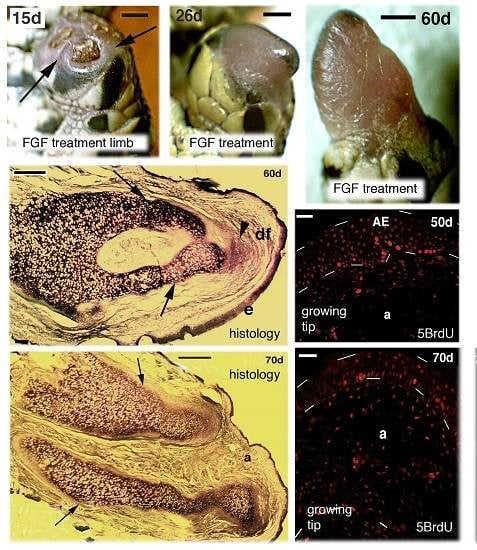
Figure 1.
Macroscopic view showing the aspect of regenerating hind-limbs at progressive days (d) post-amputation. (A–C,G,H) FGF1-treatment. (D–F,I) FGF2 treatments. Bars in all figures represent 1 mm. (A–E) and (G–H) show regenerating limbs after leg (mid tibia-fibula) amputation; (F,I) are regenerating limbs after thigh (mid femur) amputation; (A) at 6 days post-amputation the stump surface is covered by a scab (arrows); (B) at 15 days regenerating case with wound epidermis and underlined mesenchyme (arrows) sealing almost completely the stump surface; (C), in this case at 16 days a blastema (arrow) is fully formed; (D–I), gross aspect of elongating conical blastemas in representative cases at different days post-amputation; (H) the longest case at 60 days post-amputation features some flattening; (J) control of amputated limb at 25 days post-amputation.
Figure 1.
Macroscopic view showing the aspect of regenerating hind-limbs at progressive days (d) post-amputation. (A–C,G,H) FGF1-treatment. (D–F,I) FGF2 treatments. Bars in all figures represent 1 mm. (A–E) and (G–H) show regenerating limbs after leg (mid tibia-fibula) amputation; (F,I) are regenerating limbs after thigh (mid femur) amputation; (A) at 6 days post-amputation the stump surface is covered by a scab (arrows); (B) at 15 days regenerating case with wound epidermis and underlined mesenchyme (arrows) sealing almost completely the stump surface; (C), in this case at 16 days a blastema (arrow) is fully formed; (D–I), gross aspect of elongating conical blastemas in representative cases at different days post-amputation; (H) the longest case at 60 days post-amputation features some flattening; (J) control of amputated limb at 25 days post-amputation.
Figure 2.
Histological images of scarring controls at 40 days (A,B) with 5BrdU immunolabeling (C,D). (A) longitudinal section of a scarring limb that is occupied by an irregular fibrous connective tissue with some cartilaginous nodules (arrows) in the center. The wound epithelium presents un-stainable areas in the stratum corneum. Bar, 50 μm; (B) detail of the apical region of the scarring outgrowth showing the cells forming the fibrous connective tissue where sparse blood vessels are present under the apical wound epidermis. The arrow indicates the apical cartilage. Bar, 20 μm; (C) 5brdU immunolabeling showing few and sparse labeled cells in the apical connective tissue present underneath the wound epidermis. Bar, 20 μm; (D) control section of the apical connective tissue present beneath the wound epidermis. Bar, 10mm Legends: a, apical connective tissue (blastema); CO, control section; fc, fibrous connective tissue; v, blood vessel; w, wound epidermis. Dashes underline the epidermis.
Figure 2.
Histological images of scarring controls at 40 days (A,B) with 5BrdU immunolabeling (C,D). (A) longitudinal section of a scarring limb that is occupied by an irregular fibrous connective tissue with some cartilaginous nodules (arrows) in the center. The wound epithelium presents un-stainable areas in the stratum corneum. Bar, 50 μm; (B) detail of the apical region of the scarring outgrowth showing the cells forming the fibrous connective tissue where sparse blood vessels are present under the apical wound epidermis. The arrow indicates the apical cartilage. Bar, 20 μm; (C) 5brdU immunolabeling showing few and sparse labeled cells in the apical connective tissue present underneath the wound epidermis. Bar, 20 μm; (D) control section of the apical connective tissue present beneath the wound epidermis. Bar, 10mm Legends: a, apical connective tissue (blastema); CO, control section; fc, fibrous connective tissue; v, blood vessel; w, wound epidermis. Dashes underline the epidermis.
Figure 3.
Histological longitudinal sections of regenerating limbs at 40 days treated with FGF1 (A,B), 50 days treated with FGF2 (C,D) and 60 days with FGF1 (E,F) post-amputation; (A) section of a limb outgrowth derived from a tibia-fibula amputation that shows a central mass of immature and unstained cartilaginous tissue partially sub-divided into two regions that show a mature stained cartilage (metachromatic) in the more proximal areas (arrowheads), in continuity with the tibia bone. The two arrows indicate the plane of amputation. Bar, 200 μm; (B) close-up to the tip of the previous figure showing the small apical immature cartilage (arrowheads) in continuation with distal perichondrium cells, reaching the apical connective tissue region located underneath the apical wound epidermis. The latter shows a thin corneous layer. Bar, 50 μm. The inset shows a detail of the apical wound epidermis with the corneous layer (arrowhead). Bar, 10 μm; (C) other section of a conical blastema, showing the axial rod of cartilage containing mature chondrocytes (arrowhead), and surrounded by immature chondrocytes (non metachromatic to methylene blue). Bar, 200 μm; (D) apical region showing the thick wound epidermis and the cartilaginous nodules (arrows) containing centrally-located metachromatic chondrocytes, and surrounded by a perichondrium (arrowheads). Bar, 50 μm. The inset shows a tendon-like belt (arrowheads). Bar, 10 μm; (E) section showing the mature regenerated axial cartilage divided into two elements (arrows) representing the tibia and fibula. The apical blastema after 60 days of regeneration is mainly turned into a fibrous connective tissue (arrowhead). Bar, 200 μm; (F) detail of the proximal region of the previous section contacting the bone of the larger tibia. Bar, 100 μm. Legends: a, apical connective tissue (blastema); c, corneous layer; AE, apical wound epidermis; b, bone; ca, regenerated cartilage; df, dense fibrous connective tissue; e, new epidermis; ic, immature cartilage; mu, regenerated muscles; pc, proximal cartilage; pr, perichondrial cells; sc, scale; t, tendon-like; w, wound (regenerating) epidermis.
Figure 3.
Histological longitudinal sections of regenerating limbs at 40 days treated with FGF1 (A,B), 50 days treated with FGF2 (C,D) and 60 days with FGF1 (E,F) post-amputation; (A) section of a limb outgrowth derived from a tibia-fibula amputation that shows a central mass of immature and unstained cartilaginous tissue partially sub-divided into two regions that show a mature stained cartilage (metachromatic) in the more proximal areas (arrowheads), in continuity with the tibia bone. The two arrows indicate the plane of amputation. Bar, 200 μm; (B) close-up to the tip of the previous figure showing the small apical immature cartilage (arrowheads) in continuation with distal perichondrium cells, reaching the apical connective tissue region located underneath the apical wound epidermis. The latter shows a thin corneous layer. Bar, 50 μm. The inset shows a detail of the apical wound epidermis with the corneous layer (arrowhead). Bar, 10 μm; (C) other section of a conical blastema, showing the axial rod of cartilage containing mature chondrocytes (arrowhead), and surrounded by immature chondrocytes (non metachromatic to methylene blue). Bar, 200 μm; (D) apical region showing the thick wound epidermis and the cartilaginous nodules (arrows) containing centrally-located metachromatic chondrocytes, and surrounded by a perichondrium (arrowheads). Bar, 50 μm. The inset shows a tendon-like belt (arrowheads). Bar, 10 μm; (E) section showing the mature regenerated axial cartilage divided into two elements (arrows) representing the tibia and fibula. The apical blastema after 60 days of regeneration is mainly turned into a fibrous connective tissue (arrowhead). Bar, 200 μm; (F) detail of the proximal region of the previous section contacting the bone of the larger tibia. Bar, 100 μm. Legends: a, apical connective tissue (blastema); c, corneous layer; AE, apical wound epidermis; b, bone; ca, regenerated cartilage; df, dense fibrous connective tissue; e, new epidermis; ic, immature cartilage; mu, regenerated muscles; pc, proximal cartilage; pr, perichondrial cells; sc, scale; t, tendon-like; w, wound (regenerating) epidermis.
![Jfmk 02 00025 g003]()
Figure 4.
Longitudinal sections of regenerating limbs treated with FGF1 at 60 days (A–C) and 70 days (D) post-amputation. (A) distal region of an outgrowth containing two separated cartilaginous elements (arrows), the upper considered a regenerated tibia, and the lower a regenerated fibula. A dense fibrous connective tissue (fc) surrounds the cartilage, forming the perichondrium. In front of them a cartilaginous nodule (arrowhead) is seen. The apical wound epithelium (w) shows numerous epidermal papillae (small pegs). Bar, 200 μm; (B) separated regenerated cartilages of the tibia (upper arrow) and fibula (lower arrow), surrounded by a thick perichondrium (arrows) in the long outgrowth illustrated in Fig 1H, examined at 70 days post-amputation. The tibia cartilage is attached to the original tibia bone (b). Bar, 50 μm; (C) detail of calcified cartilage (cc) localized near the perichondrium (arrow) of the cartilaginous tibia. Bar, 20 μm; (D) detail of apical wound epidermis of the outgrowth of figure B, featuring the numerous epidermal papillae (arrows). Bar, 200 μm.
Figure 4.
Longitudinal sections of regenerating limbs treated with FGF1 at 60 days (A–C) and 70 days (D) post-amputation. (A) distal region of an outgrowth containing two separated cartilaginous elements (arrows), the upper considered a regenerated tibia, and the lower a regenerated fibula. A dense fibrous connective tissue (fc) surrounds the cartilage, forming the perichondrium. In front of them a cartilaginous nodule (arrowhead) is seen. The apical wound epithelium (w) shows numerous epidermal papillae (small pegs). Bar, 200 μm; (B) separated regenerated cartilages of the tibia (upper arrow) and fibula (lower arrow), surrounded by a thick perichondrium (arrows) in the long outgrowth illustrated in Fig 1H, examined at 70 days post-amputation. The tibia cartilage is attached to the original tibia bone (b). Bar, 50 μm; (C) detail of calcified cartilage (cc) localized near the perichondrium (arrow) of the cartilaginous tibia. Bar, 20 μm; (D) detail of apical wound epidermis of the outgrowth of figure B, featuring the numerous epidermal papillae (arrows). Bar, 200 μm.
![Jfmk 02 00025 g004]()
Figure 5.
Histological (A,B) and 5BrdU-immunofluorescent (C–F) images of a regenerating outgrowth at 50 days after mid femur transection (FGF2 treatment). (A) Distal part of regenerated femur cartilage surrounded by a fibrous and dense connective tissue. Bar, 100 μm; (B) detail of an apical cartilaginous nodule surrounded by perichondrium (arrowheads), located near the tip of the femur cartilage. Bar 20 μm; (C) labeled cells (red dots) are distributed in the apical region of the outgrowth (see location in the pink inset drawing featuring a limb blastema with the central cartilaginous rod, dotted, derived from the transected femur). The wound epidermis and the tip of the regenerated cartilage are indicated by dashes. Bar, 50 μm; (D) detail of the apex of the outgrowth with most labeled cells in the apical wound epidermis outlined by dashes. Bar, 20 μm; (E) detail of labeled cells within the apical region (blastema), that are present near the tip of the regenerated cartilaginous rod (dashed). Bar, 20 μm; (F) detail of a cartilage nodule present at the tip of the regenerated outgrowth, and surrounded by labeled cells of the perichondrium (arrows). Bar, 20 μm. Legends: a, apical region (blastema); AE, apical wound epidermis; ca, regenerated cartilaginous rod of the transected femur; dc, dense connective tissue; fc, femur cartilage. nc, nodule cartilaginous; w, wound epidermis.
Figure 5.
Histological (A,B) and 5BrdU-immunofluorescent (C–F) images of a regenerating outgrowth at 50 days after mid femur transection (FGF2 treatment). (A) Distal part of regenerated femur cartilage surrounded by a fibrous and dense connective tissue. Bar, 100 μm; (B) detail of an apical cartilaginous nodule surrounded by perichondrium (arrowheads), located near the tip of the femur cartilage. Bar 20 μm; (C) labeled cells (red dots) are distributed in the apical region of the outgrowth (see location in the pink inset drawing featuring a limb blastema with the central cartilaginous rod, dotted, derived from the transected femur). The wound epidermis and the tip of the regenerated cartilage are indicated by dashes. Bar, 50 μm; (D) detail of the apex of the outgrowth with most labeled cells in the apical wound epidermis outlined by dashes. Bar, 20 μm; (E) detail of labeled cells within the apical region (blastema), that are present near the tip of the regenerated cartilaginous rod (dashed). Bar, 20 μm; (F) detail of a cartilage nodule present at the tip of the regenerated outgrowth, and surrounded by labeled cells of the perichondrium (arrows). Bar, 20 μm. Legends: a, apical region (blastema); AE, apical wound epidermis; ca, regenerated cartilaginous rod of the transected femur; dc, dense connective tissue; fc, femur cartilage. nc, nodule cartilaginous; w, wound epidermis.
![Jfmk 02 00025 g005]()
Figure 6.
Immunofluorescence for 5BrdU of different regions of 50 days old outgrowths, obtained after mid femur transection and FGF2 treatment. (A) Distribution of labeled cells in the cartilage rod (in the inset, the indicative position is shown with an arrow pointing the regenerated cartilage rod formed within the outgrowth; the arrowhead refers to regenerating scales). The arrows indicate the perichondrium. Bar, 50 μm; (B) detail of the region localized between the cartilage rod and the close tendon-like belt (the inset drawing shows the indicative position with an arrow pointing near the regenerated cartilage rod; the arrowhead refers to the proximal repairing muscles). The arrows indicate the perichondrium while the dashes outline the dense tendon-like belt. Bar, 50 μm; (C) regenerating scales (their indicative position is shown by the arrowhead seen in the inset in (A). Bar, 20 μm; (D) detail of repairing muscles located at the base of the outgrowth (their indicative position is shown by the arrowhead in the inset in (B). Bar, 20 μm. Legends: ca, regenerated cartilage; dc, dense connective tissue of the dermis; e, regenerating scale epidermis; ls, limb stump; mu, regenerating muscles; o, limb outgrowth; t, tendon-like belt.
Figure 6.
Immunofluorescence for 5BrdU of different regions of 50 days old outgrowths, obtained after mid femur transection and FGF2 treatment. (A) Distribution of labeled cells in the cartilage rod (in the inset, the indicative position is shown with an arrow pointing the regenerated cartilage rod formed within the outgrowth; the arrowhead refers to regenerating scales). The arrows indicate the perichondrium. Bar, 50 μm; (B) detail of the region localized between the cartilage rod and the close tendon-like belt (the inset drawing shows the indicative position with an arrow pointing near the regenerated cartilage rod; the arrowhead refers to the proximal repairing muscles). The arrows indicate the perichondrium while the dashes outline the dense tendon-like belt. Bar, 50 μm; (C) regenerating scales (their indicative position is shown by the arrowhead seen in the inset in (A). Bar, 20 μm; (D) detail of repairing muscles located at the base of the outgrowth (their indicative position is shown by the arrowhead in the inset in (B). Bar, 20 μm. Legends: ca, regenerated cartilage; dc, dense connective tissue of the dermis; e, regenerating scale epidermis; ls, limb stump; mu, regenerating muscles; o, limb outgrowth; t, tendon-like belt.
![Jfmk 02 00025 g006]()
Figure 7.
Immunofluorescence for 5BrdU of different regions of 40 days old outgrowths after mid tibia-fibula transection and FGF1 treatment. (
A) Parallel section of
Figure 2B showing the numerous 5BrdU-labeled cells detected in the apical connective tissue and wound epidermis (outlined by dashes). Most labeled cells are present in the apical perichondrium while little labeling is seen in the immature cartilage (outlined by dashes). Bar, 50 μm; (
B) detail of the very tip of the same outgrowth shown in (A) evidencing the numerous labeled epidermal cells (arrows). Bar 20 μm; (
C) lateral region of the outgrowth (its indicative position is shown by an arrow in the inset drawing; the arrowhead points to the bone marrow of the tibia). Numerous labeled cells are seen in the epidermis (outlined by dashes), dermis and among repairing muscles are seen. The arrows indicate the perichondrium surrounding the central cartilaginous rod. Bar, 50 μm; (
D) detail on labeled cells within the bone marrow (its position is indicated by an arrowhead in the inset of figure C. Bar, 20 μm. Legends: a, apical connective tissue (blastema); AE, apical wound epidermis; bm, bone marrow; ca, regenerated cartilaginous rod; d, dermis; e, epidermis; f, femur (stump); ic, immature apical cartilage; mu, regenerating muscles; o, outgrowth; pr, apical perichondrium; w, wound epidermis.
Figure 7.
Immunofluorescence for 5BrdU of different regions of 40 days old outgrowths after mid tibia-fibula transection and FGF1 treatment. (
A) Parallel section of
Figure 2B showing the numerous 5BrdU-labeled cells detected in the apical connective tissue and wound epidermis (outlined by dashes). Most labeled cells are present in the apical perichondrium while little labeling is seen in the immature cartilage (outlined by dashes). Bar, 50 μm; (
B) detail of the very tip of the same outgrowth shown in (A) evidencing the numerous labeled epidermal cells (arrows). Bar 20 μm; (
C) lateral region of the outgrowth (its indicative position is shown by an arrow in the inset drawing; the arrowhead points to the bone marrow of the tibia). Numerous labeled cells are seen in the epidermis (outlined by dashes), dermis and among repairing muscles are seen. The arrows indicate the perichondrium surrounding the central cartilaginous rod. Bar, 50 μm; (
D) detail on labeled cells within the bone marrow (its position is indicated by an arrowhead in the inset of figure C. Bar, 20 μm. Legends: a, apical connective tissue (blastema); AE, apical wound epidermis; bm, bone marrow; ca, regenerated cartilaginous rod; d, dermis; e, epidermis; f, femur (stump); ic, immature apical cartilage; mu, regenerating muscles; o, outgrowth; pr, apical perichondrium; w, wound epidermis.
![Jfmk 02 00025 g007]()
Figure 8.
Immunofluorescence for 5BrdU in different regions of 50 days old outgrowths (tibia-fibula transection and FGF2 treatment). (A) Apical region of the outgrowth, showing intense labeling in the apical epidermis (outlined by dashes), apical connective tissue, and in the cartilaginous nodules (outlined by dashes). Little labeling is observed in the cartilaginous rod, outlined by dashes in the lower part of the figure (indicative position shown in the drawing inset). Bar, 50 μm; (B) detail of the apical region of the outgrowth, showing the numerous labeled cells present in the wound epidermis and underlying connective tissue (dashes outline the tip of the cartilaginous rod). Bar 20 μm; (C) epidermal peg (outlined by dashes) containing numerous labeled cells at the beginning of scale regeneration. Bar, 20 μm; (D) regenerated scale with numerous labeled cells localized in the stratum basale. Bar, 20 μm. Legends: a, apical connective tissue (blastema); AE, apical wound epidermis; ca, regenerated cartilage (rod); d, dermis; h, hinge region; I, inner scale surface; nc, nodulus cartilaginous; os, outer scale surface (dorsal); p, epidermal peg (beginning of scale formation); w, wound epidermis (regenerating).
Figure 8.
Immunofluorescence for 5BrdU in different regions of 50 days old outgrowths (tibia-fibula transection and FGF2 treatment). (A) Apical region of the outgrowth, showing intense labeling in the apical epidermis (outlined by dashes), apical connective tissue, and in the cartilaginous nodules (outlined by dashes). Little labeling is observed in the cartilaginous rod, outlined by dashes in the lower part of the figure (indicative position shown in the drawing inset). Bar, 50 μm; (B) detail of the apical region of the outgrowth, showing the numerous labeled cells present in the wound epidermis and underlying connective tissue (dashes outline the tip of the cartilaginous rod). Bar 20 μm; (C) epidermal peg (outlined by dashes) containing numerous labeled cells at the beginning of scale regeneration. Bar, 20 μm; (D) regenerated scale with numerous labeled cells localized in the stratum basale. Bar, 20 μm. Legends: a, apical connective tissue (blastema); AE, apical wound epidermis; ca, regenerated cartilage (rod); d, dermis; h, hinge region; I, inner scale surface; nc, nodulus cartilaginous; os, outer scale surface (dorsal); p, epidermal peg (beginning of scale formation); w, wound epidermis (regenerating).
![Jfmk 02 00025 g008]()
Figure 9.
Immunofluorescence for 5BrdU of different regions of 70 days old outgrowths after tibia-fibula transection (FGF1 treatment). (A) Diffusely distributed labeled cells in the apical region of the outgrowth. The regenerating epidermis is outlined by dashes. Below, the tip of the regenerated cartilaginous rod is also outlined by dashes. Bar, 50 μm; (B) detail of the tip of the outgrowth containing sparse labeled cells, especially in the wound epidermis (outlined by dashes). The dashed line below indicates the cartilaginous rod. Bar 20 μm; (C) detail on regenerating scale epidermis (outlined by dashes) containing various labeled cells (their indicative position is shown by the arrow in the inset of figure D). Bar, 20 μm; (D) detail of the scarce labeled cells present in the cartilage rod of a the regenerated fibula (indicative position shown by the arrowhead in the inset). Bar, 20 μm. Legends: a, apical connective tissue; AE, apical wound epidermis; ca, regenerated cartilage; d, dermis; e, epidermis of the forming scale; ls, stump of the limb; o, limb outgrowth; w, wound epidermis.
Figure 9.
Immunofluorescence for 5BrdU of different regions of 70 days old outgrowths after tibia-fibula transection (FGF1 treatment). (A) Diffusely distributed labeled cells in the apical region of the outgrowth. The regenerating epidermis is outlined by dashes. Below, the tip of the regenerated cartilaginous rod is also outlined by dashes. Bar, 50 μm; (B) detail of the tip of the outgrowth containing sparse labeled cells, especially in the wound epidermis (outlined by dashes). The dashed line below indicates the cartilaginous rod. Bar 20 μm; (C) detail on regenerating scale epidermis (outlined by dashes) containing various labeled cells (their indicative position is shown by the arrow in the inset of figure D). Bar, 20 μm; (D) detail of the scarce labeled cells present in the cartilage rod of a the regenerated fibula (indicative position shown by the arrowhead in the inset). Bar, 20 μm. Legends: a, apical connective tissue; AE, apical wound epidermis; ca, regenerated cartilage; d, dermis; e, epidermis of the forming scale; ls, stump of the limb; o, limb outgrowth; w, wound epidermis.
![Jfmk 02 00025 g009]()