Acute Effects of Passive Stretching with and Without Vibration on Hip Range of Motion, Temperature, and Stiffness Parameters in Male Elite Athletes
Abstract
:1. Introduction
2. Materials and Methods
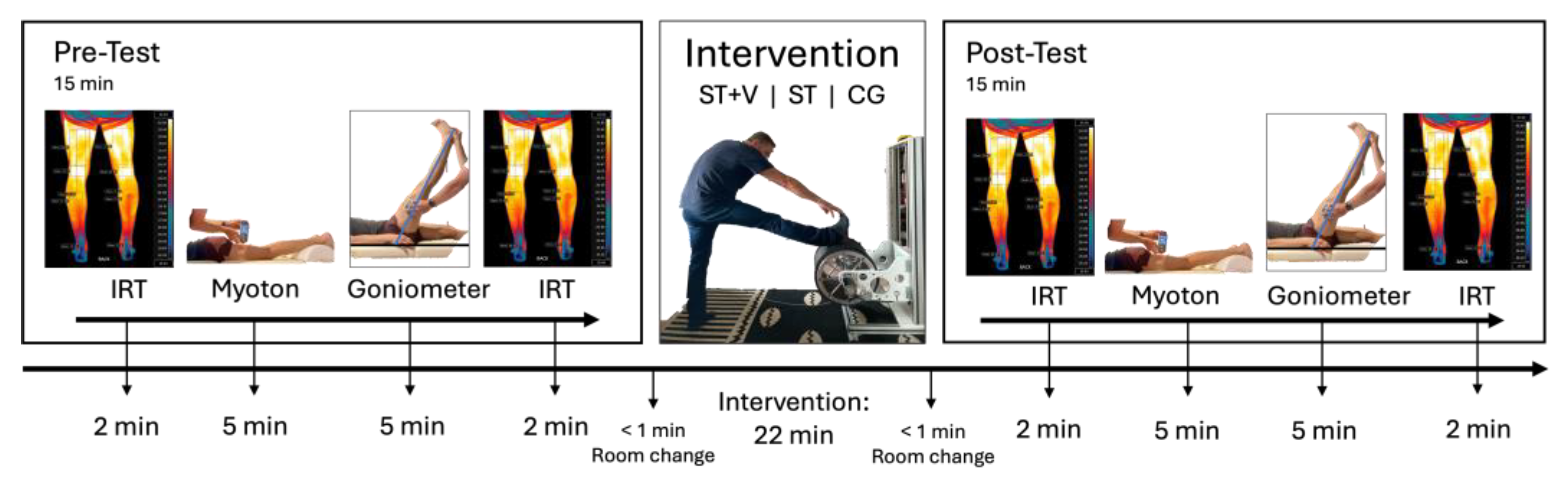
2.1. Experimental Approach
2.2. Participants
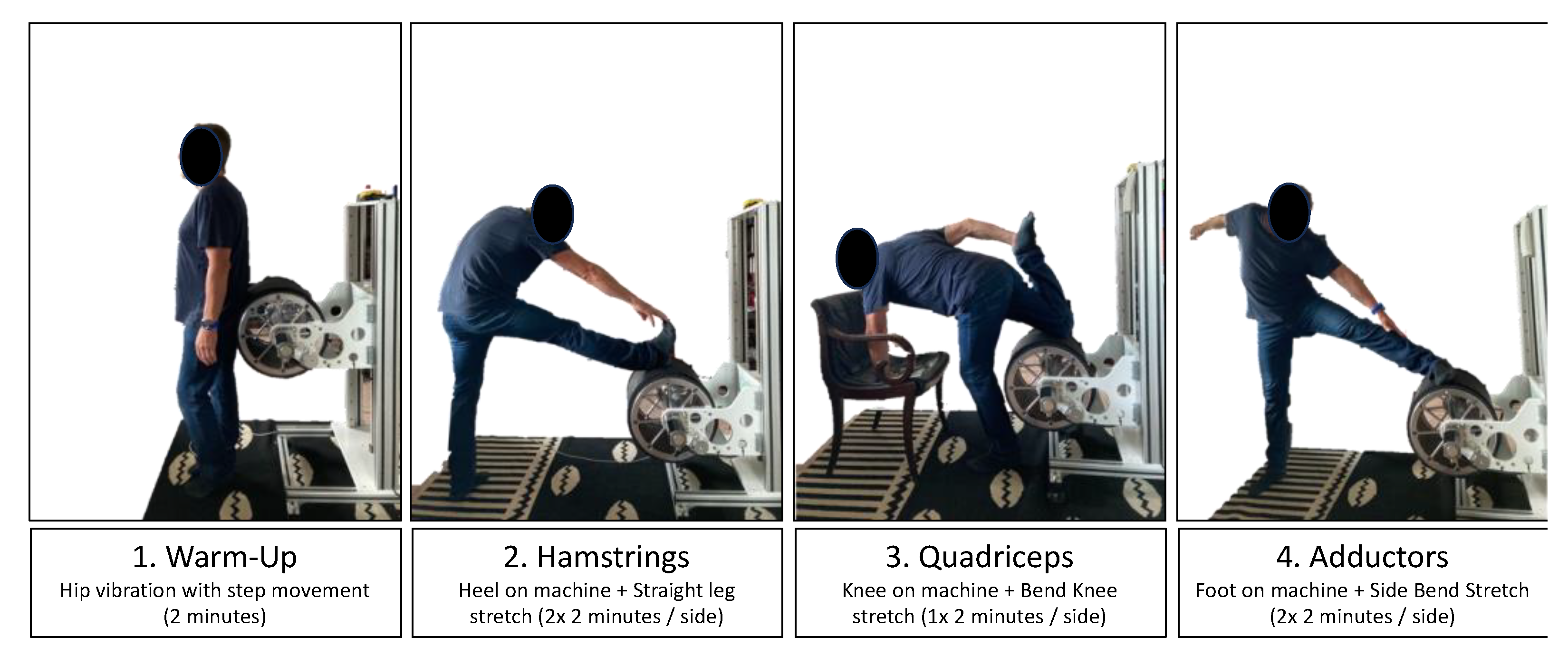
2.3. Device to Superimpose Vibration on Stretching
2.4. Infrared Thermography
2.5. Myotonometry
2.6. Range of Motion Testing via Goniometry
2.7. Stretching Protocol
2.8. Statistical Analysis
3. Results
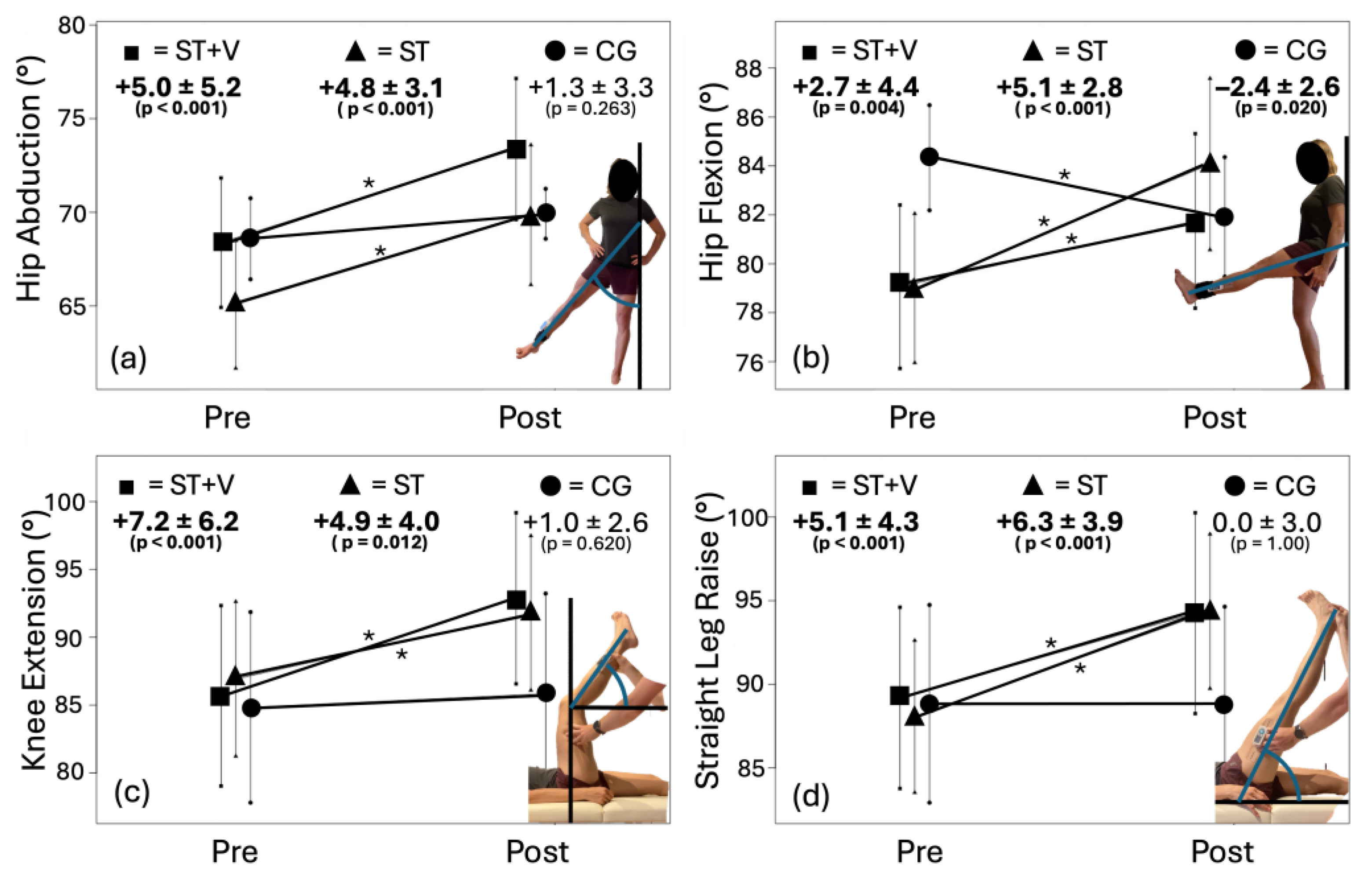
3.1. Range of Motion
3.2. Myotonometry
3.3. Skin Temperature
4. Discussion
4.1. Underlying Mechanisms of ROM Increases: Stiffness Effects
4.2. Temperature Effects
4.3. Limitations
4.4. Practical Relevance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konrad, A.; Alizadeh, S.; Daneshjoo, A.; Anvar, S.H.; Graham, A.; Zahiri, A.; Goudini, R.; Edwards, C.; Scharf, C.; Behm, D.G. Chronic effects of stretching on range of motion with consideration of potential moderating variables: A systematic review with meta-analysis. J. Sport Health Sci. 2023, 13, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Blazevich, A.J.; Kay, A.D.; McHugh, M. Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: A systematic review. Appl. Physiol. Nutr. Metab. 2016, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Konrad, A.; Tilp, M. The Time Course of Muscle-Tendon Unit Function and Structure Following Three Minutes of Static Stretching. J. Sports Sci. Med. 2020, 19, 52–58. [Google Scholar]
- Folpp, H.; Deall, S.; Harvey, L.A.; Gwinn, T. Can apparent increases in muscle extensibility with regular stretch be explained by changes in tolerance to stretch? Aust. J. Physiother. 2006, 52, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sato, S.; Kiyono, R.; Yahata, K.; Yoshida, R.; Fukaya, T.; Nishishita, S.; Knorad, A. Relationship between changes in passive properties and muscle strength after static stretching. J. Bodyw. Mov. Ther. 2021, 28, 535–539. [Google Scholar] [CrossRef]
- Roberts, H.M.; Law, R.J.; Thom, J.M. The time course and mechanisms of change in biomarkers of joint metabolism in response to acute exercise and chronic training in physiologic and pathological conditions. Eur. J. Appl. Physiol. 2019, 119, 2401–2420. [Google Scholar] [CrossRef] [PubMed]
- Konrad, A.; Nakamura, M.; Paternoster, F.K.; Tilp, M.; Behm, D.G. A comparison of a single bout of stretching or foam rolling on range of motion in healthy adults. Eur. J. Appl. Physiol. 2022, 122, 1545–1557. [Google Scholar] [CrossRef]
- Konrad, A.; Stafilidis, S.; Tilp, M. Effects of acute static, ballistic, and PNF stretching exercise on the muscle and tendon tissue properties. Scand. J. Med. Sci. Sports 2017, 27, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Konrad, A.; Reiner, M.M.; Thaller, S.; Tilp, M. The time course of muscle-tendon properties and function responses of a five-minute static stretching exercise. Eur. J. Sport Sci. 2019, 19, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Nakamura, M.; Fukaya, T.; Konrad, A.; Mizuno, T. Acute and Long-Term Effects of Static Stretching on Muscle-Tendon Unit Stiffness: A Systematic Review and Meta-Analysis. J. Sports Sci. Med. 2023, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Issurin, V. Vibrations and their applications in sport: A review. J. Sports Med. Phys. Fit. 2005, 45, 324–336. [Google Scholar]
- Fuller, J.T.; Thomson, R.L.; Howe, P.R.; Buckley, J.D. Effect of vibration on muscle perfusion: A systematic review. Clin. Physiol. Funct. Imaging 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Oliveri, D.J.; Lynn, K.; Hong, C.Z. Increased skin temperature after vibratory stimulation. Am. J. Phys. Med. Rehabil. 1989, 68, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.D.; Palombo, K.T.M.; Feland, J.B.; Blotter, J.D. Effects of Whole-Body Vibration on Flexibility and Stiffness: A Literature Review. Int. J. Exerc. Sci. 2019, 12, 735–747. [Google Scholar] [CrossRef]
- Jochum, D.; Konrad, A.; Lohmann, L.H.; Cochrane, D.J.; Rittweger, J.; Vogel, V.; Warneke, K. The merit of superimposed vibration for flexibility and passive stiffness—A systematic review with multilevel meta-analysis. J. Sport Health Sci. 2024, in press. [Google Scholar]
- Warneke, K.; Plöschberger, G.; Lohmann, L.H.; Lichtenstein, E.; Jochum, D.; Siegel, S.D.; Zech, A.; Behm, D.G. Foam rolling and stretching do not provide superior acute flexibility and stiffness improvements compared to any other warm-up intervention: A systematic review with meta-analysis. J. Sport Health Sci. 2024, 13, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.A.; Rabelo, A.S.; Couto, B.P.; Motta-Santos, D.; Drummond, M.D.M.; Gonçalves, R.; Silva, R.A.D.; Szmuchrowski, L.A. Acute effects of single bout of stretching exercise and mechanical vibration in hamstring muscle. J. Exerc. Physiol. Online 2017, 20, 46–57. [Google Scholar]
- De Nardi, M.; Facheris, C.; Ruggeri, P.; La Torre, A.; Codella, R. High-impact Routines to Ameliorate Trunk and Lower Limbs Flexibility in Women. Int. J. Sports Med. 2020, 41, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- McNeal, J.R.; Edgerly, S.; Sands, W.A.; Kawaguchi, J. Acute effects of vibration-assisted stretching are more evident in the non-dominant limb. Eur. J. Sport Sci. 2011, 11, 45–50. [Google Scholar] [CrossRef]
- Maeda, N.; Urabe, Y.; Kotoshiba, S.; Komiya, M.; Morikawa, M.; Nishikawa, Y.; Sasadai, J. Acute effects of local vibration stretching on ankle range of motion, vertical jump performance and dynamic balance after landing. Isokinet. Exerc. Sci. 2021, 29, 139–145. [Google Scholar] [CrossRef]
- Olivares-Arancibia, J.; Solis-Urra, P.; Rodriguez-Rodriguez, F.; Santos-Lozano, A.; Sanchez-Martinez, J.; Martin-Hernandez, J.; Zurita-Corvalan, N.; Sadarangani, K.; Cristi-Montero, C. A single bout of whole-body vibration improves hamstring flexibility in university athletes: A randomized controlled trial. J. Hum. Sport Exerc. 2018, 13, 776–788. [Google Scholar] [CrossRef]
- Nakano, J.; Yamabayashi, C.; Scott, A.; Reid, W.D. The effect of heat applied with stretch to increase range of motion: A systematic review. Phys. Ther. Sport 2012, 13, 180–188. [Google Scholar] [CrossRef]
- Bozic, P.R.; Pazin, N.R.; Berjan, B.B.; Planic, N.M.; Cuk, I.D. Evaluation of the Field Tests of Flexibility of the Lower Extremity: Reliability and the Concurrent and Factorial Validity. J. Strength. Cond. Res. 2010, 24, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Albasini, A.; Krause, M.; Rembitzki, I.V. Using Whole Body Vibration in Physical Therapy and Sport E-Book: Clinical Practice and Treatment Exercises; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Weigert, M.; Nitzsche, N.; Kunert, F.; Lösch, C.; Baumgärtel, L.; Schulz, H. Acute exercise-associated skin surface temperature changes after resistance training with different exercise intensities. Int. J. Kinesiol. Sports Sci. 2018, 6, 12–18. [Google Scholar] [CrossRef]
- Garcia-Bernal, M.I.; Heredia-Rizo, A.M.; Gonzalez-Garcia, P.; Cortes-Vega, M.D.; Casuso-Holgado, M.J. Validity and reliability of myotonometry for assessing muscle viscoelastic properties in patients with stroke: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 5062. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Lee, H. The Measurement of Stiffness for Major Muscles with Shear Wave Elastography and Myoton: A Quantitative Analysis Study. Diagnostics 2021, 11, 524. [Google Scholar] [CrossRef]
- Shacklock, M. Clinical Neurodynamics: A New System of Neuromusculoskeletal Treatment; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Cochrane, D.J. Vibration exercise: The potential benefits. Int. J. Sports Med. 2011, 32, 75–99. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Behm, D.G.; Alizadeh, S.; Daneshjoo, A.; Anvar, S.H.; Graham, A.; Zahiri, A.; Goudini, R.; Edwards, C.; Culleton, R.; Scharf, C.; et al. Acute Effects of Various Stretching Techniques on Range of Motion: A Systematic Review with Meta-Analysis. Sports Med. Open 2023, 9, 107. [Google Scholar] [CrossRef]
- Cai, P.; Liu, L.; Li, H. Dynamic and static stretching on hamstring flexibility and stiffness: A systematic review and meta-analysis. Heliyon 2023, 9, e18795. [Google Scholar] [CrossRef] [PubMed]
- Atha, J.; Wheatley, D.W. Joint mobility changes due to low frequency vibration and stretching exercise. Br. J. Sports Med. 1976, 10, 26–34. [Google Scholar] [CrossRef]
- Colson, S.S.; Roffino, S.; Mutin-Carnino, M.; Carnino, A.; Petit, P.D. The effect of dynamic whole-body vibration warm-up on lower extremity performance. Sci. Sport 2016, 31, 19–26. [Google Scholar] [CrossRef]
- Despina, T.; George, D.; George, T.; Sotiris, P.; Alessandra, D.C.; George, K.; Maria, R.; Stavros, K. Short-term effect of whole-body vibration training on balance, flexibility and lower limb explosive strength in elite rhythmic gymnasts. Hum. Mov. Sci. 2014, 33, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Gerodimos, V.; Zafeiridis, A.; Karatrantou, K.; Vasilopoulou, T.; Chanou, K.; Pispirikou, E. The acute effects of different whole-body vibration amplitudes and frequencies on flexibility and vertical jumping performance. J. Sci. Med. Sport 2010, 13, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Komiya, M.; Urabe, Y.; Sasadai, J.; Fujishita, H.; Sakai, S.; Maeda, N. The effects of two different whole-body-vibration frequencies on ankle dorsiflexion angle, vertical jump height, and postural stability after landing. Isokinet. Exerc. Sci. 2019, 27, 267–274. [Google Scholar] [CrossRef]
- Kurt, C.; Pekunlu, E. Acute effect of whole body vibration on isometric strength, squat jump, and flexibility in well-trained combat athletes. Biol. Sport 2015, 32, 115–122. [Google Scholar] [CrossRef]
- Pereira, B.M.; Magalhaes, F.A.; Lacerda, A.C.R.; Andrade, A.G.P.; Peixoto, G.H.D.; Chagas, M.H. Comparison of four local vibratory stimuli on mechanical and sensorial variables related to muscle-tendon unit response. Transl. Sports Med. 2020, 3, 440–446. [Google Scholar] [CrossRef]
- Jemni, M.; Mkaouer, B.; Marina, M.; Asllani, A.; Sands, W.A. Acute static vibration-induced stretching enhanced muscle viscoelasticity but did not affect maximal voluntary contractions in footballers. J. Strength. Cond. Res. 2014, 28, 3105–3114. [Google Scholar] [CrossRef] [PubMed]
- Kinser, A.M.; Ramsey, M.W.; O’Bryant, H.S.; Ayres, C.A.; Sands, W.A.; Stone, M.H. Vibration and stretching effects on flexibility and explosive strength in young gymnasts. Med. Sci. Sports Exerc. 2008, 40, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.G.; Lee, M.M.; Song, C.H. A comparison of the effects of different stretching methods on flexibility, muscle activity, and pain threshold in ballet dancers; a preliminary randomized controlled trial. J. Bodyw. Mov. Ther. 2020, 24, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Sands, W.A.; McNeal, J.R.; Stone, M.H.; Russell, E.M.; Jemni, M. Flexibility enhancement with vibration: Acute and long-term. Med. Sci. Sports Exerc. 2006, 38, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Kemertzis, M.A.; Lythgo, N.D.; Morgan, D.L.; Galea, M.P. Ankle flexors produce peak torque at longer muscle lengths after whole-body vibration. Med. Sci. Sports Exerc. 2008, 40, 1977–1983. [Google Scholar] [CrossRef]
- Tsuji, T.; Kitano, N.; Tsunoda, K.; Himori, E.; Okura, T.; Tanaka, K. Short-term effects of whole-body vibration on functional mobility and flexibility in healthy, older adults: A randomized crossover study. J. Geriatr. Phys. Ther. 2014, 37, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Liu, C.; Shiang, T.Y. Warm-up effects from concomitant use of vibration and static stretching after cycling. J. Sports Med. Phys. Fitness 2017, 57, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Yamamoto, A.; Matsuo, S.; Hatano, G.; Miyazaki, M.; Fukaya, T.; Fujiwara, M.; Asai, Y.; Suzuki, S. Dynamic stretching has sustained effects on range of motion and passive stiffness of the hamstring muscles. J. Sports Sci. Med. 2019, 18, 13. [Google Scholar] [PubMed]
- Reiner, M.M.; Tilp, M.; Nakamura, M.; Konrad, A. Is muscle stiffness a determinant for range of motion in the leg muscles? Biol. Sport 2024, 41, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Souron, R.; Zambelli, A.; Espeit, L.; Besson, T.; Cochrane, D.J.; Lapole, T. Active versus local vibration warm-up effects on knee extensors stiffness and neuromuscular performance of healthy young males. J. Sci. Med. Sport 2019, 22, 206–211. [Google Scholar] [CrossRef]
- Alter, M.J. Science of Flexibility; Human Kinetics: Champaign, IL, USA, 2004. [Google Scholar]
- Pantaleo, T.; Duranti, R.; Bellini, F. Effects of vibratory stimulation on muscular pain threshold and blink response in human subjects. Pain 1986, 24, 239–250. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, U.F.; de Araújo, L.C.; de Andrade, P.R.; Dos Santos, H.H.; Moreira, D.G.; Sillero-Quintana, M.; de Almeida Ferreira, J.J. Skin temperature changes during muscular static stretching exercise. J. Exerc. Rehabil. 2018, 14, 451–459. [Google Scholar] [CrossRef]
- Schleip, R. Fascial plasticity–A new neurobiological explanation: Part 1. J. Bodyw. Mov. Ther. 2003, 7, 11–19. [Google Scholar] [CrossRef]
- Kruger, L. Cutaneous Sensory System. In Sensory Systems: II: Senses Other than Visio; Wolfe, J.M., Ed.; Birkhäuser Boston: Boston, MA, USA, 1988; pp. 14–15. [Google Scholar]
- Jenner, J.; Stephens, J. Cutaneous reflex responses and their central nervous pathways studied in man. J. Physiol. 1982, 333, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Kearney, R.E.; Chan, C.W. Reflex response of human arm muscles to cutaneous stimulation of the foot. Brain Res. 1979, 170, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Alizadeh, S.; Anvar, S.H.; Drury, B.; Granacher, U.; Moran, J. Non-local Acute Passive Stretching Effects on Range of Motion in Healthy Adults: A Systematic Review with Meta-analysis. Sports Med. 2021, 51, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Akehurst, H.; Grice, J.E.; Angioi, M.; Morrissey, D.; Migliorini, F.; Maffulli, N. Whole-body vibration decreases delayed onset muscle soreness following eccentric exercise in elite hockey players: A randomised controlled trial. J. Orthop. Surg. Res. 2021, 16, 589. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Pascoe, D.; Mercer, J.; Weerd, L. Physiology of Thermal Signals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jochum, D.; Vogel, V.; Warneke, K. Acute Effects of Passive Stretching with and Without Vibration on Hip Range of Motion, Temperature, and Stiffness Parameters in Male Elite Athletes. J. Funct. Morphol. Kinesiol. 2025, 10, 17. https://doi.org/10.3390/jfmk10010017
Jochum D, Vogel V, Warneke K. Acute Effects of Passive Stretching with and Without Vibration on Hip Range of Motion, Temperature, and Stiffness Parameters in Male Elite Athletes. Journal of Functional Morphology and Kinesiology. 2025; 10(1):17. https://doi.org/10.3390/jfmk10010017
Chicago/Turabian StyleJochum, Daniel, Viola Vogel, and Konstantin Warneke. 2025. "Acute Effects of Passive Stretching with and Without Vibration on Hip Range of Motion, Temperature, and Stiffness Parameters in Male Elite Athletes" Journal of Functional Morphology and Kinesiology 10, no. 1: 17. https://doi.org/10.3390/jfmk10010017
APA StyleJochum, D., Vogel, V., & Warneke, K. (2025). Acute Effects of Passive Stretching with and Without Vibration on Hip Range of Motion, Temperature, and Stiffness Parameters in Male Elite Athletes. Journal of Functional Morphology and Kinesiology, 10(1), 17. https://doi.org/10.3390/jfmk10010017





