable and appeared to be equally or more accurate than other methods previously employed to measure wrist and hand range of motion. The difference with our study was, however, that subjects were tested in an active as well as in a passive condition [
16]. According to Voight
et al., subjects replicated movements more accurately after active target presentation than after passive [
19], while other authors found no difference between active and passive repositioning [
21,
48].
Proprioceptive information plays an important role in joint stabilization. The stability of motion or changes in motion sequence may be an expression of altered or disturbed proprioception. According to Voight
et al. [
19] proprioception of the glenohumeral joint is significantly altered in the presence of shoulder muscle fatigue. They measured active and passive repositioning of the upper extremity accurately following a fatigue protocol [
19]. With respect to this theory, it has to be kept in mind that some of the elite tennis players conducted the proprioceptive tests after warming-up or after having played a tennis game.
Concerning the absence of a significant difference in strength and proprioception of the wrist between subjects with and without a PLM, it is possible that its functional ability has been taken over by the other forearm flexors in subjects where it is absent.
Furthermore, absence may not necessarily be the same as loss, particularly in patients with pathology involving the other wrist flexors or thumb abductors. In such cases, removal of the PLM may further weaken the already damaged hand.
One of the limitations of the study was that we did not measure the pinch strength or thumb abduction. Some studies have reported the importance of the opposition movement and thumb abduction of the PLM, which is why these parameters might better be included in further investigations. It was difficult to measure the functional contribution of the PLM separate from the other wrist flexors and thumb abductors. We used grip strength for measuring the hand function because it provides us with quantitative evidence and is easily understood by patients. The present study was also limited in the study population that was too small because of its strong selection criteria to participate. Some of the subjects were still on high school or university, which means that they probably use their finger muscles for handwriting much more than adults. This could be of influence on the data. In order to determine whether the removal of the PLM results in change in grip and pinch strength of these patients, it is necessary to perform these tests before and after surgical removal of this muscle.
The ideal sample for this study would be a group of patients in whom the PLM has been used for reconstruction surgery in another region than the wrist. A pre- and post-operative assessment of grip and pinch strength could reflect the functional loss due to removal of the PLM. However, it is extremely difficult to get a large population of such patients.
The strength of this study was that it demonstrated the feasibility of the test protocol in terms of execution and that it examined more aspects of proprioception than most previous studies did. We have to keep in mind that a weakly-developed or anomalous PLM may have been mistaken as absent. However, a comparison between cadaveric and in vivo studies showed no significant difference in prevalence [
2]. Radiological investigation such as an ultrasonography or magnetic resonance imaging would be a sure way of detecting even an anomalous tendon, but the performance of such an investigation in a large population might render the protocol less feasible.