What Is ‘Muscle Health’? A Narrative Review and Conceptual Framework
Abstract
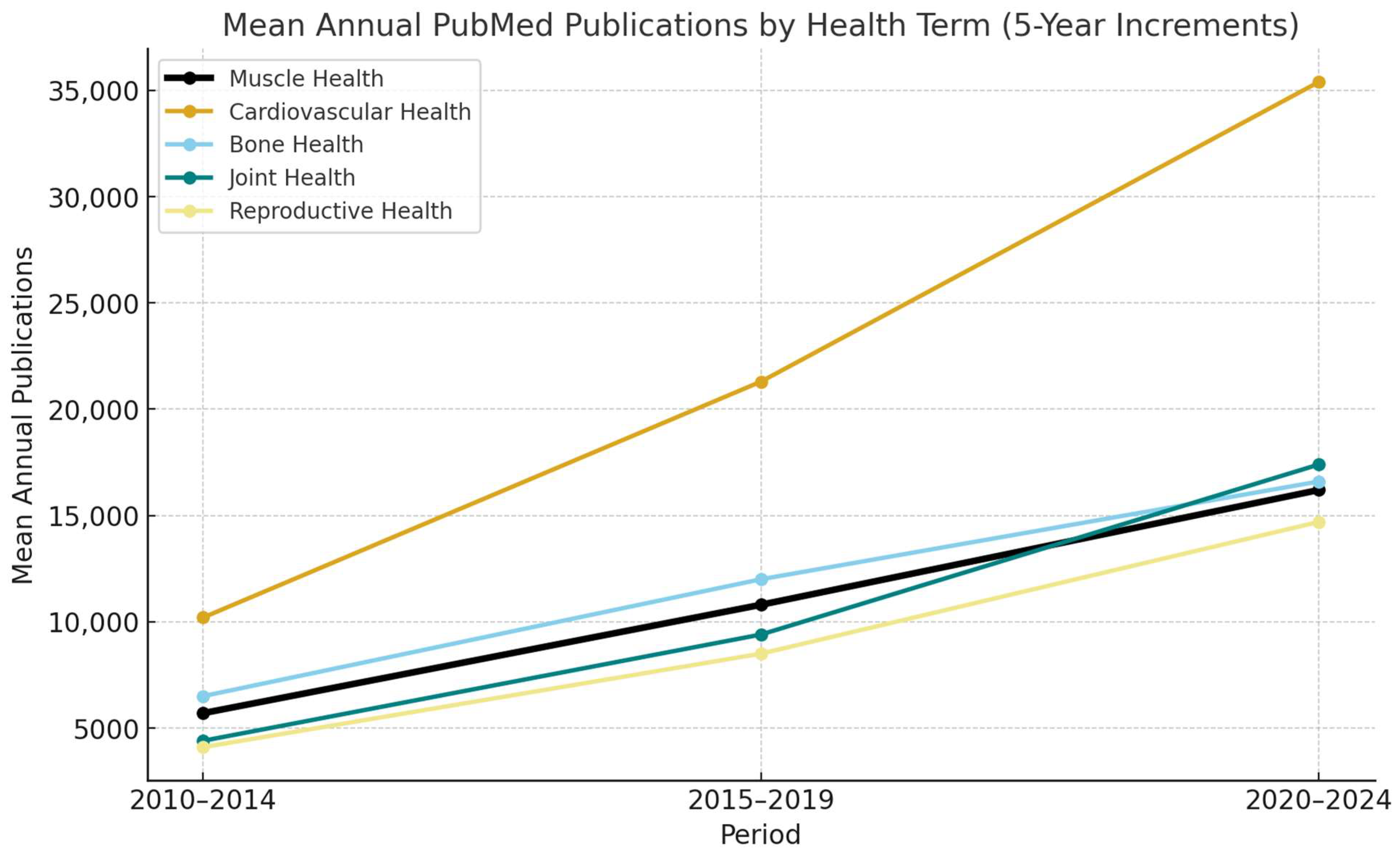
1. Introduction
1.1. What Is Health?
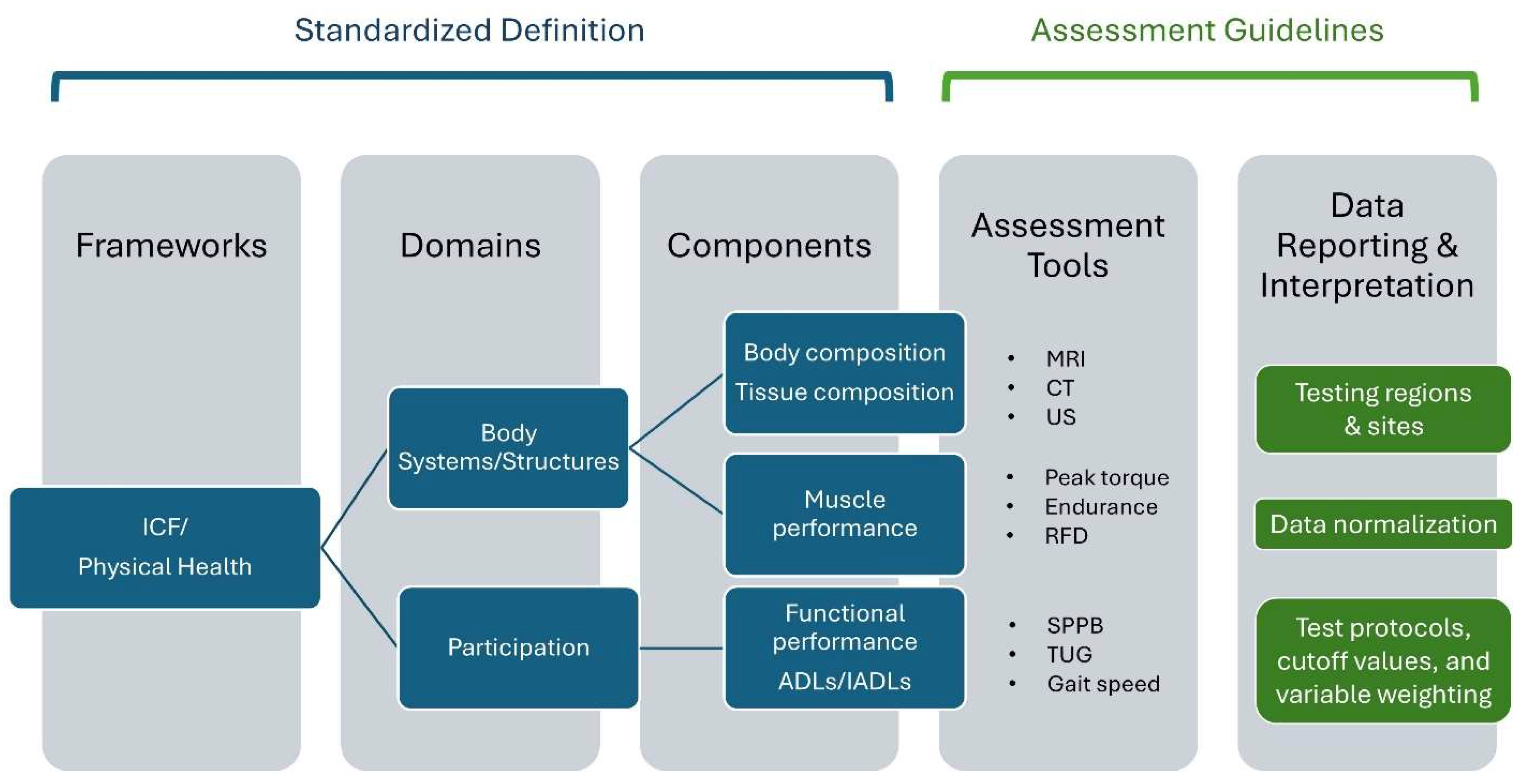
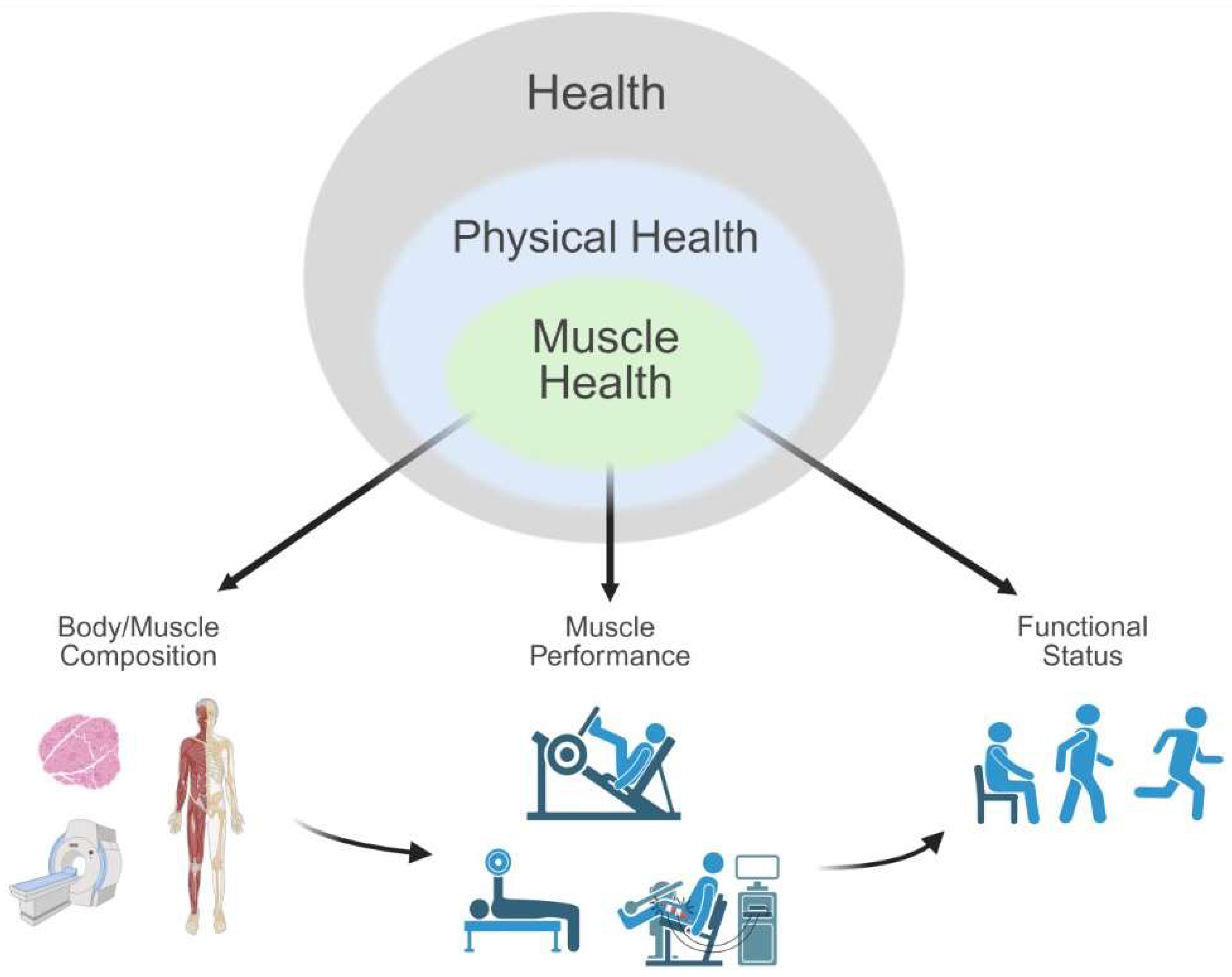
1.2. In Search of a Muscle Health Assessment Framework
2. Materials and Methods
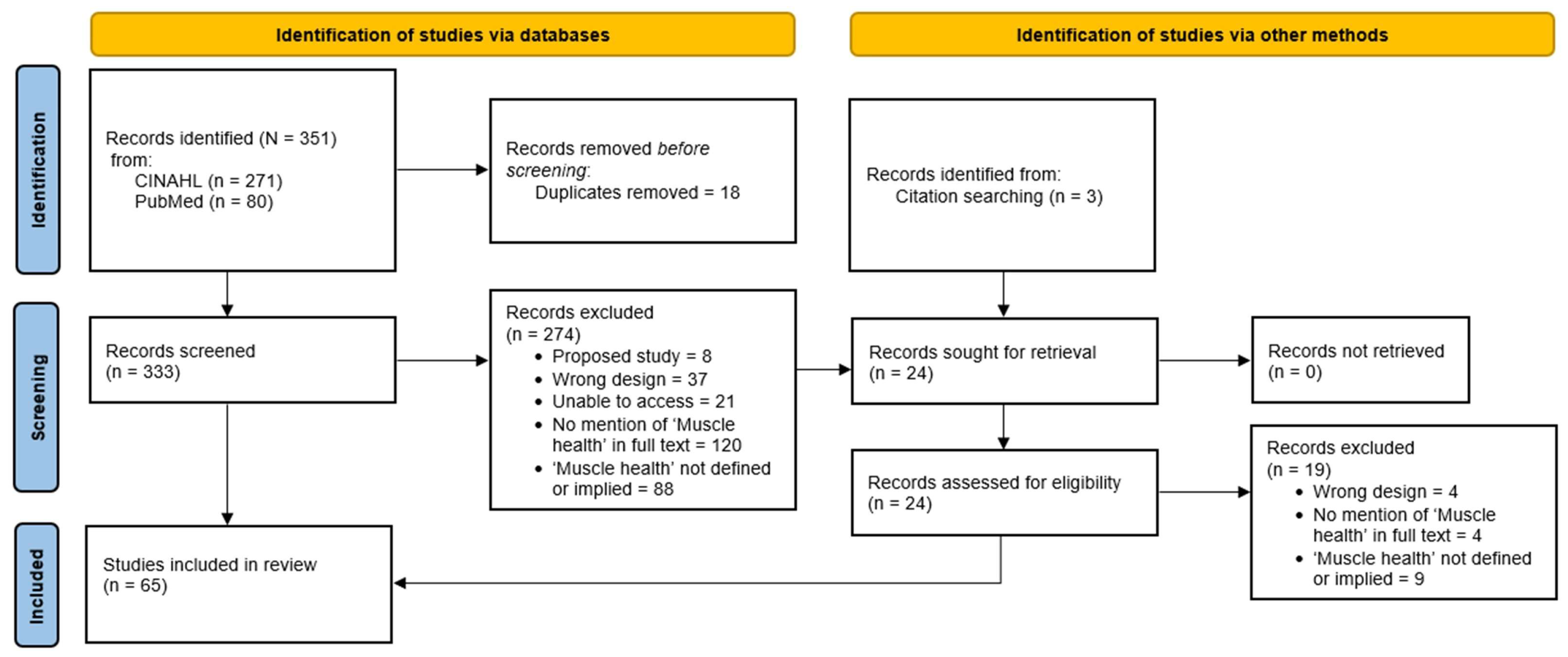
2.1. Reporting and Registration
2.2. Eligibility
2.3. Information Sources and Search Strategy
2.4. Data Extraction
2.5. Data Analysis and Interpretation
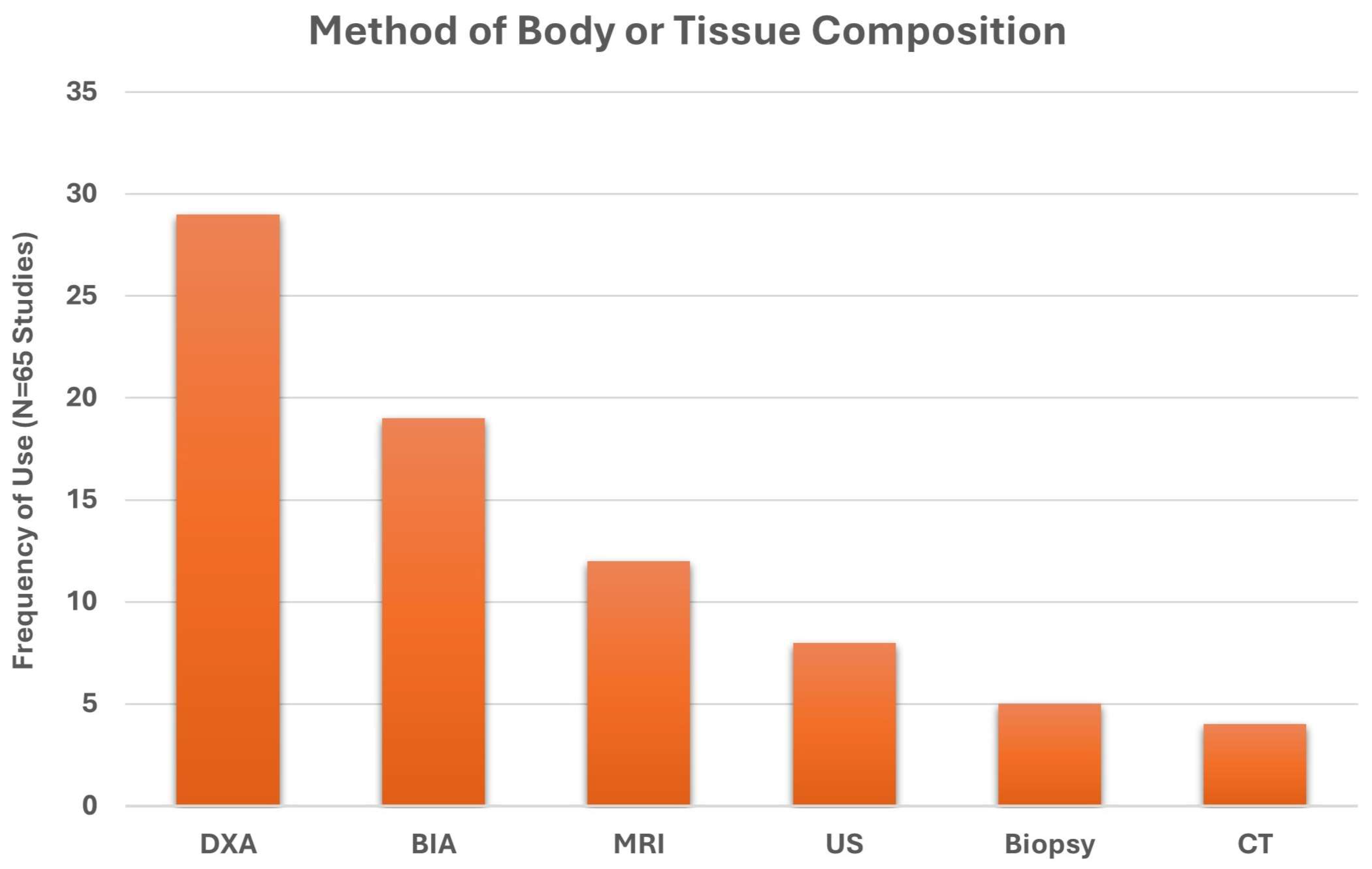
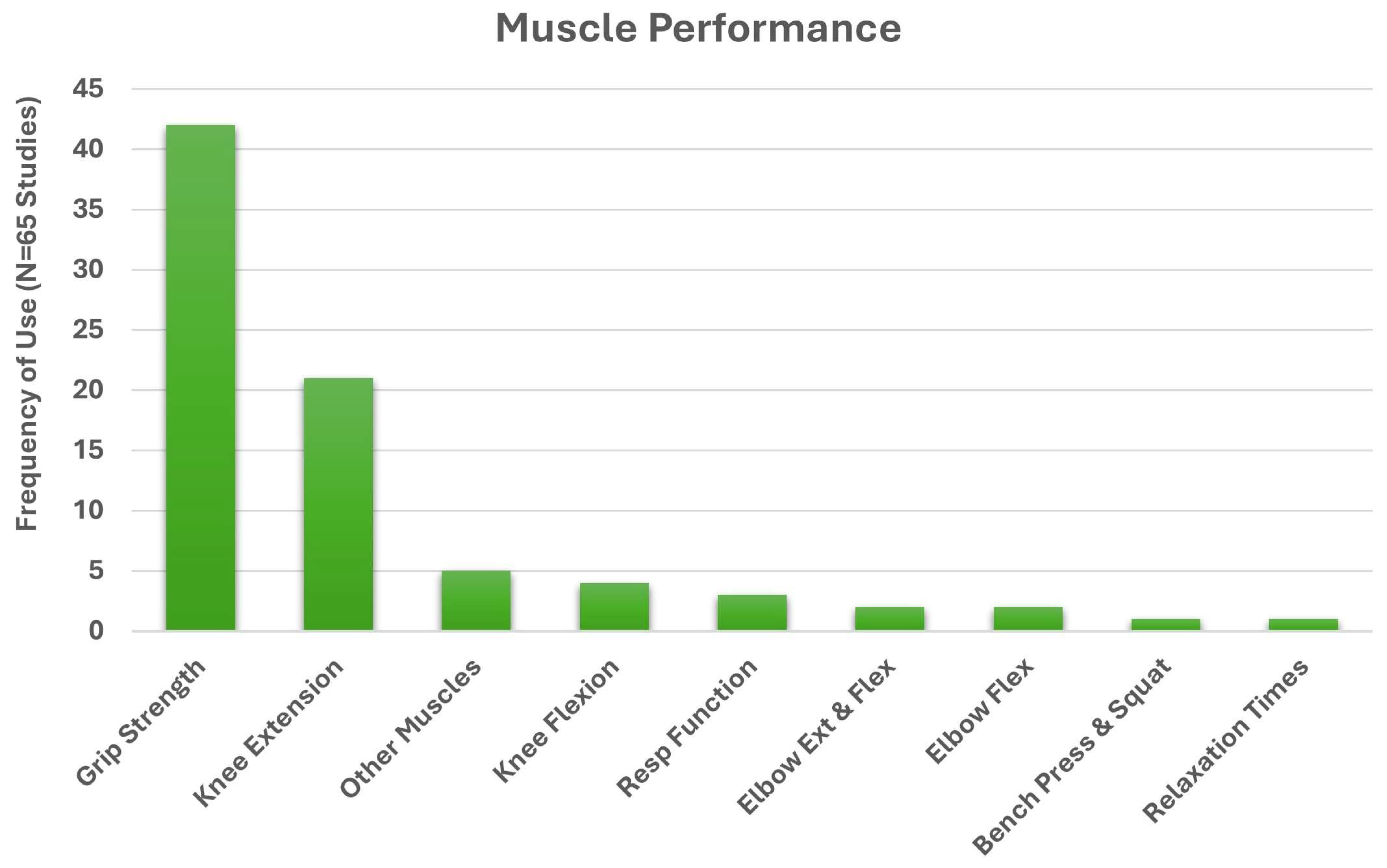
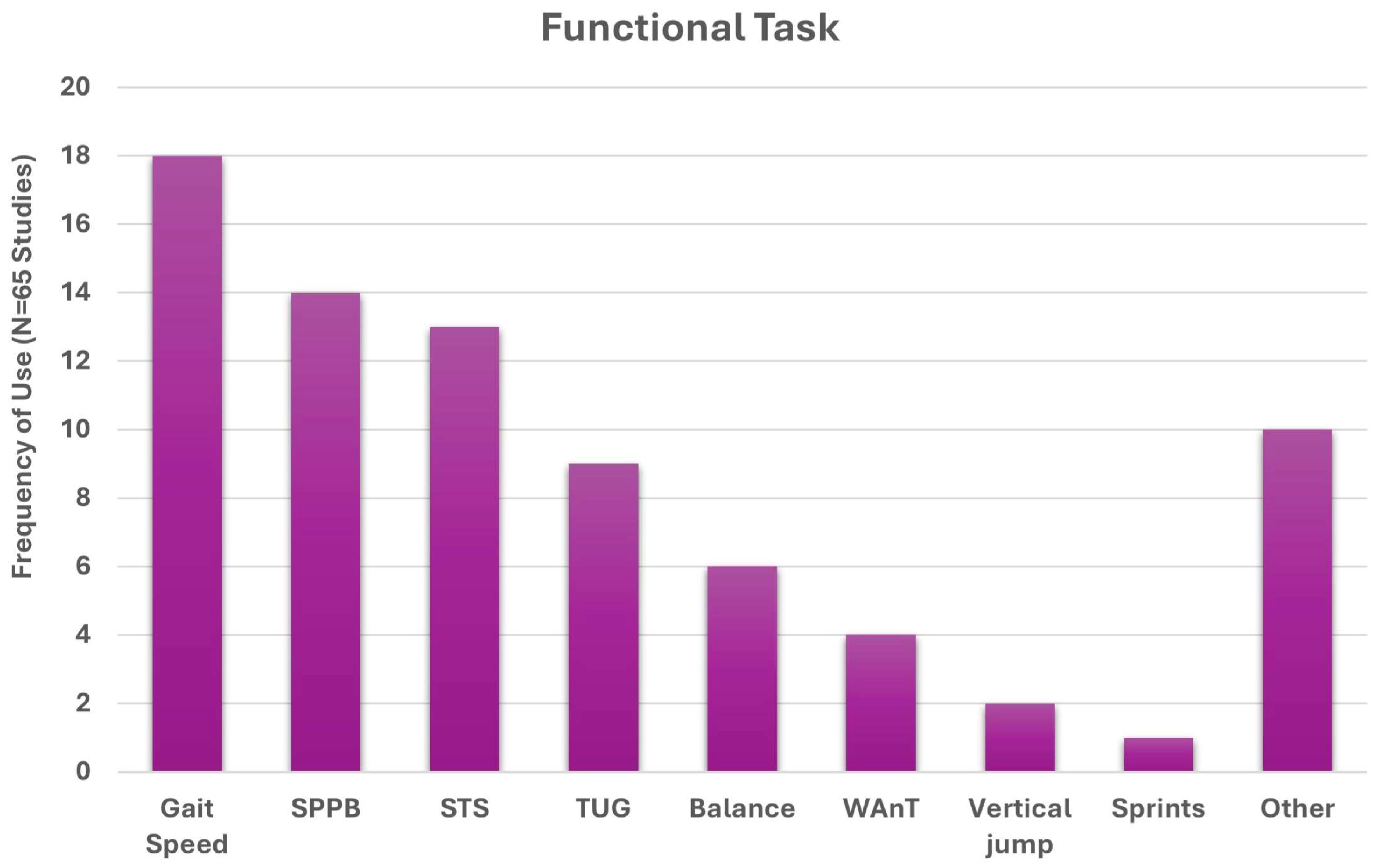
3. Results
3.1. Search Results
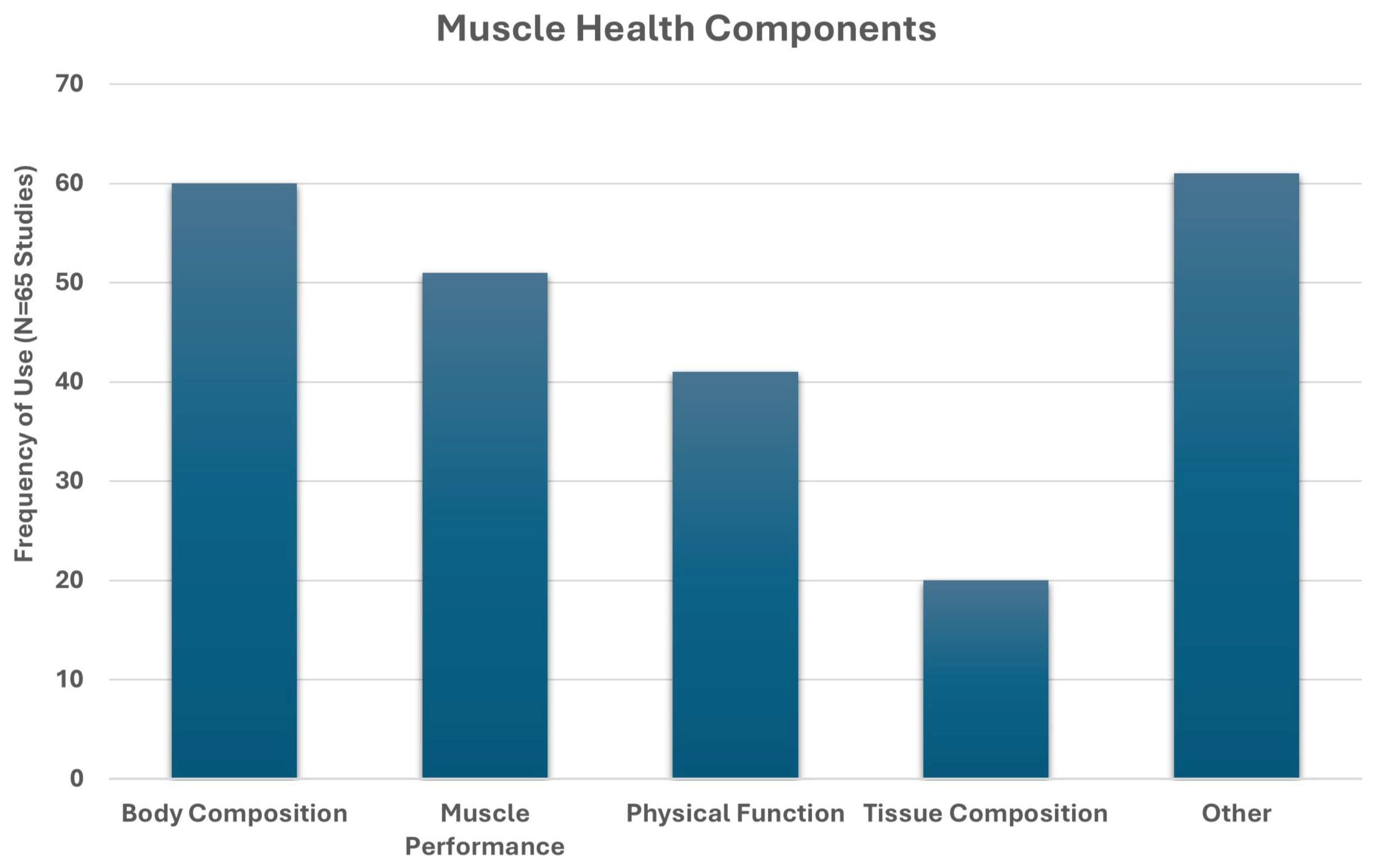
3.2. Identified Study Characteristics
| Study | Population | Defined | Measured | Body Composition | Tissue Composition | Performance | Functional Tasks | Other |
|---|---|---|---|---|---|---|---|---|
| Anderson et al., 2022 [31] | Patients undergoing surgery for lumbar spine pathology, N = 54 (32 M:22 F), 51.5 ± 16.9 yrs | “Muscle health and function is influenced by structural features such as size (cross-sectional area) and tissue composition (e.g., amount of fatty infiltration within the muscle compartment)”, “…paraspinal muscle health (size and composition)…” | Muscle size, composition, and gene expression | CSA, mCSA, F-CSA, the proportion of fat within the muscle compartment (MRI) | Muscle, adipose, loose collagen, and dense collagen composition (tissue biopsy) | 42 genes associated with adipogenic/metabolic, atrophic, fibrogenic, inflammatory, and myogenic pathways, 40S Ribosomal Protein (RPS18) and Beta-Actin (ACTB) as controls | ||
| Bathgate et al., 2018 [35] | One pair of male monozygotic twins, 52 yrs | “Skeletal muscle health—Whole muscle size, strength, and power were assessed. Additionally, protein and gene expression were measured for various markers of fiber type, metabolism, growth, repair, and inflammation.” | Skeletal muscle size, composition, strength, and power, molecular markers of muscle health, cardiorespiratory and pulmonary health, and blood profiles | CSA, MT (B-mode US) Lean mass, FM, total body fat percentage, visceral adipose tissue, bone mineral content, and bone mineral density (DXA) | Echo intensity (US) Muscle fiber composition—MHC isoforms, MyMHC expression, cellular metabolism (muscle biopsy, VL) Skeletal muscle fiber type, oxidative metabolism, citrate synthase, angiogenesis, vascular endothelial growth factor, muscular growth and repair, mechano-growth factor, insulin-like growth factor, myoblast determination protein 1, inflammatory responses (QRT-PCR) | Knee extension (dynamometry) and grip strength | Five sprints (Monark ergometer) and WAnT (Anaerobic capacity) | Cardiorespiratory: Resting heart rate, blood pressure, VO2max, and pulmonary function Muscle biopsy, AMPK protein expression Tracked normal physical activity patterns and dietary intake |
| Bauer et al., 2024 [75] | Community-dwelling older adults ≥70 yrs with urinary tract symptoms, N = 641 (264 M:377 F), 75.5 ± 4.4 yrs | “…age-related declines in skeletal muscle health, such as loss of muscle mass, volume, and strength/power, and related physical performance.” | Body size, muscle mass and volume, strength, power, physical function, cognition, and QoL | WBLM (D3-creatine) and thigh fat-free muscle volume (MRI) | Knee extension peak power (Keiser Air 420 exercise machine) and grip strength | 400 m usual walking speed, SPPB, and four-square step test | Mobility Assessment Tool-short form, MoCA, CESD-10, EQ-5D, and CHAMPS Lower urinary tract symptoms: Lower Urinary Tract Dysfunction Research Network Symptom Index-10 Total energy expenditure, BMI | |
| Berry et al., 2019 [36] | Adults with lower back pain, N = 14 (7 M:7 F), 52.8 ± 14.8 yrs | “The primary outcome measures of muscle health were mCSA and FF.” | mCSA, FF, strength, pain, and disability | mCSA (MRI) | FF (MRI) | Maximum lumbar extension strength (dynamometry) | Range of motion (isokinetic dynamometer), 100 mm visual analog scale, Oswestry Disability Index | |
| Bhandari et al., 2025 [88] | Cancer survivors >2 years in remission and off therapy, N = 20 (10 M:10 F), 35 (18–67) yrs | “Exercise has been shown to improve muscle health, including muscle mass, strength, and function…” | Muscle mass, composition, strength, function, metabolic variables | Whole body fat and fat-free mass, segmental muscle mass, visceral adipose tissue (BIA) | RF, GM, GL: CSA, muscle thickness, IMAT (US) | Grip strength | SPPB | Blood: HbA1c, fasting glucose, HOMA-IR, myostatin BMI, waist circumference |
| Cegielski et al., 2022 [39] | Healthy adults, N = 37 (21 M:16 F), 72 ± 5 yrs | “…functional muscle health parameters (e.g., handgrip strength, leg strength, muscle mass by DXA imaging) …” “… established measures of muscle health (handgrip strength, 1-RM and MVC)…” | Muscle mass, strength, function, and metabolic variables | Thigh FFM (DXA) | Muscle thickness, fascicle length, and pennation angle (US) | Unilateral leg extension 1-RM, MVC (dynamometry) and grip strength | SPPB | Blood: MPS, MPB, and ASR (COSIAM) Muscle biopsy Urine sample collected to measure D3-creatinine |
| Davis et al., 2021 [41] | Men over a 15-year span, N = 522, 50.0 (IQR: 38.3–59.7) yrs | “Low muscle mass and poor muscle strength and function are key characteristics of poor muscle health.” | Muscle mass, strength, and function | SMI, whole body composition, and ALM (DXA) | TUG | Self-reported dietary data: food frequency questionnaire Self-reported physical activity: Baecke Physical Activity Questionnaire BMI | ||
| Distefano et al., 2024 [89] | Knee osteoarthritis patients, N = 655 (280 M:375 F), 76.1 ± 4.9 yrs | “Muscle health, including muscle composition, power, and energetics…” | Muscle mass, fat mass, power, function, cardiovascular function, metabolic variables | Whole body muscle mass (D3-creatine), visceral adipose tissue, abdominal subcutaneous adipose tissue (MRI) | Thigh FFM volume, Thigh muscle fat infiltration (MRI) | Knee extensor peak power (pneumatic), peak power/thigh muscle volume | SPPB, gait speed (400 m) | Physical activity and fitness QoL: MAT-sf questionnaire Mitochondrial energetics: ATPmax, OXPHOS (biopsy) BMI |
| Engelen et al., 2022 [43] | Normal weight moderate and severe COPD patients, N = 32 (18 M:14 F), 66.8 ± 4.4 yrs | “…and improves muscle health (mass and function as secondary outcomes).” | Muscle mass and strength, lung function, and metabolic variables | Whole body and extremity FM and FFM (DXA) | Grip strength | Blood: glucose, C-reactive protein, amino acids, fatty acids, various other health markers Respiratory muscle function: inspiratory pressure, forced expiratory volume, forced vital capacity Physical Activity Scale for the Elderly questionnaire, Saint George Respiratory Questionnaire BMI, waist circumference | ||
| Ferguson et al., 2024 [76] | Patients receiving extracorporeal membrane oxygenation, N = 23 (10 M:13 F), 48 ± 14 yrs | “…muscle health (size and quality)…” | Muscle size, quality, strength, function, and nutritional data | CSA (US), mCSA (MRI) | Quadriceps thickness and RF echogenicity (US) | Knee extension MVIC (hand-held dynamometer) and muscle strength (Medical Research Council sum score with ICU mobility scale) | Highest level of mobility (ICU mobility scale) | Nutrition data: energy and protein delivery BMI |
| Finkel et al., 2021 [45] | Males with Duchenne Muscular Dystrophy, N = 31, 6.1 ± 1.1 (4–8) yrs | “…lower leg muscle health as determined by the MRI transverse relaxation time constant (T2) from a composite of five muscles.” | T2 relaxation time of lower leg muscles, muscle function, metabolic variables, and gene expression | FF (MRI) | Gait speed (10 m walk/run test), 4 stair climb, time to stand, and North Star Ambulatory Assessment | Blood: cytokine panel of multiple inflammatory markers Gene expression: NF-κB-target genes Heart rate, BMI | ||
| Jackson et al., 2022 [49] | Healthy women, N = 53, 55.8 ± 5.3 yrs | “…muscle health (muscle mass, grip strength, five-chair rise test, 4 m gait speed test)”: muscle mass, strength, and physical function (i.e., muscle health).” | Muscle mass, strength, function, and dietary intake | SMI (BIA) | Grip strength | Gait speed (4 m walk test) and five-time chair stand test | Intake of energy, protein, carbohydrate, and fat Risk for Sarcopenia BMI | |
| Jacob et al., 2022 [50] | Healthy adults, N = 274 (118 M:156 F), 41.9 ± 16.1 (18–70) yrs | “…indices of muscle health should be evaluated in samples of healthy adults to determine the optimum reference values of muscle morphology, function and functional capability.” | Morphology, function, and functional capacity | VL muscle thickness, pennation angle, fascicle length, echo intensity, and contractile properties (US and tensiomyography) | Grip strength | Five-time chair stand test and 1 min chair rise test | Femur length, thigh girth Physical activity level: IPAQ, BPAQ | |
| Locquet et al., 2019 [53] | Adults ≥65 yrs, N = 232 (98 M:134 F), 75.5 ± 5.4 yrs (76.0 ± 5.1 yrs M, 75.1 ± 5.6 yrs F) | “Muscle health—SMI (kg/m2), grip strength, physical performance…” | Mass, strength, physical performance, nutritional assessment, cognitive assessment, and physical activity | SMI and areal bone mineral density (DXA) | Grip strength | SPPB | Osteoporosis diagnosis: trabecular bone score Mini-Nutritional Assessment Mini-Mental State Examination Self-reported level of physical activity, fracture risk BMI | |
| Olpe et al., 2024 [78] | Patients with cancer, N = 269 (161 M:108 F), 68.8 ± 13.3 yrs | “…muscle health markers (i.e., handgrip strength, computed tomography (CT)-based muscle mass and radiodensity)…” | Muscle size, composition, strength, and metabolic variables | Skeletal muscle area, SMI, muscle radiodensity, intramuscular adipose tissue (CT) | Grip strength | Blood: Plasma albumin and c-reactive protein Malnutrition risk BMI | ||
| Papaioannou et al., 2021 [55] | Physically active adults, N = 191 (69 M:122 F), 67.4 ± 1.5 yrs M, 67.4 ± 1.6 yrs F | “…based on three indicators of muscle health: muscle mass was assessed using bioelectrical impedance and handgrip strength and 5 times sit-to-stand (5-STS).” | Muscle mass, strength, physical function, and dietary intake | SMI (BIA), SMM (Janseen Equation) | Grip strength | Five-time chair stand test | Dietary data: 90-item food-frequency questionnaire, Healthy diet score Adherence to physical activity (Actigraph GT3x) Blood: High-sensitivity c-reactive protein Risk for Sarcopenia | |
| Parker et al., 2021 [56] | Adults during preoperative pancreatic cancer treatment, N = 97 (52 M:45 F), 66.4 ± 7.9 yrs | “SMI and SMD were the endpoints of this study; together, they reflect skeletal muscle health.” | Muscle quantity and quality | CSA, SMI—scans performed at T0 and T1 (CT) | SMD (CT) | BMI Risk for Sarcopenia | ||
| Pratt et al., 2021 [59] | Healthy older adults, N = 300 (150 M:150 F), 64.1 ± 8.5 (50–83) yrs | “…our findings demonstrate the potential of circulating CAF as an accessible indicator of skeletal muscle health in older adults.” | Muscle mass, strength, and metabolic variables | ALM (DXA) | Grip strength | Plasma: CAF Risk of Sarcopenia | ||
| Shin et al., 2022 [62] | Adults with chronic kidney disease, N = 149 (97 M:52 F), 65 ± 11 yrs | “PhA appears to be a reliable marker for estimating muscle health and HRQoL in patients with CKD.” “…muscle health, inflammatory and muscle-related markers…” “…BIA-derived PhA in estimating the muscle health in patients with CKD. We observed that PhA was related to SMI, handgrip strength, and gait speed; “ | Body composition, muscle strength and function, and metabolic variables | FFM, SMM, SMI, intracellular water, extracellular water, and total body water (BIA) | Grip strength | Gait speed (6 m walk test) | Blood: Hemoglobin, albumin, high-sensitivity C-reactive protein, hemoglobin A1c, intact parathyroid hormone, total cholesterol, calcium, phosphorus, sodium, potassium, chloride, total carbon dioxide, blood urea nitrogen, creatinine, and eGFR QoL and risk of Sarcopenia BMI | |
| Song et al., 2022 [63] | Patients who underwent 1-level lumbar microdiscectomy, N = 163 (102 M:61 F), 47.8 ± 15.4 | “Good” muscle health was defined as score of 2, and “poor” muscle health was defined as score of 0 to 1.” “For the good muscle health group, mean PL-CSA/BMI was 169.4 mm2/kg/m2, and mean Goutallier class was 1.5.” | Muscle size | Normalized total psoas area (MRI) | Goutallier classification (MRI) | |||
| Song et al., 2023 [83] | Healthy participants with and without a history of spine surgery, N = 178 (84 M:94 F), 65.3 ± 12.7 yrs | Muscle health parameters—Goutallier grade, PL-CSA, PL-CSA/BMI, LIV “…novel MRI-based lumbar muscle health grading system incorporating paralumbar cross-sectional areas and Goutallier classification…” | Body size, muscle size, and composition | Paralumbar-CSA, Paralumbar-CSA/BMI ratio, lumbar indentation value (MRI) | Goutallier classification (MRI) | BMI | ||
| Su et al., 2022 [64] | Chinese men and women (≥65 years), N = 2994 (1424 M:1570 F), 71.9 ± 4.9 yrs | “Our data shows that serum concentrations of individual AAs can be considered biomarkers of muscle health in the older people…” | Body composition, muscle strength and function, and metabolic variables | Lean muscle mass and ALM (DXA) | Grip strength | Gait speed (6 m walk test) and five-time chair stand test | Blood: serum amino acids concentrations Dietary inflammatory index and risk of Sarcopenia BMI | |
| Tan et al., 2022 [65] | Community-dwelling ambulatory older multi-ethnic Asian patients with Type-2 Diabetes Mellitus, N = 387 (184 M:164 F), 68.4 ± 5.6 yrs (60–89 yrs) | “…muscle health parameters including muscle mass, strength and gait speed…” | Muscle mass, strength, and function | Muscle mass and SMI (BIA) | Grip strength | Gait speed (6 m walk test) | Physical activity: IPAQ, PASE QoL: World Health Organization Quality of Life scale Systolic and diastolic blood pressures Blood: HbA1c, total cholesterol, HDL, LDL, TG BMI | |
| Vingren et al., 2018 [69] | Men living with Human Immunodeficiency Virus undergoing 60-day inpatient substance abuse treatment, N = 16, 42 ± 11 yrs | “…muscle health markers (mass, strength, power).” | Muscle mass, strength, power, and biochemical analysis | Muscle mass estimation (using anthropometric measurements) | Max strength and power (bench press, standing isometric squat) | Vertical jump performance | Blood: IFNγ, IL-1β, IL-2, IL-4, IL-6, IL-10, and tumor necrosis factor (TNF)-α, vascular cell adhesion molecule–1 and cortisol Skinfold thickness, body segment circumferences (upper-arm and forearm) | |
| Virk et al., 2021 [70] | Patients with lumbar spine pathology requiring operation, N = 307 (166 M:141 F), 56.1 ± 16.7 yrs | “…muscle health measurements including lumbar indentation value (LIV), paralumbar cross-sectional area divided by body mass index (PL-CSA/BMI), and Goutallier classification of fatty atrophy.” | Muscle size, quality | LIV and PL-CSA/BMI ratio (MRI) | Goutallier classification of fatty atrophy (MRI) | HRQOLs questionnaires: visual analog pain scale back, visual analog pain scale leg, PROMIS scores, Oswestry disability index, short-form 12 mental health score, and short-form 12 physical health score BMI | ||
| Virk et al., 2021 [71] | Patients with lumbar spine pathology requiring operation, N = 308 (168 M:140 F), 57.7 ± 18.2 yrs | “We measured muscle health by the lumbar indentation value (LIV), Goutallier classification (GC), and ratio of paralumbar muscle cross-sectional area over body mass index (PL-CSA/BMI). A muscle health grade was derived based on whether a measurement showed a statistically significant impact on visual analog scale back and leg pain.” | Muscle size, health related QoL | LIV and PL-CSA/BMI ratio (MRI) | Goutallier classification of fatty atrophy (MRI) | HRQOLs questionnaires: visual analog pain scale back, visual analog pain scale leg, PROMIS scores, Oswestry disability index, short-form 12 mental health score, and short-form 12 physical health score BMI | ||
| Yuan et al., 2024 [86] | Older adults in long-term care facilities, N = 74 (22 M:52 F), 84.9 ± 7.0 yrs | Muscle health-related indicator: lean mass (SLM, SMM, ASMM, and SMI), handgrip strength, five-time chair stand, and SPPB | Muscle mass, strength, function, and QoL | SLM, SMM, ASM, and SMI (BIA) | Grip strength | Gait speed (6 m walk test), five-time chair stand test, and SPPB | Calf circumference Energy and macronutrient intake QoL | |
| Zhao et al., 2023 [87] | Chinese community-dwelling older women > 65 yrs: N = 57, 70.6 ± 4.9 yrs Normal older women: N = 10, 70.4 ± 4.4 yrs Older women with pre-Sarcopenia or sarcopenia: N = 9, 70.9 ± 3.8 yrs Older women with exercise habits: N = 10, 70 ± 3.7 yrs | “In this study, several indicators were selected to reflect muscle health including muscle mass, grip strength, 30 s chair stand, arm curl with a dumbbell, and preferred and maximal gait speed….” | Body size, muscle mass, strength, function | Upper and lower limb skeletal muscle mass and appendicular muscle mass (DXA) | Grip strength | Gait speed (preferred and maximal), chair stand test (30 s), and arm curl reps (2 kg) | BMI | |
| Zhu et al., 2015 [73] | Healthy older postmenopausal women, N = 196, 74.3 ± 2.7 yrs | “Over the 2 y, we observed a reduction in the upper arm and calf muscle areas and a decrease in hand-grip strength in women in both the protein and the placebo groups, indicating deterioration in muscle health with aging.” | Muscle mass and function | ASMM (DXA) and upper arm and calf muscle CSA (peripheral quantitative CT) | Ankle dorsiflexion, knee flexor, knee extensor, hip abductor, hip flexor, hip extensor, and hip adductor strength (strain gauge) and grip strength | TUG | Dietary intake, 24 h urinary nitrogen, and levels of physical activity BMI |
| Study | Population | Measured | Body Composition | Tissue Composition | Performance | Functional Tasks | Other |
|---|---|---|---|---|---|---|---|
| Andreo-López et al., 2023 [74] | Adults with type 1 diabetes mellitus, N = 62 (21 M:41 F), 38 ± 14 yrs | Body size, composition, strength, and metabolic variables | FFM, FM, total body water, extracellular water, body cellular mass index, SMI, ASMI, and FFM index (BIA) | Grip strength | Blood: Fasting blood glucose, total cholesterol, LDL and HDL cholesterol, triglycerides, albumin, prealbumin, and C reactive protein, glycated hemoglobin A1c, daily total dose insulin, daily total dose insulin per kilogram, and insulin sensitivity factor Lifestyle Parameters: 14-item PREDIMED questionnaire, IPAQ Risk for Sarcopenia BMI | ||
| Arentson-Lantz et al., 2019 [32] | Healthy older adults, N = 17 (11 M: 6 F), 68 ± 2 yrs | Muscle mass, composition, and metabolic variables | WBLM, WBFM, and LLM (DXA) | CSA and single fiber volume (biopsy with immunohistochemical analysis) | Isokinetic knee extension peak torque (dynamometry) | Dietary intake and step count Blood: blood glucose and plasma insulin (ELISA) BMI | |
| Arentson-Lantz et al., 2019 [33] | Healthy older (60–80 years) adults, N = 20 (12 M: 8 F), 68.5 ± 1.5 yrs | Body composition, strength, physical function, and metabolic variables | WBLM, WBFM, and LLM (DXA) | Isokinetic knee extension peak torque (dynamometry) | SPPB and peak aerobic capacity (cycle ergonomic test) | Mean Daily Energy and Macronutrient Intake Blood: blood glucose and serum insulin (ELISA) BMI | |
| Arentson-Lantz et al., 2020 [34] | Healthy older (60–80 years) adults, N = 20 (14 M: 6 F), 67.8 ± 1.1 yrs | Body composition, strength, physical function, and dietary intake | WBLM, WBFM, and LLM (DXA) | CSA and single fiber volume (immunohistochemical analysis), protein content—signaling protein expression levels and single fiber characteristics (muscle biopsy—radioimmunoprecipitation assay buffer), | Isokinetic knee extension peak torque (dynamometry) | SPPB and peak aerobic capacity (cycle ergonomic test) | Mean Daily Energy and Macronutrient Intake BMI |
| Bislev et al., 2019 [37] | Postmenopausal women, N = 104, 64.5 yrs (61–68) | Mass, function, physical performance, QoL, and metabolic variables | ALM and FM (DXA) | Maximum voluntary isometric muscle strength, maximum force production (elbow flexion and elbow extension, knee flexion [dynamometry]), and grip strength | TUG, postural stability, and chair rising test | Blood: PTH, 25(OH)D, phosphate, ionized calcium, magnesium, creatinine, and thyroid stimulating hormone Urine: Calcium, phosphate, and magnesium Self-reported physical activity, primary hyperparathyroidism-QoL, and SF36v2 BMI | |
| Bislev et al., 2020 [38] | Healthy postmenopausal women with secondary hyperparathyroidism and vitamin D insufficiency, N = 81, 65 (IQR: 61–68.4) yrs | Muscle strength and function, cardiovascular health, and metabolic variables | ASMI and FMI (DXA) | Maximum voluntary isometric muscle strength, maximum force production (elbow flexion and elbow extension, knee flexion [dynamometry]), and grip strength | TUG | Blood: 25(OH)D, 1,25(OH)2D, PTH, Ca2+, magnesium, phosphate, eGFR, total cholesterol, HDL, LDL, and triglycerides Urine: Creatinine, plasma glucose and lipid profile: hydroxybutyrate, acetate, acetoacetate, acetone, alanine, betaine, carnitine, choline, citrate, creatine, creatinine, dimethylamine, formate, glucose, glutamate, glutamine, glycerol, glycine, isoleucine, lactate, leucine, lysine, methionine, o-phosphocholine, ornithine, phenylalanine, proline, pyruvate, succinate, threonine, trimethylamine n-oxide, tyrosine, urea, valine, τ-methylhistidine Calcium intake Cardiovascular health: blood pressure and arterial stiffness BMI | |
| Cha et al., 2022 [40] | CKD patients, N = 150 (97 M: 53 F), 65.0 ± 10.8 yrs | Muscle mass, performance, strength, and metabolic variables | Body composition (BIA) | Grip strength | Gait speed (6 m walk test) | Blood: Indoxyl sulfate, TNF-α, IL-6, myostatin, serum creatinine, eGFR Kidney disease QoL, IPAQ | |
| Engelen et al., 2023 [42] | Moderate to severe COPD patients and healthy controls, N = 416 (190 M: 226 F), 68.1 yrs (65.5–71.0) | Muscle mass, strength, respiratory function and metabolic variables | WBFM, extremity FM, FFM, and bone mineral density of spine and hip, ASMI and visceral adipose tissue (DXA) | Maximal leg extension force—one-leg reciprocal extensions (dynamometry), and grip strength. | Blood: Arginine, citrulline, glutamate, glutamine, glycine, histidine, hydroxyproline, isoleucine, leucine, ornithine, phenylalanine, tau-methyl-histidine, taurine, tryptophan, tyrosine, and valine Gynoid to android ratio (DXA) Habitual dietary intake and physical activity level, level of dyspnea, COPD assessment test Respiratory muscle function (hand-held mouth pressure device). BMI | ||
| English et al., 2016 [44] | Middle-aged adults, N = 19 (12 M: 7 F), 51.5 ± 1 yrs | Muscle mass, function, and quality | WBLM, WBFM, LLM, and body fat percentage (DXA) | Muscle quality (knee extensor peak torque divided by LLM) | Unilateral knee and ankle extensor strength and knee muscle endurance (dynamometer) | Peak aerobic capacity (cycle ergometer) | Dietary intake, Cell signaling and skeletal muscle protein synthesis (muscle biopsy) BMI |
| Fujie et al., 2024 [90] | Elderly women, N = 81, 67.2 ± 5.3 yrs | Muscle mass, quality, strength, and metabolic variables | Quadriceps muscle CSA (MRI), thickness, and echogenicity (US) | 1- Repetition Maximum leg extension and biceps curl | Blood: Total cholesterol, HDL, triglycerides, angiotensin II, endothelin-1, complement component 1q, creatinine, and plasma renin activity Blood pressure, heart rate, carotid-femoral pulse wave velocity, carotid β-stiffness | ||
| Gil et al., 2022 [46] | Hospitalized COVID-19 survivors N = 80 (41 M: 39 F), 59 ± 14 yrs | Muscle strength and size | CSA (US) | Grip strength | Self-perception of health BMI | ||
| Granic et al., 2018 [47] | Community-dwelling participants, N = 722 (289 M: 433 F), 85+ yrs | Strength, function, protein intake, and physical activity | FM and FFM (BIA) | Grip strength | TUG | Protein intake: 24 h multiple-pass dietary recall Self-reported physical activity BMI | |
| Groenendijk et al., 2020 [48] | Geriatric hip fracture patients, N = 40 (11 M: 29 F), 82 ± 8.0 yrs | Muscle mass and strength | ASMM (BIA), muscle thickness (US) | Grip strength | Nutritional status and dietary intake Risk for Sarcopenia | ||
| Huang et al., 2023 [77] | Healthy Chinese children 6–9 yrs, N = 426 (243 M: 183 F), median 8.0 yrs (IQR = 7.3–8.8 yrs) | Muscle mass, strength, and metabolic variables | ASMM (DXA) | Grip strength | Blood: plasma retinol, plasma ɑ-tocopherol Energy and nutrient intake BMI | ||
| Kang et al., 2024 [91] | Elderly adults >60 yrs, N = 100 (12 M: 88 F), 65 ± 4 yrs | Muscle strength, physical function, and muscle related hormones | Muscle mass (DXA) | Knee extension torques (isokinetic dynamometry) and grip strength | SPPB, TUG, gait speed | Blood: myostatin, follistatin, and high-sensitivity C-reactive protein | |
| Kang et al., 2024 [92] | Older adults, N = 575 (274 M: 301 F), 50–95 yrs | Body composition, muscle and fat mass, strength, and metabolic variables | FM, lean soft tissue, appendicular skeletal muscle mass, visceral adipose tissue, android and gynoid FM ratio (DXA) | Concentric peak torque (isokinetic dynamometer) and grip strength | Blood: amino acid concentrations, C-reactive protein, aspartate, glutamate, hydroxyproline, asparagine, glutamine, citrulline, serine, glycine, arginine, threonine, alanine, taurine, proline, tau-methylhistidine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, histidine, lysine, tyrosine Respiratory muscle function: Maximal inspiratory pressure PASE and cognitive questionnaire Dietary intake BMI, blood pressure | ||
| Kao et al., 2025 [93] | Adults ≥ 65 yrs at risk of malnutrition and sarcopenia, N = 97 (24 M: 73 F), 72.4 ± 5.2 yrs | Body composition, strength, function, and metabolic variables | ASM, body fat %, skeletal muscle mass (BIA) | Grip strength | SPPB, 5-time STS, 6 m walk time | Blood: fasting glucose, HbA1c, insulin, homocysteine, creatine, other health measures for cardiometabolic risk factors, renal and liver function SARC-F, SARC-combined with calf circumference, mini nutritional assessment-short form, mini-mental state examination, geriatric depression scale-15 Waist and hip circumference, total body water, BMI | |
| Korzepa et al., 2025 [94] | Healthy middle-to-older adults, N = 22 (11 M; 11 F), 61.3 ± 6.5 (50–70) yrs | Body composition, and metabolic variables | Body fat % (DXA) | Blood: plasma glucose, insulin, AA concentration, appetite hormones Respiratory exchange ratio, resting metabolic rate BMI | |||
| Lee et al., 2025 [95] | Healthy older adults, N = 119 (39 M: 61 F), (65–85) yrs | Body composition, strength, endurance, function, and metabolic variables | Body fat % (BIA) | 30 s arm curl test and grip strength | 10 m walk test, 30 s STS, TUG, and 3 min incremental step-test | Blood: HbA1c, creatinine, glucose, testosterone, cystatin C, insulin, and measures for liver function, kidney function, blood lipids, and other biomarkers | |
| Li et al., 2021 [51] | Chinese older adults with low lean mass, N = 123 (61 M: 62 F), 70 ± 4 yrs | Lean muscle mass, strength and physical performance | ASMI and lean mass (DXA) | Grip strength | SPPB | Daily dietary intake and physical activity level BMI | |
| Locquet et al., 2018 [52] | Community-dwelling older subjects, N = 288 (118 M: 170 F), 74.7 ± 5.7 yrs | Muscle mass, strength and physical performance | SMI and areal bone mineral density (DXA) | Grip strength | SPPB | Skeletal status, fracture risk, and risk of Sarcopenia BMI | |
| Matsumoto et al., 2023 [54] | Stroke patients with sarcopenia hospitalized, N = 241 (107 M: 134 F), 79.3 ± 10 yrs | Muscle mass, strength, and metabolic variables | SMI (BIA) | Grip strength | Blood: Albumin, c-reactive protein, and hemoglobin Functional independence measure score, ADL assessment, nutritional intake, and risk of Sarcopenia BMI | ||
| Peng et al., 2022 [57] | Middle aged and older adults, N = 103 (35 M: 68 F), 64.0 ± 8.2 yrs | Muscle size, composition, strength, performance, and metabolic variables | Total FM and FFM (BIA), and relative ASMM (MRI) | IMAT and CSA (MRI) | Grip strength | Gait speed (6 m walk test) | Blood: Serum albumin, alanine aminotransferase, uric acid, total cholesterol, HDL, LDL, triglyceride, serum creatinine, high-sensitivity C-reactive protein, and fasting glucose; Whole blood glycated hemoglobin Cognitive function, nutritional and mood status IPAQ, BMI |
| Peng et al., 2024 [79] | Adults with inadequate protein intake, N = 97 (18 M: 79 F), 64.7 ± 4.8 yrs | Muscle size, strength, physical function, metabolic variables and quality of life | Relative ASMM (BIA) | Body fat percentage (BIA) | Grip strength | Usual gait speed (6 m), 6 min walk test, and five-time chair stand test | Blood: Albumin, creatinine, alanine aminotransferase, total cholesterol, HDL, LDL, uric acid, fasting glucose, dehydroepiandrosterone sulfate, insulin-like growth factor-1, homocysteine, high-sensitive c-reactive protein, vitamin D3, glycated hemoglobin, myostatin, and leptin Cognition: MoCA, CES-D, IPAQ Nutritional status SF-36, BMI |
| Pérez-Piñero et al., 2021 [58] | Caucasian men and postmenopausal women, N = 45 (8 M: 37 F), 58.9 ± 6.1 (50–75) yrs | Muscle mass, function, strength, quality, and metabolic variables | FM, lean mass, muscle mass, and ASMM (DXA) | Muscle quality (muscle mass between the peak torques) | Knee extension torques (isokinetic and isometric dynamometry) and grip strength | Blood pressure, health-related QoL, SF-36, dietary intake BMI | |
| Raghupathy et al., 2023 [80] | Adults and children, N = 962 (428 M: 534 F), 60 ± 9 (5–70) yrs | Body size, muscle composition, quality, strength, physical activity level, and blood markers of inflammation | ALM (DXA), subcutaneous and visceral adipose tissue (CT) | Upper extremity muscle quality (strength per kilogram of lean mass) | Knee extension (hand-held isometric dynamometry) and grip strength | Blood: IL-6, monocyte chemoattractant protein-1, resistin, and adiponectin (ELISA) Physical activity BMI | |
| Rousseau et al., 2015 [60] | Adults with thermal burns, N = 15 (11 M: 4 F), 50 (25–64) yrs | Muscle strength and metabolic variables | Bone mineral density (DXA) | Knee muscle strength (isokinetic dynamometry) | Blood: 25OH–D, 1,25(OH)2–D, calcium, fibroblast growth factor 2, PTH, phosphate, creatine, collagen type 1 cross-linked C-telopeptide, serum type 1 procollagen N-terminal and serum bone alkaline phosphatase | ||
| Sabir et al., 2023 [81] | Norwegian adults, N = 1317 (578 M: 739 F), 67–70 yrs | Muscle mass, body composition, strength, physical activity, and habitual dietary intake | SMM, ASMM, ASMI, total body FM and percentage (BIA) | Grip strength | Habitual dietary intake Self-reported physical activity BMI | ||
| Schneider et al., 2015 [61] | Healthy adults in microgravity environments, N = 11 (9 M: 2 F), 40 ± 7 yrs | Mechanical properties of skeletal muscles and tendons | Oscillation frequency (Hz), dynamic stiffness (N/m), elasticity, mechanical stress relaxation (ms) time, creep (Deborah number) (MyotonPRO device) BMI | ||||
| Seo et al., 2024 [82] | Healthy adult golfers, N = 57 (27 M: 30 F), ~59 ± 9.5 (26–64) yrs | Body size, body composition, muscle strength, golf performance, physical function, and metabolic variables | SMM and FM (BIA) | Knee extension and flexion strength (dynamometry) and grip strength | Golf drive distance, club-head speed, ball speed, 2 min push-up test, and MFT balance test | Blood: lactic acid, creatine, lactate dehydrogenase, creatine kinase, blood urea nitrogen, red blood cell, white blood cell, hemoglobin, platelet, hematocrit, glucose, aspartate aminotransferase, alanine transaminase, and gamma-glutamyl transferase Dietary intake and levels of physical activity Blood pressure, heart rate, BMI | |
| Van Ancum et al., 2020 [66] | Community-dwelling adults, N = 197 (57 M: 140 F), 67.9 (57–75.1) yrs | Body composition, muscle mass, strength, and function | SMM, SMI, ALM, ALM/height2, SMM and ALM relative to body weight (BIA) | Grip strength | Gait speed (4 m walk test) | Self-reported levels of physical activity, ADL, and risk of Sarcopenia BMI | |
| Van Dongen et al., 2020 [67] | Community-dwelling older adults, N = 168, (66 M:102 F), 75 ± 6 yrs | Body composition and mass, muscle strength and function | Lean body mass, ALM, and FM (DXA) | Lower limb 3-Repetition Maximum test (leg press and leg extension machines) and knee extension strength (dynamometry) | Gait speed (6 min walk test and 4 m walk test), SPPB, and TUG | QoL, ADL, nutritional status, dietary intake, and risk of Sarcopenia BMI | |
| Vesey et al., 2020 [68] | Children and adolescents with conditions that impacted musculoskeletal health, N = 17, 15.7 ± 2.9 yrs | Body composition and function | Whole body: FM, lean mass, bone mineral content, and bone mineral density Lumbar spine: bonce mineral content and bone mineral density (DXA) | Gait speed (6 min walk test), chair stand test, balance test, and single leg jump test | BMI | ||
| Vitale et al., 2020 [72] | Healthy older adults, N = 9 (3 M: 6 F), 68 ± 7 (62.9–73.1) yrs | Body composition, muscle strength and function | Lean mass, FM, ASMI (DXA) and CSA of thigh (MRI) | Maximum isometric strength of knee flexor and extensor (dynamometry) and grip strength | Chair stand test (30 s) and Mini balance evaluation systems test | BMI | |
| Xiong et al., 2024 [84] | Older adults with high fall risk, N = 160, 68.5 ± 8.9 (65–85) yrs | Muscle mass and function | Bone mineral density and lower limb muscle mass (DXA) | Berg balance scale, TUG, chair stand test (30 s), and fall-risk assessment tool | Fall-risk questionnaire | ||
| Yoshimura et al., 2024 [85] | Stroke patients, N = 955 (511 M: 443 F), 73.2 ± 13.3 yrs | Muscle mass, strength, and metabolic variables | SMI (BIA) | Grip strength | Blood: Albumin, hemoglobin, c-reactive protein Energy and protein intake and pre-stroke ADL |

4. Discussion
4.1. Common Elements of Muscle Health
4.2. Implications for Muscle Health Assessment
4.3. Toward a Standardized Approach to Assessing Muscle Health
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADL | Activities of daily living |
| IADL | Instrumental activities of daily living |
| ICF | International Classification of Functioning, Disability and Health |
| CT | Computed tomography |
| SPPB | Short physical performance battery |
| TUG | Timed up-and-go |
| BMI | Body-mass index |
| DXA | Dual-energy X-ray absorptiometry |
| BIA | Bio-electrical impedance |
| MRI | Magnetic resonance imaging |
| GLIS | Global Leadership Initiative in Sarcopenia |
References
- Miljkovic, I.; Zmuda, J.M. Epidemiology of Myosteatosis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 260–264. [Google Scholar] [CrossRef]
- Correa-de-Araujo, R.; Harris-Love, M.O.; Miljkovic, I.; Fragala, M.S.; Anthony, B.W.; Manini, T.M. The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report. Front. Physiol. 2017, 8, 87. [Google Scholar] [CrossRef]
- Chao, Y.-P.; Fang, W.-H.; Chen, W.-L.; Peng, T.-C.; Yang, W.-S.; Kao, T.-W. Exploring muscle health deterioration and Its determinants among community-dwelling older adults. Front. Nutr. 2022, 9, 817044. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Landi, F.; Chew, S.T.H.; Atherton, P.J.; Molinger, J.; Ruck, T.; Gonzalez, M.C. Advances in muscle health and nutrition: A toolkit for healthcare professionals. Clin. Nutr. 2022, 41, 2244–2263. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Prado, C.M.; Gonzalez, M.C. Skeletal muscle-focused guideline development: Hierarchical model incorporating muscle form, function, and clinical outcomes. Appl. Physiol. Nutr. Metab. 2023, 48, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Falcon, L.; Harris-Love, M.O. Sarcopenia and the new ICD-10-CM code: Screening, staging, and diagnosis considerations. Fed. Pract. 2017, 34, 24–32. [Google Scholar] [CrossRef]
- Chester, J.G.; Rudolph, J.L. Vital signs in older patients: Age-related changes. J. Am. Med. Dir. Assoc. 2011, 12, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Schlicht, J.A.; Wingood, M.; Heled, Y.; Weitzel, K.; Rogers, M.E.; Seffens, P.R. The physical activity vital sign for older adults: Time for an update. J. Am. Med. Dir. Assoc. 2024, 25, 105020. [Google Scholar] [CrossRef]
- Carlson, C.; Merel, S.E.; Yukawa, M. Geriatric syndromes and geriatric assessment for the generalist. Med. Clin. N. Am. 2015, 99, 263–279. [Google Scholar] [CrossRef]
- Peterson, M.D.; Collins, S.; Meier, H.C.S.; Brahmsteadt, A.; Faul, J.D. Grip strength is inversely associated with DNA methylation age acceleration. J. Cachexia Sarcopenia Muscle 2023, 14, 108–115. [Google Scholar] [CrossRef]
- Chai, L.; Zhang, D.; Fan, J. Comparison of grip strength measurements for predicting all-cause mortality among adults aged 20+ years from the NHANES 2011–2014. Sci. Rep. 2024, 14, 29245. [Google Scholar] [CrossRef]
- Kaczorowska, A.; Kozieł, S.; Ignasiak, Z. Hand grip strength and quality of life among adults aged 50–90 years from South West Poland. Sci. Rep. 2025, 15, 882. [Google Scholar] [CrossRef]
- World Health Organization. Basic Documents: (Including Amendments Adopted Up to 31 May 2019), 49th ed.; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-000051-3. [Google Scholar]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36)—I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife hand grip strength as a predictor of old age disability. JAMA 1999, 281, 558–560. [Google Scholar] [CrossRef]
- Larsson, L.; Grimby, G.; Karlsson, J. Muscle strength and speed of movement in relation to age and muscle morphology. J. Appl. Physiol. 1979, 46, 451–456. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability and Health (ICF); World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Millán-Calenti, J.C.; Tubío, J.; Pita-Fernández, S.; González-Abraldes, I.; Lorenzo, T.; Fernández-Arruty, T.; Maseda, A. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch. Gerontol. Geriatr. 2010, 50, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.M.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 3–25. [Google Scholar] [CrossRef]
- Hirani, V.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Seibel, M.J.; Waite, L.M.; Handelsman, D.J.; Cumming, R.G. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing 2017, 46, 413–420. [Google Scholar] [CrossRef]
- Broadwin, J.; Goodman-Gruen, D.; Slymen, D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J. Am. Geriatr. Soc. 2001, 49, 1641–1645. [Google Scholar] [CrossRef]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. Ser. A 2005, 60, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Meskers, C.G.M.; Reijnierse, E.M.; Numans, S.T.; Kruizinga, R.C.; Pierik, V.D.; van Ancum, J.M.; Slee-Valentijn, M.; Scheerman, K.; Verlaan, S.; Maier, A.B. Association of handgrip strength and muscle mass with dependency in (instrumental) activities of daily living in hospitalized older adults -the EMPOWER study. J. Nutr. Health Aging 2019, 23, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Fox, K.M.; Gandra, S.R.; Delmonico, M.J.; Chiou, C.-F.; Anthony, M.S.; Sewall, A.; Goodpaster, B.; Satterfield, S.; Cummings, S.R.; et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J. Am. Geriatr. Soc. 2009, 57, 1411–1419. [Google Scholar] [CrossRef]
- Koipysheva, E.A.; Lebedinsky, V.Y.; Koipysheva, M.A. Physical Health (Definition, Semantic Content, Study Prospects). In The European Proceedings of Social & Behavioural Sciences; Nicosia: Irkutsk, Russia, 2018; pp. 601–605. ISBN 2357-1330. [Google Scholar]
- Leonardi, M.; Lee, H.; Kostanjsek, N.; Fornari, A.; Raggi, A.; Martinuzzi, A.; Yáñez, M.; Almborg, A.-H.; Fresk, M.; Besstrashnova, Y.; et al. 20 Years of ICF—International Classification of Functioning, Disability and Health: Uses and Applications around the World. Int. J. Environ. Res. Public Health 2022, 19, 11321. [Google Scholar] [CrossRef] [PubMed]
- Forstner, M. Conceptual models of disability: The development of the consideration of non-biomedical aspects. Disabilities 2022, 2, 540–563. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Anderson, B.; Ordaz, A.; Zlomislic, V.; Allen, R.T.; Garfin, S.R.; Schuepbach, R.; Farshad, M.; Schenk, S.; Ward, S.R.; Shahidi, B. Paraspinal muscle health is related to fibrogenic, adipogenic, and myogenic gene expression in patients with lumbar spine pathology. BMC Musculoskelet. Disord. 2022, 23, 608. [Google Scholar] [CrossRef]
- Arentson-Lantz, E.; Galvan, E.; Wacher, A.; Fry, C.S.; Paddon-Jones, D. 2000 steps/say does not fully protect skeletal muscle health in older adults during bed rest. J. Aging Phys. Act. 2019, 27, 191–197. [Google Scholar] [CrossRef]
- Arentson-Lantz, E.J.; Galvan, E.; Ellison, J.; Wacher, A.; Paddon-Jones, D. Improving dietary protein quality reduces the negative effects of physical inactivity on body composition and muscle function. J. Gerontol. Ser. A 2019, 74, 1605–1611. [Google Scholar] [CrossRef]
- Arentson-Lantz, E.J.; Fiebig, K.N.; Anderson-Catania, K.J.; Deer, R.R.; Wacher, A.; Fry, C.S.; Lamon, S.; Paddon-Jones, D. Countering disuse atrophy in older adults with low-volume leucine supplementation. J. Appl. Physiol. 2020, 128, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Bathgate, K.E.; Bagley, J.R.; Jo, E.; Talmadge, R.J.; Tobias, I.S.; Brown, L.E.; Coburn, J.W.; Arevalo, J.A.; Segal, N.L.; Galpin, A.J. Muscle health and performance in monozygotic twins with 30 years of discordant exercise habits. Eur. J. Appl. Physiol. 2018, 118, 2097–2110. [Google Scholar] [CrossRef]
- Berry, D.B.; Padwal, J.; Johnson, S.; Englund, E.K.; Ward, S.R.; Shahidi, B. The effect of high-intensity resistance exercise on lumbar musculature in patients with low back pain: A preliminary study. BMC Musculoskelet. Disord. 2019, 20, 290. [Google Scholar] [CrossRef]
- Bislev, L.S.; Langagergaard Rødbro, L.; Sikjær, T.; Rejnmark, L. Effects of elevated parathyroid hormone levels on muscle health, postural stability and quality of life in vitamin D-insufficient healthy women: A cross-sectional study. Calcif. Tissue Int. 2019, 105, 642–650. [Google Scholar] [CrossRef]
- Bislev, L.S.; Sundekilde, U.K.; Kilic, E.; Dalsgaard, T.K.; Rejnmark, L.; Bertram, H.C. Circulating levels of muscle-related metabolites increase in response to a daily moderately high dose of a vitamin D3 supplement in women with vitamin D insufficiency—Secondary analysis of a randomized placebo-controlled trial. Nutrients 2020, 12, 1310. [Google Scholar] [CrossRef]
- Cegielski, J.; Brook, M.S.; Phillips, B.E.; Boereboom, C.; Gates, A.; Gladman, J.F.R.; Smith, K.; Wilkinson, D.J.; Atherton, P.J. The Combined Oral Stable Isotope Assessment of Muscle (COSIAM) reveals D-3 creatine derived muscle mass as a standout cross-sectional biomarker of muscle physiology vitality in older age. GeroScience 2022, 44, 2129–2138. [Google Scholar] [CrossRef]
- Cha, R.; Kang, S.H.; Han, M.Y.; An, W.S.; Kim, S.; Kim, J.C. Effects of AST-120 on muscle health and quality of life in chronic kidney disease patients: Results of RECOVERY study. J. Cachexia Sarcopenia Muscle 2022, 13, 397–408. [Google Scholar] [CrossRef]
- Davis, J.A.; Mohebbi, M.; Collier, F.; Loughman, A.; Staudacher, H.; Shivappa, N.; Hébert, J.R.; Pasco, J.A.; Jacka, F.N. The role of diet quality and dietary patterns in predicting muscle mass and function in men over a 15-year period. Osteoporos. Int. 2021, 32, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.P.K.J.; Kirschner, S.K.; Coyle, K.S.; Argyelan, D.; Neal, G.; Dasarathy, S.; Deutz, N.E.P. Sex related differences in muscle health and metabolism in chronic obstructive pulmonary disease. Clin. Nutr. 2023, 42, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.P.; Jonker, R.; Sulaiman, H.; Fisk, H.L.; Calder, P.C.; Deutz, N.E. ω-3 polyunsaturated fatty acid supplementation improves postabsorptive and prandial protein metabolism in patients with chronic obstructive pulmonary disease: A randomized clinical trial. Am. J. Clin. Nutr. 2022, 116, 686–698. [Google Scholar] [CrossRef]
- English, K.L.; Mettler, J.A.; Ellison, J.B.; Mamerow, M.M.; Arentson-Lantz, E.; Pattarini, J.M.; Ploutz-Snyder, R.; Sheffield-Moore, M.; Paddon-Jones, D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am. J. Clin. Nutr. 2016, 103, 465–473. [Google Scholar] [CrossRef]
- Finkel, R.S.; Finanger, E.; Vandenborne, K.; Sweeney, H.L.; Tennekoon, G.; Shieh, P.B.; Willcocks, R.; Walter, G.; Rooney, W.D.; Forbes, S.C.; et al. Disease-modifying effects of edasalonexent, an NF-κB inhibitor, in young boys with Duchenne muscular dystrophy: Results of the MoveDMD phase 2 and open label extension trial. Neuromuscul. Disord. 2021, 31, 385–396. [Google Scholar] [CrossRef]
- Gil, S.; De Oliveira Júnior, G.N.; Sarti, F.M.; Filho, W.J.; Longobardi, I.; Turri, J.A.O.; Shinjo, S.K.; Ferriolli, E.; Avelino-Silva, T.J.; Busse, A.L.; et al. Acute muscle mass loss predicts long-term fatigue, myalgia, and health care costs in COVID-19 survivors. J. Am. Med. Dir. Assoc. 2023, 24, 10–16. [Google Scholar] [CrossRef]
- Granic, A.; Mendonça, N.; Sayer, A.A.; Hill, T.R.; Davies, K.; Adamson, A.; Siervo, M.; Mathers, J.C.; Jagger, C. Low protein intake, muscle strength and physical performance in the very old: The Newcastle 85+ Study. Clin. Nutr. 2018, 37, 2260–2270. [Google Scholar] [CrossRef]
- Groenendijk, I.; Kramer, C.S.; Den Boeft, L.M.; Hobbelen, H.S.M.; Van Der Putten, G.-J.; De Groot, L.C.P.G.M. Hip fracture patients in geriatric rehabilitation show poor nutritional status, dietary intake and muscle health. Nutrients 2020, 12, 2528. [Google Scholar] [CrossRef]
- Jackson, K.L.; Gropper, S.S.; Hunt, D.; D’Avolio, D.; Newman, D. Effectiveness of a per-meal protein prescription and nutrition education with versus without diet coaching on dietary protein intake and muscle health in middle-aged women. Nutrients 2022, 14, 375. [Google Scholar] [CrossRef]
- Jacob, I.; Johnson, M.I.; Jones, G.; Jones, A.; Francis, P. Age-related differences of vastus lateralis muscle morphology, contractile properties, upper body grip strength and lower extremity functional capability in healthy adults aged 18 to 70 years. BMC Geriatr. 2022, 22, 538. [Google Scholar] [CrossRef]
- Li, C.; Meng, H.; Wu, S.; Fang, A.; Liao, G.; Tan, X.; Chen, P.; Wang, X.; Chen, S.; Zhu, H. Daily supplementation with whey, soy, or whey-soy blended protein for 6 months maintained lean muscle mass and physical performance in older adults with low lean mass. J. Acad. Nutr. Diet. 2021, 121, 1035–1048.e6. [Google Scholar] [CrossRef] [PubMed]
- Locquet, M.; Beaudart, C.; Bruyère, O.; Kanis, J.A.; Delandsheere, L.; Reginster, J.-Y. Bone health assessment in older people with or without muscle health impairment. Osteoporos. Int. 2018, 29, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Locquet, M.; Beaudart, C.; Reginster, J.-Y.; Bruyère, O. Association between the decline in muscle health and the decline in bone health in older individuals from the SarcoPhAge cohort. Calcif. Tissue Int. 2019, 104, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Yoshimura, Y.; Nagano, F.; Bise, T.; Kido, Y.; Shimazu, S.; Shiraishi, A. Statin use impairs muscle strength recovery in post-stroke patients with sarcopenia. Geriatr. Gerontol. Int. 2023, 23, 676–683. [Google Scholar] [CrossRef]
- Papaioannou, K.-G.; Nilsson, A.; Nilsson, L.M.; Kadi, F. Healthy eating is associated with sarcopenia risk in physically active older adults. Nutrients 2021, 13, 2813. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.H.; Gorzelitz, J.; Ngo-Huang, A.; Caan, B.J.; Prakash, L.; Garg, N.; Petzel, M.Q.B.; Schadler, K.; Basen-Engquist, K.; Katz, M.H.G. The role of home-based exercise in maintaining skeletal muscle during preoperative pancreatic cancer treatment. Integr. Cancer Ther. 2021, 20, 1534735420986615. [Google Scholar] [CrossRef]
- Peng, L.-N.; Lin, M.-H.; Lee, H.-F.; Hsu, C.-C.; Chang, S.-J.; Chen, L.-K. Clinical efficacy of oligonol® supplementation on metabolism and muscle health in middle-aged and older adults: A double-blinded randomized controlled trial. Arch. Gerontol. Geriatr. 2022, 103, 104784. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Piñero, S.; Ávila-Gandía, V.; Rubio Arias, J.A.; Muñoz-Carrillo, J.C.; Losada-Zafrilla, P.; López-Román, F.J. A 12-week randomized double-blind placebo-controlled clinical trial, evaluating the effect of supplementation with a spinach extract on skeletal muscle fitness in adults older than 50 years of age. Nutrients 2021, 13, 4373. [Google Scholar] [CrossRef]
- Pratt, J.; De Vito, G.; Narici, M.; Segurado, R.; Pessanha, L.; Dolan, J.; Conroy, J.; Boreham, C. Plasma C-terminal agrin fragment as an early biomarker for sarcopenia: Results from the GenoFit study. J. Gerontol. Ser. A 2021, 76, 2090–2096. [Google Scholar] [CrossRef]
- Rousseau, A.-F.; Foidart-Desalle, M.; Ledoux, D.; Remy, C.; Croisier, J.-L.; Damas, P.; Cavalier, E. Effects of cholecalciferol supplementation and optimized calcium intakes on vitamin D status, muscle strength and bone health: A one-year pilot randomized controlled trial in adults with severe burns. Burns 2015, 41, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Peipsi, A.; Stokes, M.; Knicker, A.; Abeln, V. Feasibility of monitoring muscle health in microgravity environments using Myoton technology. Med. Biol. Eng. Comput. 2015, 53, 57–66. [Google Scholar] [CrossRef]
- Shin, J.; Hwang, J.H.; Han, M.; Cha, R.-H.; Kang, S.H.; An, W.S.; Kim, J.C.; Kim, S.H. Phase angle as a marker for muscle health and quality of life in patients with chronic kidney disease. Clin. Nutr. 2022, 41, 1651–1659. [Google Scholar] [CrossRef]
- Song, J.; Araghi, K.; Dupont, M.M.; Shahi, P.; Bovonratwet, P.; Shinn, D.; Dalal, S.S.; Melissaridou, D.; Virk, S.S.; Iyer, S.; et al. Association between muscle health and patient-reported outcomes after lumbar microdiscectomy: Early results. Spine J. 2022, 22, 1677–1686. [Google Scholar] [CrossRef]
- Su, Y.; Elshorbagy, A.; Turner, C.; Refsum, H.; Kwok, T. The association of circulating amino acids and dietary inflammatory potential with muscle health in chinese community-dwelling older people. Nutrients 2022, 14, 2471. [Google Scholar] [CrossRef]
- Tan, N.C.; Sankari, U.; Ng, C.E.; Koh, Y.L.E. Longitudinal study on the progression of muscle status among community-dwelling ambulatory older multiethnic Asians with type 2 diabetes mellitus. BMC Geriatr. 2022, 22, 446. [Google Scholar] [CrossRef]
- Van Ancum, J.M.; Meskers, C.G.M.; Reijnierse, E.M.; Yeung, S.S.Y.; Jonkman, N.H.; Trappenburg, M.C.; Pijnappels, M.; Maier, A.B. Lack of knowledge contrasts the willingness to counteract sarcopenia among community-dwelling adults. J. Aging Health 2020, 32, 787–794. [Google Scholar] [CrossRef]
- Van Dongen, E.J.I.; Haveman-Nies, A.; Doets, E.L.; Dorhout, B.G.; De Groot, L.C.P.G.M. Effectiveness of a diet and resistance exercise intervention on muscle health in older adults: ProMuscle in practice. J. Am. Med. Dir. Assoc. 2020, 21, 1065–1072.e3. [Google Scholar] [CrossRef]
- Vesey, R.M.; Hofman, P.L.; Derraik, J.G.; Colle, P.; Biggs, J.B.; Munns, C.F.; Cutfield, W.S.; Gusso, S. Safety, feasibility and efficacy of side-alternating vibration therapy on bone and muscle health in children and adolescents with musculoskeletal disorders: A pilot trial. J. Paediatr. Child Health 2020, 56, 1257–1262. [Google Scholar] [CrossRef]
- Vingren, J.L.; Curtis, J.H.; Levitt, D.E.; Duplanty, A.A.; Lee, E.C.; McFarlin, B.K.; Hill, D.W. Adding resistance training to the standard of care for inpatient substance abuse treatment in men with human immunodeficiency virus improves skeletal muscle health without altering cytokine concentrations. J. Strength Cond. Res. 2018, 32, 76–82. [Google Scholar] [CrossRef]
- Virk, S.; Sandhu, M.; Wright-Chisem, J.; Vaishnav, A.; Albert, T.; Qureshi, S.A. The association between spondylolisthesis and decreased muscle health throughout the lumbar spine for patients with operative lumbar spinal stenosis. Eur. Spine J. 2021, 30, 2605–2612. [Google Scholar] [CrossRef]
- Virk, S.; Wright-Chisem, J.; Sandhu, M.; Vaishnav, A.; Albert, T.J.; Gang, C.H.; Qureshi, S. A novel magnetic resonance imaging-based lumbar muscle grade to predict health-related quality of life scores among patients requiring surgery. Spine 2021, 46, 259–267. [Google Scholar] [CrossRef]
- Vitale, J.A.; Bonato, M.; Borghi, S.; Messina, C.; Albano, D.; Corbetta, S.; Sconfienza, L.M.; Banfi, G. Home-based resistance training for older subjects during the COVID-19 outbreak in Italy: Preliminary results of a six-months RCT. Int. J. Environ. Res. Public Health 2020, 17, 9533. [Google Scholar] [CrossRef]
- Zhu, K.; Kerr, D.A.; Meng, X.; Devine, A.; Solah, V.; Binns, C.W.; Prince, R.L. Two-year whey protein supplementation did not enhance muscle mass and physical function in well-nourished healthy older postmenopausal women. J. Nutr. 2015, 145, 2520–2526. [Google Scholar] [CrossRef]
- Andreo-López, M.C.; Zarco-Martín, M.T.; Contreras-Bolívar, V.; Fernández-Soto, M.L. Prevalence of sarcopenia and dynapenia and related clinical outcomes in patients with type 1 diabetes mellitus. Nutrients 2023, 15, 4914. [Google Scholar] [CrossRef]
- Bauer, S.R.; Parker-Autry, C.; Lu, K.; Cummings, S.R.; Hepple, R.T.; Scherzer, R.; Covinsky, K.; Cawthon, P.M. Skeletal muscle health, physical performance, and lower urinary tract symptoms in older adults: The study of muscle, mobility, and aging. J. Gerontol. Ser. A 2024, 79, glad218. [Google Scholar] [CrossRef]
- Ferguson, C.E.; Hayes, K.; Tatucu-Babet, O.A.; Lambell, K.J.; Paul, E.; Hodgson, C.L.; Ridley, E.J. Nutrition delivery and the relationship with changes in muscle mass in adult patients receiving extracorporeal membrane oxygenation: A retrospective observational study. Aust. Crit. Care 2024, 37, 727–733. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Hong, Z.; Lin, X.; Chen, F.; Lan, J.; Zhang, Z.; Deng, H. Associations of plasma retinol and α-tocopherol levels with skeletal muscle health in Chinese children. Br. J. Nutr. 2023, 130, 2174–2181. [Google Scholar] [CrossRef]
- Olpe, T.; Wunderle, C.; Bargetzi, L.; Tribolet, P.; Laviano, A.; Stanga, Z.; Prado, C.M.; Mueller, B.; Schuetz, P. Muscle matters: Prognostic implications of malnutrition and muscle health parameters in patients with cancer. A secondary analysis of a randomised trial. Clin. Nutr. 2024, 43, 2255–2262. [Google Scholar] [CrossRef]
- Peng, L.; Lin, M.; Tseng, S.; Yen, K.; Lee, H.; Hsiao, F.; Chen, L. Protein-enriched soup and weekly exercise improve muscle health: A randomized trial in mid-to-old age with inadequate protein intake. J. Cachexia Sarcopenia Muscle 2024, 15, 1348–1357. [Google Scholar] [CrossRef]
- Raghupathy, R.; McLean, R.R.; Kiel, D.P.; Hannan, M.T.; Sahni, S. Higher abdominal adiposity is associated with higher lean muscle mass but lower muscle quality in middle-aged and older men and women: The Framingham Heart Study. Aging Clin. Exp. Res. 2023, 35, 1477–1485. [Google Scholar] [CrossRef]
- Sabir, Z.; Dierkes, J.; Hjartåker, A.; Rosendahl-Riise, H. The association of dietary patterns with muscle mass and strength in old age: The Hordaland Health Study. Eur. J. Nutr. 2023, 62, 2739–2750. [Google Scholar] [CrossRef]
- Seo, J.-W.; Jiang, S.; Ahn, S.; Kang, Y.S.; Sung, Y.; Li, X.; Jamrasi, P.; Sun, E.M.; Yoo, J.; Kim, B.-Y.; et al. Effect of mixed protein supplementation on golf performance and muscle function: A randomized, double-blind, placebo-controlled study. J. Int. Soc. Sports Nutr. 2024, 21, 2393368. [Google Scholar] [CrossRef]
- Song, J.; Shahsavarani, S.; Vatsia, S.; Katz, A.D.; Ngan, A.; Fallon, J.; Strigenz, A.; Seitz, M.; Silber, J.; Essig, D.; et al. Association between history of lumbar spine surgery and paralumbar muscle health: A propensity score-matched analysis. Spine J. 2023, 23, 1659–1666. [Google Scholar] [CrossRef]
- Xiong, X.; Zang, J.; Zhu, C.; Wei, W.; Wang, P.; Wang, J.; Gao, Q. Effects of proprioceptive neuromuscular facilitation technique on balance function and muscle health in older adults with high fall risk. J. Gerontol. Nurs. 2024, 50, 37–44. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Nagano, F.; Matsumoto, A.; Shimazu, S.; Shiraishi, A.; Kido, Y.; Bise, T.; Kuzuhara, A.; Hori, K.; Hamada, T.; et al. Low hemoglobin levels are associated with compromised muscle health: Insights from a post-stroke rehabilitation cohort. Geriatr. Gerontol. Int. 2024, 24, 305–311. [Google Scholar] [CrossRef]
- Yuan, W.; Chen, M.; Chen, Y.; Xu, D.; Li, Z.; Bai, H.; Xu, Q.; Jiang, Y.; Gu, J.; Li, S.; et al. Effects of soy protein-rich meals on muscle health of older adults in long-term care: A randomized clinical trial. Nutrition 2024, 126, 112507. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, J.; Liu, N. Developing a predictive equation of muscular age to evaluate muscle health in Chinese community-dwelling older women. Health Care Women Int. 2023, 44, 1587–1600. [Google Scholar] [CrossRef]
- Bhandari, R.; Lukas, K.; Lee, K.; Shamunee, J.; Almeida, B.; Guzman, T.; Echevarria, M.; Lindenfeld, L.; Nenninger, C.; Iukuridze, A.; et al. Feasibility of telehealth exercise and nicotinamide riboside supplementation in survivors of childhood cancer at risk for diabetes: A pilot randomized controlled trial. Pediatr. Blood Cancer 2025, 72, e31369. [Google Scholar] [CrossRef]
- Distefano, G.; Harrison, S.; Lynch, J.; Link, T.M.; Kramer, P.A.; Ramos, S.V.; Mau, T.; Coen, P.M.; Sparks, L.M.; Goodpaster, B.H.; et al. Skeletal muscle composition, power, and mitochondrial energetics in older men and women with knee osteoarthritis. Arthritis Rheumatol. 2024, 76, 1764–1774. [Google Scholar] [CrossRef]
- Fujie, S.; Horii, N.; Kajimoto, H.; Yamazaki, H.; Inoue, K.; Iemitsu, K.; Uchida, M.; Arimitsu, T.; Shinohara, Y.; Sanada, K.; et al. Impact of resistance training and chicken intake on vascular and muscle health in elderly women. J. Cachexia Sarcopenia Muscle 2025, 16, jcsm.13572. [Google Scholar] [CrossRef]
- Kang, C.-H.; Jung, E.-S.; Jung, S.-J.; Han, Y.-H.; Chae, S.-W.; Jeong, D.Y.; Kim, B.-C.; Lee, S.-O.; Yoon, S.-J. Pasteurized Akkermansia muciniphila HB05 (HB05P) improves muscle strength and function: A 12-Week, randomized, double-blind, placebo-controlled clinical trial. Nutrients 2024, 16, 4037. [Google Scholar] [CrossRef]
- Kang, M.C.; Deutz, N.E.P.; Kirschner, S.K.; Engelen, M.P.K.J. Metabolic kinetics and muscle and brain health markers in older adults, and the role of age and presence of chronic morbidities: A large cross-sectional cohort study. Clin. Nutr. 2024, 43, 36–47. [Google Scholar] [CrossRef]
- Kao, S.-L.; Wang, J.-H.; Lai, H.-Y.; Hsiao, F.-Y.; Chen, L.-K.; Loh, C.-H. Daily supplementation with protein-enriched lacto-vegetarian soups and muscle health in community-dwelling older adults: A randomized controlled trial. J. Nutr. Health Aging 2025, 29, 100477. [Google Scholar] [CrossRef]
- Korzepa, M.; Marshall, R.N.; Rogers, L.M.; Belfield, A.E.; Quinlan, J.I.; Huang, Y.; Gritsas, A.; Churchward-Venne, T.A.; Glover, E.I.; Van Loon, L.J.C.; et al. Postprandial plasma amino acid and appetite responses to a low protein breakfast supplemented with whey or pea protein in middle-to-older aged adults. Eur. J. Nutr. 2025, 64, 86. [Google Scholar] [CrossRef]
- Lee, M.-C.; Hsu, Y.-J.; Yang, H.-J.; Huang, C.-C. Enhancement of lower limb muscle strength and reduction of inflammation in the elderly: A randomized, double-blind clinical trial comparing Lacticaseibacillus paracasei PS23 probiotic with heat-treated supplementation. Nutrients 2025, 17, 463. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, vi5–vi9. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.A.H.R. The gold standard: Not a golden standard. Br. Med. J. 2005, 330, 1121. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Manini, T.; Patel, S.M.; Newman, A.; Travison, T.; Kiel, D.P.; Santanasto, A.J.; Ensrud, K.E.; Xue, Q.; Shardell, M.; et al. Putative cut-points in sarcopenia components and incident adverse health outcomes: An SDOC analysis. J. Am. Geriatr. Soc. 2020, 68, 1429–1437. [Google Scholar] [CrossRef]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia definition: The position statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Brown, T.; Kiel, D.P.; et al. Establishing the link between lean mass and grip strength cut points with mobility disability and other health outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. J. Gerontol. Ser. A 2020, 75, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Kostek, M.C.; Johns, J.; Hurley, B.F.; Conway, J.M. Can dual energy X-ray absorptiometry provide a valid assessment of changes in thigh muscle mass with strength training in older adults? Eur. J. Clin. Nutr. 2008, 62, 1372–1378. [Google Scholar] [CrossRef]
- Lee, S.; Kuk, J.L. Changes in fat and skeletal muscle with exercise training in obese adolescents: Comparison of whole-body MRI and dual energy X-ray absorptiometry. Obesity 2013, 21, 2063–2071. [Google Scholar] [CrossRef]
- Nelson, M.; Fiatarone, M.; Layne, J.; Trice, I.; Economos, C.; Fielding, R.; Ma, R.; Pierson, R.; Evans, W. Analysis of body-composition techniques and models for detecting change in soft tissue with strength training. Am. J. Clin. Nutr. 1996, 63, 678–686. [Google Scholar] [CrossRef]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Zhu, K.; Wactawski-Wende, J.; Ochs-Balcom, H.M.; LaMonte, M.J.; Hovey, K.M.; Evans, W.; Shankaran, M.; Troen, B.R.; Banack, H.R. The association of muscle mass measured by D3-creatine dilution method with dual-energy X-ray absorptiometry and physical function in postmenopausal women. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1591–1599. [Google Scholar] [CrossRef]
- Evans, W.J.; Hellerstein, M.; Orwoll, E.; Cummings, S.; Cawthon, P.M. D3-creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J. Cachexia Sarcopenia Muscle 2019, 10, 14–21. [Google Scholar] [CrossRef]
- Clark, R.V.; Walker, A.C.; Miller, R.R.; O’Connor-Semmes, R.L.; Ravussin, E.; Cefalu, W.T. Creatine (methyl-d3) dilution in urine for estimation of total body skeletal muscle mass: Accuracy and variability vs. MRI and DXA. J. Appl. Physiol. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Van den Broeck, J.; Buzzatti, L.; Jager-Wittenaar, H.; Perkisas, S.; Scafoglieri, A. The validity of ultrasound-derived equation models to predict whole-body muscle mass: A systematic review. Clin. Nutr. ESPEN 2021, 46, 133–141. [Google Scholar] [CrossRef]
- Harris-Love, M.O.; Avila, N.A.; Adams, B.; Zhou, J.; Seamon, B.; Ismail, C.; Zaidi, S.H.; Kassner, C.A.; Liu, F.; Blackman, M.R. The comparative associations of ultrasound and computed tomography estimates of muscle quality with physical performance and metabolic parameters in older men. J. Clin. Med. 2018, 7, 340. [Google Scholar] [CrossRef]
- Harris-Love, M.O.; Benson, K.; Leasure, E.; Adams, B.; McIntosh, V. The influence of upper and lower extremity strength on performance-based sarcopenia assessment tests. J. Funct. Morphol. Kinesiol. 2018, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Trappenburg, M.C.; Hogrel, J.-Y.; McPhee, J.S.; Piasecki, M.; Sipila, S.; Salpakoski, A.; Butler-Browne, G.; Pääsuke, M.; et al. Handgrip strength cannot be assumed a proxy for overall muscle strength. J. Am. Med. Dir. Assoc. 2018, 19, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Test-retest reliability of measurements of hand-grip strength obtained by dynamometry from older adults: A systematic review of research in the PubMed database. J. Frailty Aging 2017, 6, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Grip strength: An indispensable biomarker for older adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Andrews, A.W.; Thomas, M.W. Walking speed: Reference values and correlates for older adults. J. Orthop. Sports Phys. Ther. 1996, 24, 86–90. [Google Scholar] [CrossRef]
- Suetta, C.; Haddock, B.; Alcazar, J.; Noerst, T.; Hansen, O.M.; Ludvig, H.; Kamper, R.S.; Schnohr, P.; Prescott, E.; Andersen, L.L.; et al. The Copenhagen Sarcopenia Study: Lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J. Cachexia Sarcopenia Muscle 2019, 10, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Agostini, D.; Gervasi, M.; Ferrini, F.; Bartolacci, A.; Stranieri, A.; Piccoli, G.; Barbieri, E.; Sestili, P.; Patti, A.; Stocchi, V.; et al. An integrated approach to skeletal muscle health in aging. Nutrients 2023, 15, 1802. [Google Scholar] [CrossRef]
- Buford, T.W.; Anton, S.D.; Judge, A.R.; Marzetti, E.; Wohlgemuth, S.E.; Carter, C.S.; Leeuwenburgh, C.; Pahor, M.; Manini, T.M. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res. Rev. 2010, 9, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Rule, A.D.; Grossardt, B.R.; Weston, A.D.; Garner, H.W.; Kline, T.L.; Chamberlain, A.M.; Allen, A.M.; Erickson, B.J.; Rocca, W.A.; St Sauver, J.L. Older tissue age derived from abdominal computed tomography biomarkers of muscle, fat, and bone is associated with chronic conditions and higher mortality. Mayo Clin. Proc. 2024, 99, 878–890. [Google Scholar] [CrossRef]
- Correa-de-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the context of skeletal muscle function deficit: An interdisciplinary workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef]
- Kirk, B.; Cawthon, P.M.; Arai, H.; Ávila-Funes, J.A.; Barazzoni, R.; Bhasin, S.; Binder, E.F.; Bruyere, O.; Cederholm, T.; Chen, L.-K.; et al. The conceptual definition of sarcopenia: Delphi consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing 2024, 53, afae052. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Alcazar, J.; Aprahamian, I.; Batsis, J.A.; Yamada, Y.; Prado, C.M.; Reginster, J.-Y.; Sanchez-Rodriguez, D.; Lim, W.S.; Sim, M.; et al. Health outcomes of sarcopenia: A consensus report by the outcome working group of the Global Leadership Initiative in Sarcopenia (GLIS). Aging Clin. Exp. Res. 2025, 37, 100. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boncella, K.L.; Oranchuk, D.J.; Gonzalez-Rivera, D.; Sawyer, E.E.; Magnusson, D.M.; Harris-Love, M.O. What Is ‘Muscle Health’? A Narrative Review and Conceptual Framework. J. Funct. Morphol. Kinesiol. 2025, 10, 367. https://doi.org/10.3390/jfmk10040367
Boncella KL, Oranchuk DJ, Gonzalez-Rivera D, Sawyer EE, Magnusson DM, Harris-Love MO. What Is ‘Muscle Health’? A Narrative Review and Conceptual Framework. Journal of Functional Morphology and Kinesiology. 2025; 10(4):367. https://doi.org/10.3390/jfmk10040367
Chicago/Turabian StyleBoncella, Katie L., Dustin J. Oranchuk, Daniela Gonzalez-Rivera, Eric E. Sawyer, Dawn M. Magnusson, and Michael O. Harris-Love. 2025. "What Is ‘Muscle Health’? A Narrative Review and Conceptual Framework" Journal of Functional Morphology and Kinesiology 10, no. 4: 367. https://doi.org/10.3390/jfmk10040367
APA StyleBoncella, K. L., Oranchuk, D. J., Gonzalez-Rivera, D., Sawyer, E. E., Magnusson, D. M., & Harris-Love, M. O. (2025). What Is ‘Muscle Health’? A Narrative Review and Conceptual Framework. Journal of Functional Morphology and Kinesiology, 10(4), 367. https://doi.org/10.3390/jfmk10040367









