Neurosciences and Sports Rehabilitation in ACLR: A Narrative Review on Winning Alliance Strategies and Connecting the Dots
Abstract
:1. Introduction
Objective and Rationale
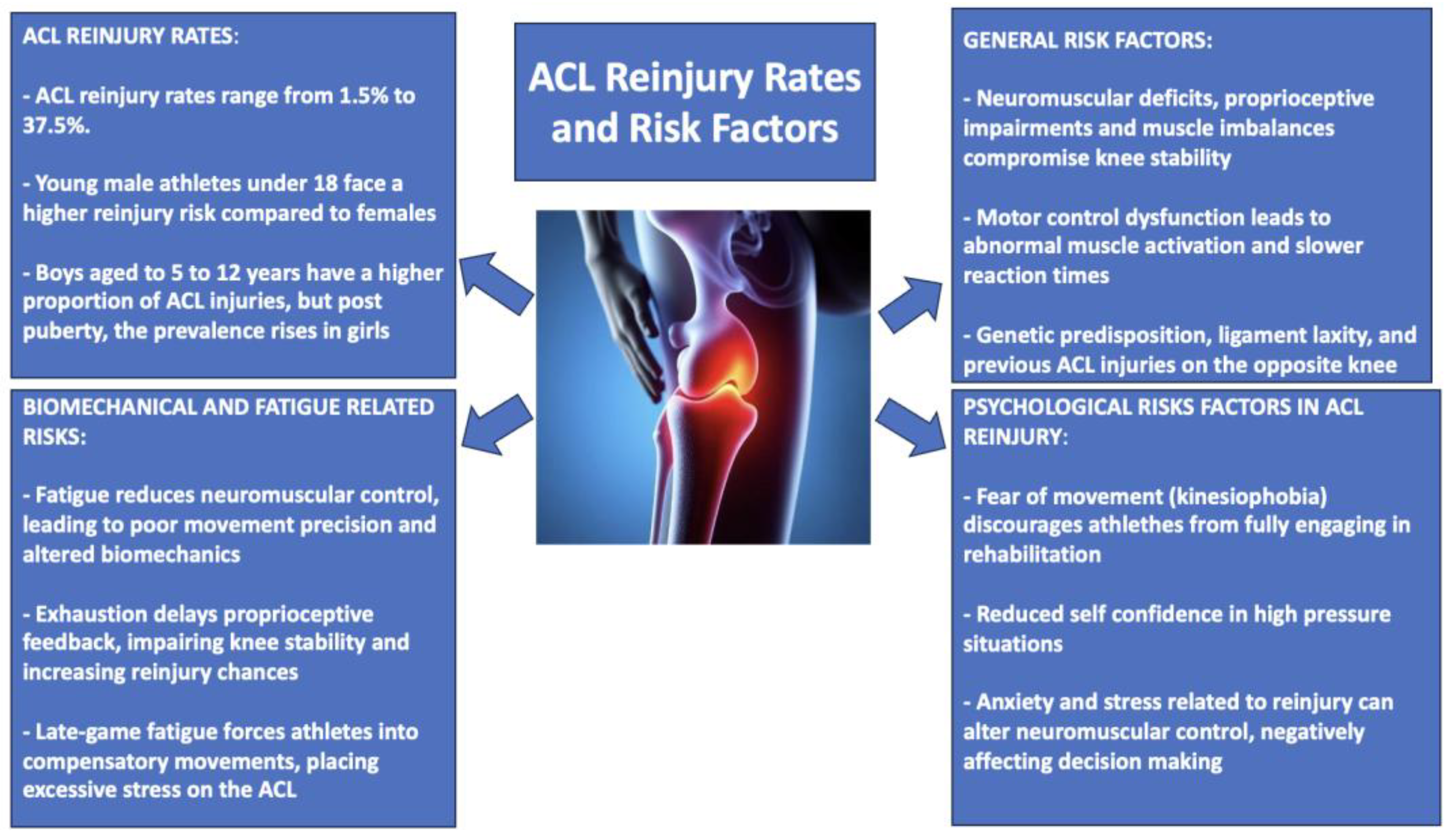
2. The Unwelcome Guest: ACL Reinjury Rates and Risk Factors
Risk Factors in Post-ACLR Rehabilitation: The Role of Neuromuscular Deficits, Fatigue, and Proprioceptive Impairments
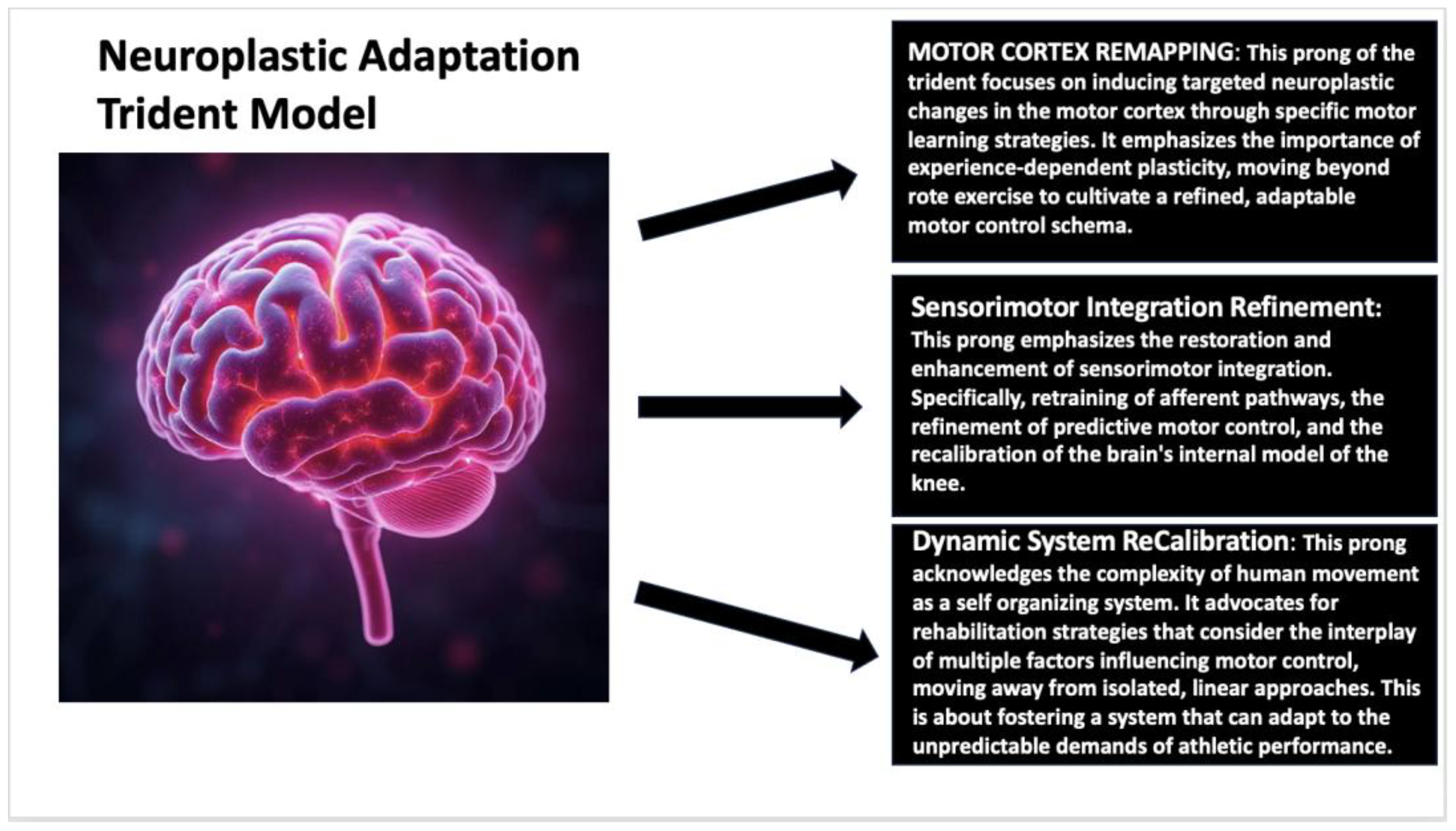
3. Reframing ACL Rehabilitation Through Neuroplasticity
3.1. Next-Generation Rehabilitation: The Convergence of Robotics, VR, and Biofeedback in ACL Recovery
3.2. Long-Term Impact vs. Practical Limitations: Evaluating Advanced Rehabilitation Technologies in ACL Recovery
4. Cerebral Compensation: How Neuroplasticity Shapes ACLR Recovery Beyond the Knee
Brain Activity and Kinesiophobia: Understanding the Neurological Impact of ACLR
5. Overcoming Persistent Deficits: Addressing Sensory, Neuromuscular, and Muscular Challenges in ACL Rehabilitation
The Next Frontier in ACL Rehabilitation: Integrating Innovation and Expertise
6. Discussion: Expanding Neurorehabilitation Insights into Sports Medicine
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gibson, B. Rehabilitation: A Post-Critical Approach; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Peterson, L.; Renstrom, P.A.F.H.; Lynch, S. Sports Injuries: Prevention, Treatment and Rehabilitation; Routledge: London, UK, 2024. [Google Scholar]
- Herrington, L.; Spencer, S. Principles of Exercise Rehabilitation. In Petty’s Principles of Musculoskeletal Treatment and Management; Petty, N.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; p. 233. [Google Scholar]
- Wilk, K.E.; Ivey, M.; Thomas, Z.M.; Lupowitz, L. Neurocognitive and Neuromuscular Rehabilitation Techniques after ACL Injury, Part 1: Optimizing Recovery in the Acute Post-Operative Phase- A Clinical Commentary. Int. J. Sports Phys. Ther. 2024, 19, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Rollo, I.; Carter, J.M.; Close, G.L.; Yangüas, J.; Gomez-Diaz, A.; Medina Leal, D.; Duda, J.L.; Holohan, D.; Erith, S.J.; Podlog, L. Role of sports psychology and sports nutrition in return to play from musculoskeletal injuries in professional soccer: An interdisciplinary approach. Eur. J. Sport Sci. 2021, 21, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, H.; Dhillon, S.; Dhillon, M.S. Current Concepts in Sports Injury Rehabilitation. Indian J. Orthop. 2017, 51, 529–536, Erratum in Indian J. Orthop. 2017, 51, 724. https://doi.org/10.4103/0019-5413.217722. [Google Scholar] [CrossRef]
- Mujika, I.; Halson, S.; Burke, L.; Farrow, D. An integrated, multifactorial approach to periodization for optimal performance in individual and team sports. Int. J. Sports Physiol. Perform. 2018, 13, 538–561. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, Z.; Tang, N.; Wang, K.; Bian, C.; Li, D.; Kuraki, V.; Schmid, F. Research on Sports Injury Rehabilitation Detection Based on IoT Models for Digital Health Care. Big Data 2024. [Google Scholar] [CrossRef]
- Kirkby Shaw, K.; Alvarez, L.; Foster, S.A.; Tomlinson, J.E.; Shaw, A.J.; Pozzi, A. Fundamental principles of rehabilitation and musculoskeletal tissue healing. Vet. Surg. 2020, 49, 22–32. [Google Scholar] [CrossRef]
- Cienfuegos, M.; Maycock, J.; Naceri, A.; Düsterhus, T.; Kõiva, R.; Schack, T.; Ritter, H. Exploring motor skill acquisition in bimanual coordination: Insights from navigating a novel maze task. Sci. Rep. 2024, 14, 18887. [Google Scholar] [CrossRef]
- Nordin, A.D.; Rymer, W.Z.; Biewener, A.A.; Schwartz, A.B.; Chen, D.; Horak, F.B. Biomechanics and neural control of movement, 20 years later: What have we learned and what has changed? J. Neuroeng. Rehabil. 2017, 14, 91. [Google Scholar] [CrossRef]
- Florio, T.M. Emergent Aspects of the Integration of Sensory and Motor Functions. Brain Sci. 2025, 15, 162. [Google Scholar] [CrossRef]
- Singh, R.E.; Iqbal, K.; White, G.; Hutchinson, T.E. A Systematic Review on Muscle Synergies: From Building Blocks of Motor Behavior to a Neurorehabilitation Tool. Appl. Bionics Biomech. 2018, 2018, 3615368. [Google Scholar] [CrossRef]
- Peng, J.; Zikereya, T.; Shao, Z.; Shi, K. The neuromechanical of Beta-band corticomuscular coupling within the human motor system. Front. Neurosci. 2024, 18, 1441002. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.G.; Wittenberg, G.F. Motor Recovery: How Rehabilitation Techniques and Technologies Can Enhance Recovery and Neuroplasticity. Semin. Neurol. 2021, 41, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Micera, S.; Caleo, M.; Chisari, C.; Hummel, F.C.; Pedrocchi, A. Advanced Neurotechnologies for the Restoration of Motor Function. Neuron 2020, 105, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, G.M. Defining neuroplasticity. Handb. Clin. Neurol. 2022, 184, 3–18. [Google Scholar] [CrossRef]
- Johnson, B.P.; Cohen, L.G. Applied strategies of neuroplasticity. Handb. Clin. Neurol. 2023, 196, 599–609. [Google Scholar] [CrossRef]
- Zotey, V.; Andhale, A.; Shegekar, T.; Juganavar, A. Adaptive Neuroplasticity in Brain Injury Recovery: Strategies and Insights. Cureus 2023, 15, e45873. [Google Scholar] [CrossRef]
- Nahum, M.; Lee, H.; Merzenich, M.M. Principles of neuroplasticity-based rehabilitation. Prog. Brain Res. 2013, 207, 141–171. [Google Scholar] [CrossRef]
- Toricelli, M.; Pereira, A.A.R.; Souza Abrao, G.; Malerba, H.N.; Maia, J.; Buck, H.S.; Viel, T.A. Mechanisms of neuroplasticity and brain degeneration: Strategies for protection during the aging process. Neural Regen. Res. 2021, 16, 58–67. [Google Scholar] [CrossRef]
- Tallent, J.; Woodhead, A.; Frazer, A.K.; Hill, J.; Kidgell, D.J.; Howatson, G. Corticospinal and spinal adaptations to motor skill and resistance training: Potential mechanisms and implications for motor rehabilitation and athletic development. Eur. J. Appl. Physiol. 2021, 121, 707–719. [Google Scholar] [CrossRef]
- Levin, M.F.; Demers, M. Motor learning in neurological rehabilitation. Disabil. Rehabil. 2021, 43, 3445–3453. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Galea, M.P.; Gonzenbach, R.; Kesselring, J. Neurorehabilitation: Applied neuroplasticity. J. Neurol. 2017, 264, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Pryde, S.J.; Williams, O.; O’Hare, M.P.; Murdock, C.; Pedlow, K. Exploring access to community neurorehabilitation for people with progressive neurological conditions: A qualitative study. Disabil. Rehabil. 2025, 47, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Paul, S.; Mourya, G.K.; Kumar, N.; Hussain, M. Recent Trends and Practices Toward Assessment and Rehabilitation of Neurodegenerative Disorders: Insights From Human Gait. Front. Neurosci. 2022, 16, 859298. [Google Scholar] [CrossRef] [PubMed]
- Gil-Castillo, J.; Barria, P.; Aguilar Cárdenas, R.; Baleta Abarza, K.; Andrade Gallardo, A.; Biskupovic Mancilla, A.; Azorín, J.M.; Moreno, J.C. A Robot-Assisted Therapy to Increase Muscle Strength in Hemiplegic Gait Rehabilitation. Front. Neurorobot. 2022, 16, 837494. [Google Scholar] [CrossRef]
- Nistor-Cseppento, C.D.; Gherle, A.; Negrut, N.; Bungau, S.G.; Sabau, A.M.; Radu, A.F.; Bungau, A.F.; Tit, D.M.; Uivaraseanu, B.; Ghitea, T.C.; et al. The Outcomes of Robotic Rehabilitation Assisted Devices Following Spinal Cord Injury and the Prevention of Secondary Associated Complications. Medicina 2022, 58, 1447. [Google Scholar] [CrossRef]
- Gil-Agudo, Á.; Megía-García, Á.; Pons, J.L.; Sinovas-Alonso, I.; Comino-Suárez, N.; Lozano-Berrio, V.; Del-Ama, A.J. Exoskeleton-based training improves walking independence in incomplete spinal cord injury patients: Results from a randomized controlled trial. J. Neuroeng. Rehabil. 2023, 20, 36, Erratum in J. Neuroeng. Rehabil. 2023, 20, 160. https://doi.org/10.1186/s12984-023-01281-x. [Google Scholar] [CrossRef]
- Demeco, A.; Zola, L.; Frizziero, A.; Martini, C.; Palumbo, A.; Foresti, R.; Buccino, G.; Costantino, C. Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review. Sensors 2023, 23, 1712. [Google Scholar] [CrossRef]
- Chenais, N.; Görgen, A. Immersive interfaces for clinical applications: Current status and future perspective. Front. Neurorobot. 2024, 18, 1362444. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, X.; Jin, Y.; Wu, B.; Vigotsky, A.D.; Fan, L.; Gu, P.; Tu, W.; Huang, L.; Jiang, S. Immersive virtual reality-based rehabilitation for subacute stroke: A randomized controlled trial. J. Neurol. 2024, 271, 1256–1266. [Google Scholar] [CrossRef]
- Goel, T.; Sharma, N.; Gehlot, A.; Srivastav, A.K. Effectiveness of immersive virtual reality training to improve sitting balance control among individuals with acute and sub-acute paraplegia: A randomized clinical trial. J. Spinal Cord. Med. 2023, 46, 964–974. [Google Scholar] [CrossRef]
- Bowman, T.; Gervasoni, E.; Arienti, C.; Lazzarini, S.G.; Negrini, S.; Crea, S.; Cattaneo, D.; Carrozza, M.C. Wearable Devices for Biofeedback Rehabilitation: A Systematic Review and Meta-Analysis to Design Application Rules and Estimate the Effectiveness on Balance and Gait Outcomes in Neurological Diseases. Sensors 2021, 21, 3444. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.K.A.; da Costa, K.S.A.; de Lucena, G.L.; de Oliveira Sousa, C.; Filho, J.F.M.; Brasileiro, J.S. Comparing exercises with and without electromyographic biofeedback in subacromial pain syndrome: A randomized controlled trial. Clin. Biomech. 2022, 93, 105596. [Google Scholar] [CrossRef]
- Liu, M.; Xu, L.; Li, H.; Chen, S.; Chen, B. Morphological and Functional Changes of the Tibialis Anterior Muscle After Combined Mirror Visual Feedback and Electromyographic Biofeedback in Poststroke Patients: A Randomized Trial. Am. J. Phys. Med. Rehabil. 2021, 100, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, W.; Li, W.; Zhang, S.; Lv, P.; Yin, Y. Effects of motor imagery based brain-computer interface on upper limb function and attention in stroke patients with hemiplegia: A randomized controlled trial. BMC Neurol. 2023, 23, 136. [Google Scholar] [CrossRef]
- Colamarino, E.; Lorusso, M.; Pichiorri, F.; Toppi, J.; Tamburella, F.; Serratore, G.; Riccio, A.; Tomaiuolo, F.; Bigioni, A.; Giove, F.; et al. DiSCIoser: Unlocking recovery potential of arm sensorimotor functions after spinal cord injury by promoting activity-dependent brain plasticity by means of brain-computer interface technology: A randomized controlled trial to test efficacy. BMC Neurol. 2023, 23, 414. [Google Scholar] [CrossRef]
- Nicolelis, M.A.L.; Alho, E.J.L.; Donati, A.R.C.; Yonamine, S.; Aratanha, M.A.; Bao, G.; Campos, D.S.F.; Almeida, S.; Fischer, D.; Shokur, S. Training with noninvasive brain-machine interface, tactile feedback, and locomotion to enhance neurological recovery in individuals with complete paraplegia: A randomized pilot study. Sci. Rep. 2022, 12, 20545. [Google Scholar] [CrossRef]
- Ardaillon, H.; Ribault, S.; Herault, C.; Pisella, L.; Lechopier, N.; Reilly, K.T.; Rode, G. Striking the Balance: Embracing Technology While Upholding Humanistic Principles in Neurorehabilitation. Neurorehabil. Neural Repair 2024, 38, 705–710. [Google Scholar] [CrossRef]
- Nwachukwu, B.U.; Adjei, J.; Rauck, R.C.; Chahla, J.; Okoroha, K.R.; Verma, N.N.; Allen, A.A.; Williams, R.J., 3rd. How Much Do Psychological Factors Affect Lack of Return to Play After Anterior Cruciate Ligament Reconstruction? A Systematic Review. Orthop. J. Sports Med. 2019, 7, 2325967119845313. [Google Scholar] [CrossRef]
- Burland, J.P.; Toonstra, J.; Werner, J.L.; Mattacola, C.G.; Howell, D.M.; Howard, J.S. Decision to Return to Sport After Anterior Cruciate Ligament Reconstruction, Part I: A Qualitative Investigation of Psychosocial Factors. J. Athl. Train. 2018, 53, 452–463. [Google Scholar] [CrossRef]
- Ross, C.A.; Clifford, A.; Louw, Q.A. Factors informing fear of reinjury after anterior cruciate ligament reconstruction. Physiother. Theory Pract. 2017, 33, 103–114. [Google Scholar] [CrossRef]
- McVeigh, F.; Pack, S.M. An exploration of sports rehabilitators’ and athletic rehabilitation therapists’ views on fear of reinjury after anterior cruciate ligament reconstruction. J. Sport Rehabil. 2015, 24, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Piedade, S.R.; Leite Arruda, B.P.; de Vasconcelos, R.A.; Parker, D.A.; Maffulli, N. Rehabilitation following surgical reconstruction for anterior cruciate ligament insufficiency: What has changed since the 1960s?—State of the art. J. ISAKOS 2023, 8, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Mashud, G.; Hasan, S.; Alam, N. Advances in control techniques for rehabilitation exoskeleton robots: A systematic review. Actuators 2025, 14, 108. [Google Scholar] [CrossRef]
- Tripp, D.; Stanish, W.; Ebel-Lam, A.; Brewer, B.; Birchard, J. Fear of reinjury, negative affect, and catastrophizing predicting return to sport in recreational athletes with anterior cruciate ligament injuries at 1 year postsurgery. Sport Exerc. Perform. Psychol. 2011, 1, 74–81. [Google Scholar] [CrossRef]
- Acuña Luna, K.P.; Hernandez-Rios, E.R.; Valencia, V.; Trenado, C.; Peñaloza, C. Deep learning-enhanced motor training: A hybrid VR and exoskeleton system for cognitive–motor rehabilitation. Bioengineering 2025, 12, 331. [Google Scholar] [CrossRef]
- Rose, T.; Nam, C.; Chen, K. Immersion of virtual reality for rehabilitation: A review. Appl. Ergon. 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Queen, R.M.; Peebles, A.T.; Miller, T.K.; Savla, J.; Ollendick, T.; Messier, S.P.; Williams, D.B., 3rd. Reduction of Risk Factors for ACL Re-injuries using an Innovative Biofeedback Approach: Rationale and Design. Contemp. Clin. Trials Commun. 2021, 22, 100769. [Google Scholar] [CrossRef]
- Muratori, L.M.; Lamberg, E.M.; Quinn, L.; Duff, S.V. Applying principles of motor learning and control to upper extremity rehabilitation. J. Hand Ther. 2013, 26, 94–103. [Google Scholar] [CrossRef]
- Czyż, S.H.; Wójcik, A.M.; Solarská, P.; Kiper, P. High contextual interference improves retention in motor learning: Systematic review and meta-analysis. Sci. Rep. 2024, 14, 15974. [Google Scholar] [CrossRef]
- Shultz, S.J.; Schmitz, R.J.; Cameron, K.L.; Ford, K.R.; Grooms, D.R.; Lepley, L.K.; Myer, G.D.; Pietrosimone, B. Anterior Cruciate Ligament Research Retreat VIII Summary Statement: An Update on Injury Risk Identification and Prevention Across the Anterior Cruciate Ligament Injury Continuum, March 14–16, 2019, Greensboro, NC. J. Athl. Train. 2019, 54, 970–984. [Google Scholar] [CrossRef]
- Machan, T.; Krupps, K. The Neuroplastic Adaptation Trident Model: A Suggested Novel Framework for ACL Rehabilitation. Int. J. Sports Phys. Ther. 2021, 16, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Fones, L.; Kostyun, R.O.; Cohen, A.D.; Pace, J.L. Patient-Reported Outcomes, Return-to-Sport Status, and Reinjury Rates After Anterior Cruciate Ligament Reconstruction in Adolescent Athletes: Minimum 2-Year Follow-up. Orthop. J. Sports Med. 2020, 8, 2325967120964471. [Google Scholar] [CrossRef] [PubMed]
- Krogsgaard, M.R.; Fischer-Rasmussen, T.; Dyhre-Poulsen, P. Absence of sensory function in the reconstructed anterior cruciate ligament. J. Electromyogr. Kinesiol. 2011, 21, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, A.J.; Grandhi, R.K.; Schneider, D.K.; Stanfield, D.; Webster, K.E.; Myer, G.D. Risk of Secondary Injury in Younger Athletes After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2016, 44, 1861–1876. [Google Scholar] [CrossRef]
- Stenroth, L.; Bartholdy, C.; Schwarz Larsen, J.; Sørensen, M.S.; Smale, K.B.; Flaxman, T.E.; Benoit, D.L.; Krogsgaard, M.R.; Alkjær, T. Altered movement strategy during functional movement after an ACL injury, despite ACL reconstruction. Front. Sports Act. Living 2022, 4, 994139. [Google Scholar] [CrossRef]
- Myer, G.D.; Ford, K.R.; Divine, J.G.; Wall, E.J.; Kahanov, L.; Hewett, T.E. Longitudinal assessment of noncontact anterior cruciate ligament injury risk factors during maturation in a female athlete: A case report. J. Athl. Train. 2009, 44, 101–109. [Google Scholar] [CrossRef]
- Shultz, S.J.; Cruz, M.R.; Casey, E.; Dompier, T.P.; Ford, K.R.; Pietrosimone, B.; Schmitz, R.J.; Taylor, J.B. Sex-Specific Changes in Physical Risk Factors for Anterior Cruciate Ligament Injury by Chronological Age and Stages of Growth and Maturation From 8 to 18 Years of Age. J. Athl. Train. 2022, 57, 830–876, Erratum in J. Athl. Train. 2023, 58, 588. https://doi.org/10.4085/1062-6050-1003.23. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Kiapour, A.M. Age-related changes in ACL morphology during skeletal growth and maturation are different between females and males. J. Orthop. Res. 2021, 39, 841–849. [Google Scholar] [CrossRef]
- Stracciolini, A.; Stein, C.J.; Zurakowski, D.; Meehan WP 3rd Myer, G.D.; Micheli, L.J. Anterior cruciate ligament injuries in pediatric athletes presenting to sports medicine clinic: A comparison of males and females through growth and development. Sports Health 2015, 7, 130–136. [Google Scholar] [CrossRef]
- Buckthorpe, M.; Danelon, F.; La Rosa, G.; Nanni, G.; Stride, M.; Della Villa, F. Recommendations for Hamstring Function Recovery After ACL Reconstruction. Sports Med. 2021, 51, 607–624. [Google Scholar] [CrossRef]
- Buckthorpe, M.; La Rosa, G.; Villa, F.D. Restoring Knee Extensor Strenght After Anterior Cruciate Ligament Reconstruction: A Clinical. Int. J. Sports Phys. Ther. 2019, 14, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Kvist, J.; Ek, A.; Sporrstedt, K.; Good, L. Fear of re-injury: A hindrance for returning to sports after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Brand, E.; Nyland, J. Patient outcomes following anterior cruciate ligament reconstruction: The influence of psychological factors. Orthopedics 2009, 32, 335. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; McEwen, P.; Letson, H.L.; Dobson, G.P. Anterior Cruciate Ligament Reconstruction Surgery: Creating a Permissive Healing Phenotype in Military Personnel and Civilians for Faster Recovery. Mil. Med. 2022, 187, 1310–1317. [Google Scholar] [CrossRef]
- Baker, L.A. Genetic Dissection of Anterior Cruciate Ligament Rupture in the Dog Model; The University of Wisconsin-Madison: Madison, WI, USA, 2019. [Google Scholar]
- Ardern, C.L.; Taylor, N.F.; Feller, J.A.; Webster, K.E. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: An updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br. J. Sports Med. 2014, 48, 1543–1552. [Google Scholar] [CrossRef]
- Cardoso, F.; Afonso, J.; Roca, A.; Teoldo da Costa, I. The association between perceptual-cognitive processes and response time in decision making in young soccer players. J. Sports Sci. 2021, 39, 926–935. [Google Scholar] [CrossRef]
- Zago, M.; David, S.; Bertozzi, F.; Brunetti, C.; Gatti, A.; Salaorni, F.; Tarabini, M.; Galvani, C.; Sforza, C.; Galli, M. Fatigue Induced by Repeated Changes of Direction in Élite Female Football (Soccer) Players: Impact on Lower Limb Biomechanics and Implications for ACL Injury Prevention. Front. Bioeng. Biotechnol. 2021, 9, 666841. [Google Scholar] [CrossRef]
- Stańczak, M.; Swinnen, B.; Kacprzak, B.; Pacek, A.; Surmacz, J. Neurophysiology of ACL Injury. Orthop. Rev. 2025, 17, 129173. [Google Scholar] [CrossRef]
- Thomas, Z.M.; Lupowitz, L.; Ivey, M.; Wilk, K.E. Neurocognitive and Neuromuscular Rehabilitation Techniques after ACL injury—Part 2: Maximizing Performance in the Advanced Return to Sport Phase. Int. J. Sports Phys. Ther. 2024, 19, 1629–1641. [Google Scholar] [CrossRef]
- Raizah, A.; Reddy, R.S.; Alshahrani, M.S.; Tedla, J.S.; Dixit, S.; Gular, K.; Gautam, A.P.; Ahmad, I.; Kandakurti, P.K. Investigating Knee Joint Proprioception and Its Impact on Limits of Stability Using Dynamic Posturography in Individuals with Bilateral Knee Osteoarthritis-A Cross-Sectional Study of Comparisons and Correlations. J. Clin. Med. 2023, 12, 2764. [Google Scholar] [CrossRef]
- Nyland, J.; Pyle, B.; Richards, J.; Yoshida, K.; Brey, J.; Carter, S. A clinical practice review of therapeutic movement-based anterior cruciate ligament reconstruction return to sports bridge program: The biological, biomechanical and behavioral rationale. Ann. Jt. 2023, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Gkikopoulos, G.; Chronopoulou, C.; Christakou, A. Examining re-injury worry, confidence and attention after a sport musculoskeletal injury. J. Sports Med. Phys. Fit. 2020, 60, 428–434. [Google Scholar] [CrossRef]
- Bertozzi, F.; Fischer, P.D.; Hutchison, K.A.; Zago, M.; Sforza, C.; Monfort, S.M. Associations Between Cognitive Function and ACL Injury-Related Biomechanics: A Systematic Review. Sports Health 2023, 15, 855–866. [Google Scholar] [CrossRef]
- Ardern, C.L.; Webster, K.E.; Taylor, N.F.; Feller, J.A. Return to sport following anterior cruciate ligament reconstruction surgery: A systematic review and meta-analysis of the state of play. Br. J. Sports Med. 2011, 45, 596–606. [Google Scholar] [CrossRef]
- An, Y.W.; An, Y.W. Neurophysiological mechanisms underlying functional knee instability following an anterior cruciate ligament injury. Exerc. Sci. 2018, 27, 109–117. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Lephart, S.M.; Pincivero, D.M.; Giraldo, J.L.; Fu, F.H. The role of proprioception in the management and rehabilitation of athletic injuries. Am. J. Sports Med. 1997, 25, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, A.; Gostian-Ropotin, L.A.; Beltrán-Velasco, A.I.; Belando-Pedreño, N.; Simón, J.A.; López-Mora, C.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F.; Clemente-Suárez, V.J. Sporting Mind: The Interplay of Physical Activity and Psychological Health. Sports 2024, 12, 37. [Google Scholar] [CrossRef]
- Gokeler, A.; Neuhaus, D.; Benjaminse, A.; Grooms, D.R.; Baumeister, J. Principles of Motor Learning to Support Neuroplasticity After ACL Injury: Implications for Optimizing Performance and Reducing Risk of Second ACL Injury. Sports Med. 2019, 49, 853–865, Erratum in Sports Med. 2019, 49, 979. https://doi.org/10.1007/s40279-019-01078-w. [Google Scholar] [CrossRef]
- Kacprzak, B.; Stańczak, M.; Surmacz, J. Anterior Cruciate Ligament (ACL) Injury Hidden in the Complex Sensorimotor System. J. Orthop. Sci. 2024, 5, 1–23. [Google Scholar]
- Palmieri-Smith, R.M.; Lepley, L.K. Quadriceps Strength Asymmetry After Anterior Cruciate Ligament Reconstruction Alters Knee Joint Biomechanics and Functional Performance at Time of Return to Activity. Am. J. Sports Med. 2015, 43, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Wojtys, E.M.; Beaulieu, M.L.; Ashton-Miller, J.A. New perspectives on ACL injury: On the role of repetitive sub-maximal knee loading in causing ACL fatigue failure. J. Orthop. Res. 2016, 34, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Gomes-Sagaz, F.; Zorrilla-Muñoz, V.; Garcia-Aracil, N. Rehabilitation Technologies by Integrating Exoskeletons, Aquatic Therapy, and Quantum Computing for Enhanced Patient Outcomes. Sensors 2024, 24, 7765. [Google Scholar] [CrossRef]
- Rambaud, A.J.; Neri, T.; Dingenen, B.; Parker, D.; Servien, E.; Gokeler, A.; Edouard, P. The modifying factors that help improve anterior cruciate ligament reconstruction rehabilitation: A narrative review. Ann. Phys. Rehabil. Med. 2022, 65, 101601. [Google Scholar] [CrossRef]
- Wilmart, R.; Garone, E.; Innocenti, B. The Use of Robotics Devices in Knee Rehabilitation: A Critical Review. Muscles Ligaments Tendons J. 2019, 9, 21. [Google Scholar] [CrossRef]
- Ju, F.; Wang, Y.; Xie, B.; Mi, Y.; Zhao, M.; Cao, J. The Use of Sports Rehabilitation Robotics to Assist in the Recovery of Physical Abilities in Elderly Patients with Degenerative Diseases: A Literature Review. Healthcare 2023, 11, 326. [Google Scholar] [CrossRef]
- Vaida, C.; Rus, G.; Tucan, P.; Machado, J.; Pisla, A.; Zima, I.; Birlescu, I.; Pisla, D. Enhancing Robotic-Assisted Lower Limb Rehabilitation Using Augmented Reality and Serious Gaming. Appl. Sci. 2024, 14, 12029. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Q.; Yu, L.; Ye, M. The immediate intervention effects of robotic training in patients after anterior cruciate ligament reconstruction. J. Phys. Ther. Sci. 2016, 28, 2031–2033. [Google Scholar] [CrossRef]
- Gulick, V.; Graves, D.; Ames, S.; Krishnamani, P.P. Effect of a Virtual Reality-Enhanced Exercise and Education Intervention on Patient Engagement and Learning in Cardiac Rehabilitation: Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e23882. [Google Scholar] [CrossRef]
- Wang, Z.R.; Wang, P.; Xing, L.; Mei, L.P.; Zhao, J.; Zhang, T. Leap Motion-based virtual reality training for improving motor functional recovery of upper limbs and neural reorganization in subacute stroke patients. Neural Regen. Res. 2017, 12, 1823–1831. [Google Scholar] [CrossRef]
- Gokeler, A.; Bisschop, M.; Myer, G.D.; Benjaminse, A.; Dijkstra, P.U.; van Keeken, H.G.; van Raay, J.J.; Burgerhof, J.G.; Otten, E. Immersive virtual reality improves movement patterns in patients after ACL reconstruction: Implications for enhanced criteria-based return-to-sport rehabilitation. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2280–2286. [Google Scholar] [CrossRef] [PubMed]
- Demeco, A.; Salerno, A.; Gusai, M.; Vignali, B.; Gramigna, V.; Palumbo, A.; Corradi, A.; Mickeviciute, G.C.; Costantino, C. The Role of Virtual Reality in the Management of Football Injuries. Medicina 2024, 60, 1000. [Google Scholar] [CrossRef] [PubMed]
- Armitano-Lago, C.; Pietrosimone, B.; Davis-Wilson, H.C.; Evans-Pickett, A.; Franz, J.R.; Blackburn, T.; Kiefer, A.W. Biofeedback augmenting lower limb loading alters the underlying temporal structure of gait following anterior cruciate ligament reconstruction. Hum. Mov. Sci. 2020, 73, 102685. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, A.W.; Kushner, A.M.; Groene, J.; Williams, C.; Riley, M.A.; Myer, G.D. A Commentary on Real-Time Biofeedback to Augment Neuromuscular Training for ACL Injury Prevention in Adolescent Athletes. J. Sports Sci. Med. 2015, 14, 1–8. [Google Scholar]
- Sturma, A.; Hruby, L.A.; Prahm, C.; Mayer, J.A.; Aszmann, O.C. Rehabilitation of Upper Extremity Nerve Injuries Using Surface EMG Biofeedback: Protocols for Clinical Application. Front. Neurosci. 2018, 12, 906. [Google Scholar] [CrossRef]
- Gokeler, A.; Bisschop, M.; Benjaminse, A.; Myer, G.D.; Eppinga, P.; Otten, E. Quadriceps function following ACL reconstruction and rehabilitation: Implications for optimisation of current practices. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1163–1174. [Google Scholar] [CrossRef]
- Lepley, L.K.; Palmieri-Smith, R.M. Pre-operative quadriceps activation is related to post-operative activation, not strength, in patients post-ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 236–246. [Google Scholar] [CrossRef]
- Ueda, Y.; Matsushita, T.; Shibata, Y.; Takiguchi, K.; Kida, A.; Araki, D.; Kanzaki, N.; Hoshino, Y.; Ono, R.; Sakai, Y.; et al. Longitudinal Quadriceps Strength Recovery After Anterior Cruciate Ligament Reconstruction With Hamstring Autograft: Patients Stratified by Preoperative Quadriceps Strength Deficit. J. Sport Rehabil. 2019, 29, 602–607. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lin, J.-J.; Guo, Y.-L.; Wang, W.-T.; Chen, Y.-J. EMG Biofeedback Effectiveness to Alter Muscle Activity Pattern and Scapular Kinematics in Subjects with and without Shoulder Impingement. J. Electromyogr. Kinesiol. 2013, 23, 267–274. [Google Scholar] [CrossRef]
- Diekfuss, J.A.; Grooms, D.R.; Bonnette, S.; DiCesare, C.A.; Thomas, S.; MacPherson, R.P.; Ellis, J.D.; Kiefer, A.W.; Riley, M.A.; Schneider, D.K.; et al. Real-time biofeedback integrated into neuromuscular training reduces high-risk knee biomechanics and increases functional brain connectivity: A preliminary longitudinal investigation. Psychophysiology 2020, 57, e13545. [Google Scholar] [CrossRef]
- Gherman, B.; Zima, I.; Vaida, C.; Tucan, P.; Pisla, A.; Birlescu, I.; Machado, J.; Pisla, D. Robotic Systems for Hand Rehabilitation—Past, Present and Future. Technologies 2025, 13, 37. [Google Scholar] [CrossRef]
- Sherman, D.A.; Glaviano, N.R.; Norte, G.E. Hamstrings Neuromuscular Function After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1751–1769. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, H.-T.; Yoo, B. Virtual Reality Sickness: A Review of Causes and Measurements. Int. J. Hum.-Comput. Interact 2020, 36, 1–25. [Google Scholar] [CrossRef]
- Ananías, J.; Vidal, C.; Ortiz-Muñoz, L.; Irarrázaval, S.; Besa, P. Use of electromyographic biofeedback in rehabilitation following anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Physiotherapy 2024, 123, 19–29. [Google Scholar] [CrossRef]
- Vitharana, T.N.; King, E.; Moran, K. Sensorimotor Dysfunction Following Anterior Cruciate Ligament Reconstruction—An Afferent Perspective: A Scoping Review. Int. J. Sports Phys. Ther. 2024, 19, 1410–1437. [Google Scholar] [CrossRef]
- Grefkes, C.; Fink, G.R. Reorganization of Cerebral Networks After Stroke: New Insights from Neuroimaging with Connectivity Approaches. Brain 2011, 134, 1264–1276. [Google Scholar] [CrossRef]
- Kokotilo, K.J.; Eng, J.J.; Curt, A. Reorganization and Preservation of Motor Control of the Brain in Spinal Cord Injury: A Systematic Review. J. Neurotrauma 2009, 26, 2113–2126. [Google Scholar] [CrossRef]
- Ding, H.; Seusing, N.; Nasseroleslami, B.; Anwar, A.R.; Strauss, S.; Lotze, M.; Grothe, M.; Groppa, S.; Muthuraman, M. The Role of Ipsilateral Motor Network in Upper Limb Movement. Front. Physiol. 2023, 14, 1199338. [Google Scholar] [CrossRef]
- Statton, M.A.; Vazquez, A.; Morton, S.M.; Vasudevan, E.V.L.; Bastian, A.J. Making Sense of Cerebellar Contributions to Perceptual and Motor Adaptation. Cerebellum 2018, 17, 111–121. [Google Scholar] [CrossRef]
- Schnittjer, A.J.; Kim, H.; Lepley, A.S.; Onate, J.A.; Criss, C.R.; Simon, J.E.; Grooms, D.R. Organization of sensorimotor activity in anterior cruciate ligament reconstructed individuals: An fMRI conjunction analysis. Front. Hum. Neurosci. 2023, 17, 1263292. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, H.; Wu, X.; Zhang, Y.; Yu, J.; Li, W. Brain Near-Infrared Study of Upstairs Movement After Anterior Cruciate Ligament Reconstruction. Front. Neurol. 2025, 15, 1500579. [Google Scholar] [CrossRef] [PubMed]
- Criss, C.R.; Lepley, A.S.; Onate, J.A.; Simon, J.E.; France, C.R.; Clark, B.C.; Grooms, D.R. Neural Correlates of Self-Reported Knee Function in Individuals After Anterior Cruciate Ligament Reconstruction. Sports Health 2023, 15, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Attalin, B.; Sagnard, T.; Laboute, E.; Forestier, N.; Rémy-Néris, O.; Picot, B. Proprioceptive Reweighting and Postural Control Are Impaired Among Elite Athletes Following Anterior Cruciate Ligament Reconstruction. Int. J. Sports Phys. Ther. 2024, 19, 1314–1323. [Google Scholar] [CrossRef]
- Giesche, F.; Vieluf, S.; Wilke, J.; Engeroff, T.; Niederer, D.; Banzer, W. Cortical Motor Planning and Biomechanical Stability During Unplanned Jump Landings in Men With Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2022, 57, 547–556. [Google Scholar] [CrossRef]
- Arnsten, A.F. Stress Signalling Pathways That Impair Prefrontal Cortex Structure and Function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Surkar, S.M.; Hoffman, R.M.; Harbourne, R.; Kurz, M.J. Cognitive-Motor Interference Heightens the Prefrontal Cortical Activation and Deteriorates the Task Performance in Children with Hemiplegic Cerebral Palsy. Arch. Phys. Med. Rehabil. 2021, 102, 225–232. [Google Scholar] [CrossRef]
- Kim, H.; Onate, J.A.; Criss, C.R.; Simon, J.E.; Mischkowski, D.; Grooms, D.R. The Relationship Between Drop Vertical Jump Action-Observation Brain Activity and Kinesiophobia After Anterior Cruciate Ligament Reconstruction: A Cross-Sectional fMRI Study. Brain Behav. 2023, 13, e2879. [Google Scholar] [CrossRef]
- Criss, C.; Onate, J.; Grooms, D. Neural Activity for Hip-Knee Control in Those with Anterior Cruciate Ligament Reconstruction: A Task-Based Functional Connectivity Analysis. Neurosci. Lett. 2020, 730, 134985. [Google Scholar] [CrossRef]
- Tsai, L.C.; McLean, S.; Colletti, P.M.; Powers, C.M. Greater Muscle Co-Contraction Results in Increased Tibiofemoral Compressive Forces in Females Who Have Undergone Anterior Cruciate Ligament Reconstruction. J. Orthop. Res. 2012, 30, 2007–2014. [Google Scholar] [CrossRef]
- Brewer, B.W.; Van Raalte, J.L.; Cornelius, A.E. An Interactive Cognitive-Behavioural Multimedia Program Favourably Affects Pain and Kinesiophobia During Rehabilitation After Anterior Cruciate Ligament Surgery: An Effectiveness Trial. Int. J. Sport Exerc. Psychol. 2022, 20, 1133–1155. [Google Scholar] [CrossRef]
- Gür, O.; Başar, S. The effect of virtual reality on pain, kinesiophobia and function in total knee arthroplasty patients: A randomized controlled trial. Knee 2023, 45, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.D.; Ritzmann, R.; Centner, C. Effect of an Anterior Cruciate Ligament Rupture on Knee Proprioception Within 2 Years After Conservative and Operative Treatment: A Systematic Review with Meta-Analysis. Sports Med. 2022, 52, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, M.S.; Bali, K.; Prabhakar, S. Proprioception in anterior cruciate ligament deficient knees and its relevance in anterior cruciate ligament reconstruction. Indian J. Orthop. 2011, 45, 294–300. [Google Scholar] [CrossRef]
- Tayfur, B.; Charuphongsa, C.; Morrissey, D.; Miller, S.C. Neuromuscular Function of the Knee Joint Following Knee Injuries: Does It Ever Get Back to Normal? A Systematic Review with Meta-Analyses. Sports Med. 2021, 51, 321–338. [Google Scholar] [CrossRef]
- Henriksson, M.; Ledin, T.; Good, L. Postural control after ACL reconstruction and functional rehabilitation. Am. J. Sports Med. 2001, 29, 359–366. [Google Scholar] [CrossRef]
- Souissi, S.; Chaouachi, A.; Burnett, A.; Hue, O.; Bouhlel, E.; Chtara, M.; Chamari, K. Leg asymmetry and muscle function recovery after anterior cruciate ligament reconstruction in elite athletes: A pilot study on slower recovery of the dominant leg. Biol. Sport 2020, 37, 175–184. [Google Scholar] [CrossRef]
- Faxon, J.L.; Sanni, A.A.; McCully, K.K. Hamstrings and Quadriceps Muscles Function in Subjects with Prior ACL Reconstruction Surgery. J. Funct. Morphol. Kinesiol. 2018, 3, 56. [Google Scholar] [CrossRef]
- Högberg, J.; Piussi, R.; Wernbom, M.; Della Villa, F.; Simonsson, R.; Samuelsson, K.; Thomeé, R.; Senorski, E.H.H. No association between hamstrings-to-quadriceps strength ratio and second ACL injuries after accounting for prognostic factors: A cohort study of 574 patients after ACL reconstruction. Sports Med. Open 2024, 10, 7. [Google Scholar] [CrossRef]
- Barrios, J.A.; Crossley, K.M.; Davis, I.S. Gait retraining to reduce the knee adduction moment through real-time visual feedback of dynamic knee alignment. J. Biomech. 2010, 43, 2208–2213. [Google Scholar] [CrossRef]
- Filbay, S.R.; Grindem, H. Evidence-based recommendations for the management of anterior cruciate ligament (ACL) rupture. Best Pract. Res. Clin. Rheumatol. 2019, 33, 33–47. [Google Scholar] [CrossRef]
- Rigamonti, L.; Estel, K.; Gehlen, T.; Wolfarth, B.; Lawrence, J.B.; Back, D.A. Use of artificial intelligence in sports medicine: A report of 5 fictional cases. BMC Sports Sci. Med. Rehabil. 2021, 13, 1–7. [Google Scholar]
- Rony, M.K.K.; Kayesh, I.; Bala, S.D.; Akter, F.; Parvin, M.R. Artificial intelligence in future nursing care: Exploring perspectives of nursing professionals—A descriptive qualitative study. Heliyon 2024, 10, e25718. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.; Morris, N.; Barnert, J.; Lawson, D.; Witt, I.; Herzog, W. Forecasting neuromuscular recovery after anterior cruciate ligament injury: Athlete recovery profiles with generalized additive modeling. J. Orthop. Res. 2022, 40, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Gokeler, A.; Grassi, A.; Hoogeslag, R.; van Houten, A.; Lehman, T.; Bolling, C.; Buckthorpe, M.; Norte, G.; Benjaminse, A.; Heuvelmans, P.; et al. Return to sports after ACL injury 5 years from now: 10 things we must do. J. Exp. Orthop. 2022, 9, 73, Erratum in J. Exp. Orthop. 2022, 9, 111. https://doi.org/10.1186/s40634-022-00548-x. [Google Scholar] [CrossRef]
- Cupido, C.; Peterson, D.; Sutherland, M.S.; Ayeni, O.; Stratford, P.W. Tracking patient outcomes after anterior cruciate ligament reconstruction. Physiother. Can. 2014, 66, 199–205. [Google Scholar] [CrossRef]
- Brinlee, A.W.; Dickenson, S.B.; Hunter-Giordano, A.; Snyder-Mackler, L. ACL Reconstruction Rehabilitation: Clinical Data, Biologic Healing, and Criterion-Based Milestones to Inform a Return-to-Sport Guideline. Sports Health 2022, 14, 770–779. [Google Scholar] [CrossRef]
- Grooms, D.R.; Chaput, M.; Simon, J.E.; Criss, C.R.; Myer, G.D.; Diekfuss, J.A. Combining Neurocognitive and Functional Tests to Improve Return-to-Sport Decisions Following ACL Reconstruction. J. Orthop. Sports Phys. Ther. 2023, 53, 415–419. [Google Scholar] [CrossRef]
- Evancho, A.; Tyler, W.J.; McGregor, K. A review of combined neuromodulation and physical therapy interventions for enhanced neurorehabilitation. Front. Hum. Neurosci. 2023, 17, 1151218. [Google Scholar] [CrossRef]
- Moreno-Duarte, I.; Morse, L.R.; Alam, M.; Bikson, M.; Zafonte, R.; Fregni, F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage 2014, 85 Pt 3, 1003–1013. [Google Scholar] [CrossRef]
- García-Alén, L.; Ros-Alsina, A.; Sistach-Bosch, L.; Wright, M.; Kumru, H. Noninvasive Electromagnetic Neuromodulation of the Central and Peripheral Nervous System for Upper-Limb Motor Strength and Functionality in Individuals with Cervical Spinal Cord Injury: A Systematic Review and Meta-Analysis. Sensors 2024, 24, 4695. [Google Scholar] [CrossRef]
- Grippe, T.; Desai, N.; Arora, T.; Chen, R. Use of non-invasive neurostimulation for rehabilitation in functional movement disorders. Front. Rehabil. Sci. 2022, 3, 1031272. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Green, M.; Tram, J.; Wang, E.; Murphy, M.; Abd-Elsayed, A.A.; Chakravarthy, K. Latest Advancements in Transcutaneous Electrical Nerve Stimulation (TENS) and Electronic Muscle Stimulation (EMS): Revisiting an Established Therapy with New Possibilities. J. Pain Res. 2025, 18, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Sale, M.V.; Reid, L.B.; Cocchi, L.; Pagnozzi, A.M.; Rose, S.E.; Mattingley, J.B. Brain changes following four weeks of unimanual motor training: Evidence from behavior, neural stimulation, cortical thickness, and functional MRI. Hum. Brain Mapp. 2017, 38, 4773–4787. [Google Scholar] [CrossRef] [PubMed]
- Forogh, B.; Aslanpour, H.; Fallah, E.; Babaei-Ghazani, A.; Ebadi, S. Adding high-frequency transcutaneous electrical nerve stimulation to the first phase of post anterior cruciate ligament reconstruction rehabilitation does not improve pain and function in young male athletes more than exercise alone: A randomized single-blind clinical trial. Disabil. Rehabil. 2019, 41, 514–522. [Google Scholar] [CrossRef]
- Mendes, J.J.A., Jr.; Vieira, M.E.M.; Pires, M.B.; Stevan, S.L., Jr. Sensor Fusion and Smart Sensor in Sports and Biomedical Applications. Sensors 2016, 16, 1569. [Google Scholar] [CrossRef]
- Zhu, R.; Zheng, M.; Liu, S.; Guo, J.; Cao, C. Effects of Perceptual-Cognitive Training on Anticipation and Decision-Making Skills in Team Sports: A Systematic Review and Meta-Analysis. Behav. Sci. 2024, 14, 919. [Google Scholar] [CrossRef]
- Taylor, J.B.; Nguyen, A.D.; Paterno, M.V.; Huang, B.; Ford, K.R. Real-time optimized biofeedback utilizing sport techniques (ROBUST): A study protocol for a randomized controlled trial. BMC Musculoskelet. Disord. 2017, 18, 71. [Google Scholar] [CrossRef]
- Harwin, W.S.; Murgia, A.; Stokes, E.K. Assessing the effectiveness of robot facilitated neurorehabilitation for relearning motor skills following a stroke. Med. Biol. Eng. Comput. 2011, 49, 1093–1102. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mohamed, K.; Zeeshan, S.; Dong, X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database 2020, 2020, baaa010. [Google Scholar] [CrossRef]
- Donisi, L.; Cesarelli, G.; Pisani, N.; Ponsiglione, A.M.; Ricciardi, C.; Capodaglio, E. Wearable Sensors and Artificial Intelligence for Physical Ergonomics: A Systematic Review of Literature. Diagnostics 2022, 12, 3048. [Google Scholar] [CrossRef]
- Si, J.; Thelkar, A.R. Leveraging Artificial Neural Networks for Enhanced Athlete Performance Evaluation through IMU Data Analysis. Heliyon 2024, 10, e34826. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.; Ullah, A. Advanced biomechanical analytics: Wearable technologies for precision health monitoring in sports performance. Digit. Health 2024, 10, 20552076241256745. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.J.; Meierbachtol, A.; George, S.Z.; Chmielewski, T.L. Fear of Reinjury in Athletes. Sports Health 2017, 9, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Sheean, A.J.; DeFoor, M.T.; Spindler, K.P.; Arner, J.W.; Athiviraham, A.; Bedi, A.; DeFroda, S.; Ernat, J.J.; Frangiamore, S.J.; Nuelle, C.W.; et al. The Psychology of ACL Injury, Treatment, and Recovery: Current Concepts and Future Directions. Sports Health 2024, 19417381241226896. [Google Scholar] [CrossRef]
- Meredith, S.J.; Rauer, T.; Chmielewski, T.L.; Fink, C.; Diermeier, T.; Rothrauff, B.B.; Svantesson, E.; Hamrin Senorski, E.; Hewett, T.E.; Sherman, S.L.; et al. Return to Sport After Anterior Cruciate Ligament Injury: Panther Symposium ACL Injury Return to Sport Consensus Group. Orthop. J. Sports Med. 2020, 8, 2325967120930829. [Google Scholar] [CrossRef]
- Grooms, D.R.; Page, S.J.; Nichols-Larsen, D.S.; Chaudhari, A.M.; White, S.E.; Onate, J.A. Neuroplasticity Associated With Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2017, 47, 180–189. [Google Scholar] [CrossRef]


| Rehabilitation Focus | Definition | Key Mechanism | Rehabilitation Approach | Impact on ACL Recovery | Practical Application | Limitations of the Advanced Techniques. |
|---|---|---|---|---|---|---|
| Neuroplasticity in ACL Rehab [15,17,18] | The brain’s ability to reorganize and form new neural connections after injury. | Reorganization of neural pathways and motor skills. | Incorporating proprioceptive and cognitive training alongside physical exercises. | Enhances recovery by restoring communication between knee and brain, improving movement patterns. | Using complex exercises combining balance and strength to enhance brain–knee communication. | Requires high patient engagement and consistency; neural adaptation varies between individuals. |
| Proprioception and Sensory Feedback [80,81] | The ability to detect joint position and movement, essential for coordination and injury prevention. | Activation of sensory receptors near the knee, promoting brain-knee communication. | Exercises on unstable surfaces or tasks requiring precise movements. | Restores knee stability and reduces risk of future injuries. | Balance exercises on a Bosu ball or wobble board to improve joint awareness. | May not be suitable for all rehabilitation stages; risk of instability-related setbacks. |
| Cognitive Function and Decision-Making [70] | Cognitive function involves mental processes like attention, memory, and problem-solving, essential for making quick, informed decisions, especially under pressure. Effective decision-making in athletes relies on these cognitive abilities, influencing performance and injury prevention. | Cognitive load (e.g., multi-tasking or unexpected stimuli) improves decision-making and reaction time. | Incorporating tasks like counting during drills or responding to unpredictable stimuli. | Improves reaction speed and confidence, reducing reinjury risk during dynamic sports situations. | Drills that require athletes to respond to unexpected movements or make quick decisions during rehabilitation. | High cognitive demands may increase mental fatigue; effectiveness depends on individual cognitive ability. |
| Robotic-Assisted Rehabilitation [89,90] | Robotic devices help in executing precise, regulated movements to aid muscle recovery. | Exact biomechanical movements to improve motor function and neuromuscular control. | Use of exoskeletons or robotic trainers to guide exercises and monitor progress. | Enhances recovery through muscle strengthening, joint stability, and motor skill re-learning. | Robotic-assisted walking devices to improve gait and functional movements after ACL reconstruction. | High cost and limited accessibility; may not replicate sport-specific dynamic movements. |
| VR Training [93,94] | Immersive virtual environments provide scenario-based training to improve movement control. | Induces neural changes for better coordination, proprioception, and knee biomechanics. | VR-based drills simulating unpredictable sports movements. | Improves knee biomechanics and psychological readiness for return to sport, reducing fear of reinjury. | VR simulations of game-like scenarios (e.g., basketball pivoting) to prepare athletes for return to play. | Requires specialized equipment; limited real-world transferability for some movement patterns. |
| Biofeedback Systems [96,97,98,99] | Provides real-time feedback on muscle activity and movement patterns to aid recovery. | Monitoring of muscle activity, joint position, and weight distribution. | Use of EMG and force plate biofeedback to guide muscle activation and balance. | Improves proprioception, neuromuscular control, and corrects compensatory movements, reducing reinjury risk. | EMG biofeedback to monitor and enhance quadriceps activation post-surgery to promote better knee stability. | Requires specialized training and equipment; effectiveness depends on patient compliance and proper use. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrò, R.S.; Calderone, A.; Fiorente, N. Neurosciences and Sports Rehabilitation in ACLR: A Narrative Review on Winning Alliance Strategies and Connecting the Dots. J. Funct. Morphol. Kinesiol. 2025, 10, 119. https://doi.org/10.3390/jfmk10020119
Calabrò RS, Calderone A, Fiorente N. Neurosciences and Sports Rehabilitation in ACLR: A Narrative Review on Winning Alliance Strategies and Connecting the Dots. Journal of Functional Morphology and Kinesiology. 2025; 10(2):119. https://doi.org/10.3390/jfmk10020119
Chicago/Turabian StyleCalabrò, Rocco Salvatore, Andrea Calderone, and Nicola Fiorente. 2025. "Neurosciences and Sports Rehabilitation in ACLR: A Narrative Review on Winning Alliance Strategies and Connecting the Dots" Journal of Functional Morphology and Kinesiology 10, no. 2: 119. https://doi.org/10.3390/jfmk10020119
APA StyleCalabrò, R. S., Calderone, A., & Fiorente, N. (2025). Neurosciences and Sports Rehabilitation in ACLR: A Narrative Review on Winning Alliance Strategies and Connecting the Dots. Journal of Functional Morphology and Kinesiology, 10(2), 119. https://doi.org/10.3390/jfmk10020119







