Effects of Dominant and Nondominant Limb Immobilization on Muscle Activation and Physical Demand during Ambulation with Axillary Crutches
Abstract
:1. Introduction
2. Materials and Methods
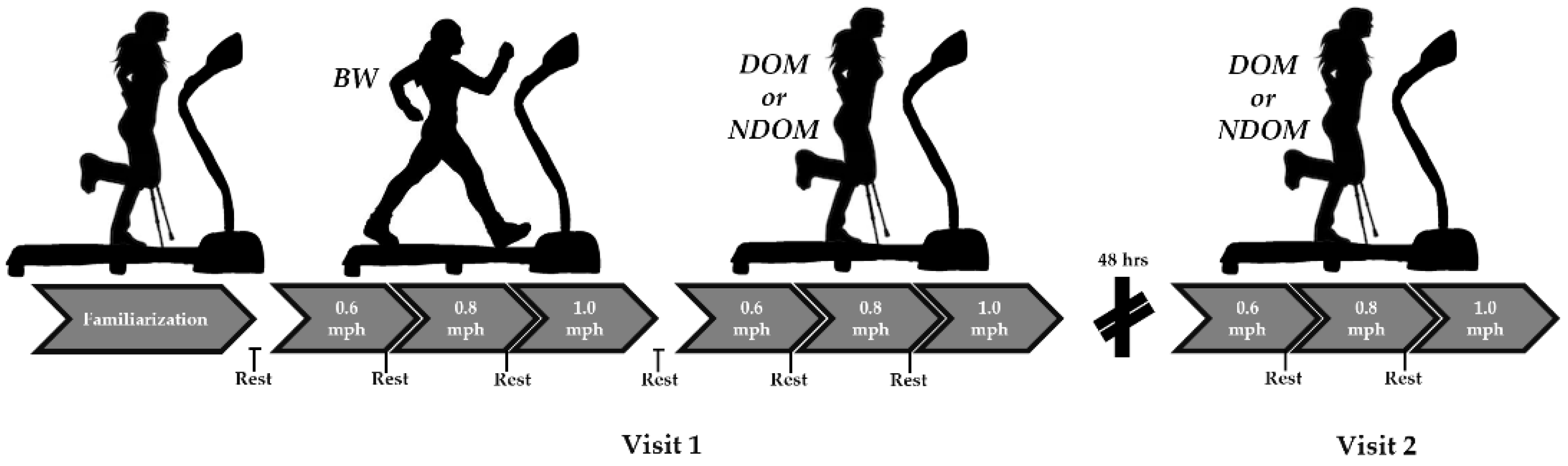
2.1. Study Design
2.2. Participants
2.3. Surface Electromyograph (sEMG) Sensor Placement
- ▪
- Medial gastrocnemius (MG): Participants laid prone with their knee fully extended; the sensor(s) were placed on the most prominent medial bulge of the muscle.
- ▪
- Soleus (SOL): Participants sat upright wither their knee at 90 degrees in passive flexion with their heel on the floor; the sensors were placed at two thirds of the line between the medial condyle of the femur to the medial malleolus.
- ▪
- Tibialis anterior (TA): Participants sat upright with their knee at 90 degrees in passive flexion with their heel on the floor; the sensors were placed at about a third on the line between the tip of the fibula and the tip of the medial malleolus.
- ▪
- Biceps brachii (BB): Participants sat in a chair with the elbow passively flexed at 90 degrees and the dorsal side of the forearm in a horizontal position; the sensors were placed on the line between the medial acromion and the fossa cubit at one third from the fossa cubit.
- ▪
- Triceps brachii (TB): Participants sat upright with their shoulder at approximately 90 degrees abduction with the arm 90 degrees flexed and the palm of the hand pointing downwards; the electrodes were placed half-way on the line between the posterior crista of the acromion and the olecranon at two finger widths medial to the line.
- ▪
- Ground strap: A ground reference strap was placed on each medial malleolus.
2.4. Procedures
2.5. Data Analysis
3. Results
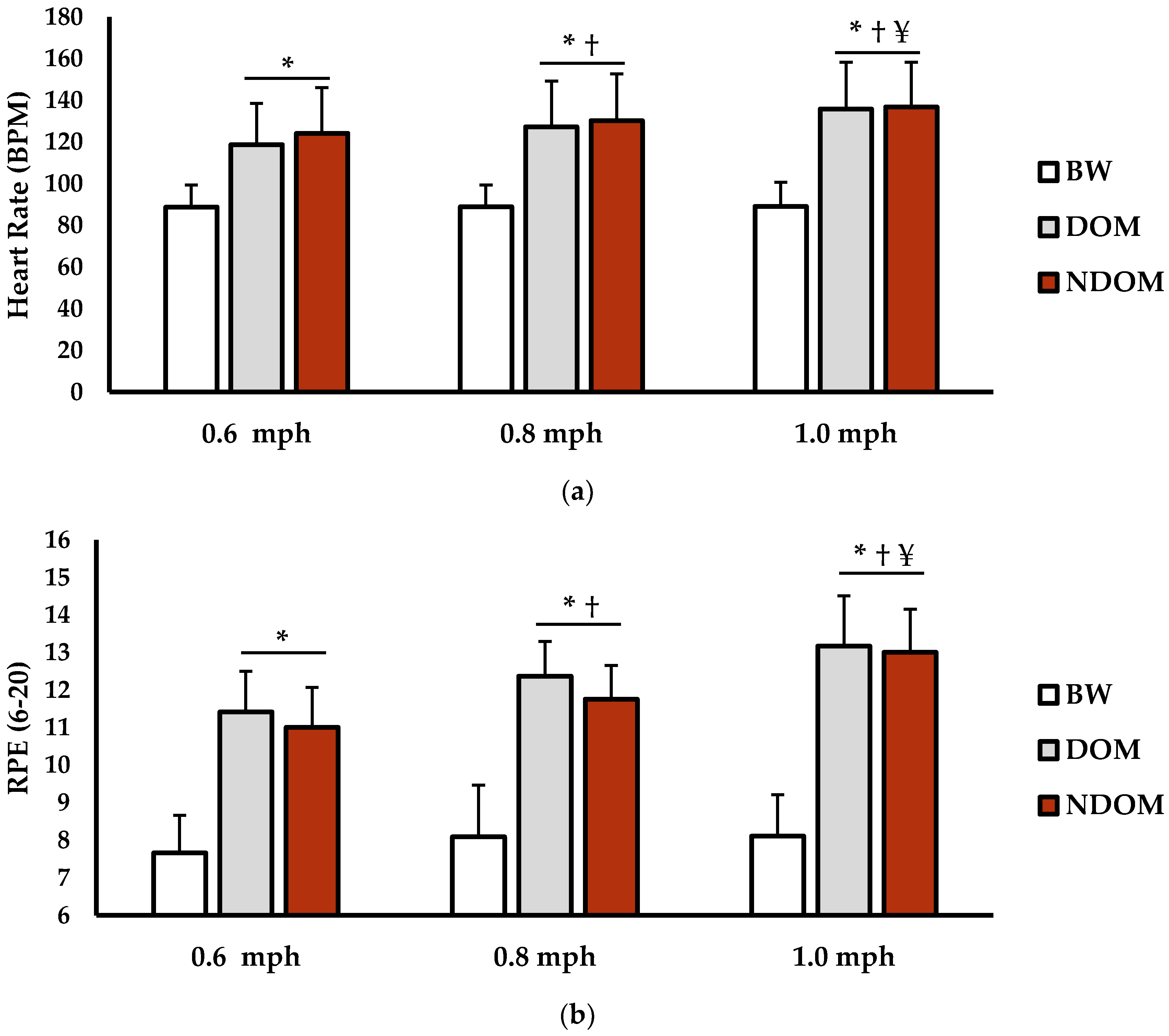
3.1. Physical Demand Analysis
3.2. Muscle Activation Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitts, S.R.; Niska, W.; Burt, C.W. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl. Health Stat. Rep. 2008, 7, 1–38. [Google Scholar]
- Lambers, K.; Ootes, D.; Ring, D. Incidence of patients with lower extremity injuries presenting to US emergency departments by anatomic region, disease category, and age. Clin. Orthop. Relat. Res. 2012, 470, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Kaye, H.S.; Kang, T.; LaPlante, M.P. Mobility Device Use in the United States; Disability Statistics Report 14; National Institute on Disability and Rehabilitation Research, US Department of Education: Washington, DC, USA, 2000.
- Foley, M.P.; Prax, B.; Crowell, R.; Boone, T. Effects of assistive devices on cardiorespiratory demands in older adults. Phys. Ther. 1996, 76, 1313–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valderrabano, V.; Nigg, B.M.; Hintermann, B.; Goepfert, B.; Dick, W.; Frank, C.B.; Herzog, W.; Tscharner, V.v. Muscular lower leg asymmetry in middle-aged people. Foot Ankle Int. 2007, 28, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Polk, J.D.; Stumpf, R.M.; Rosengren, K.S. Limb dominance, foot orientation and functional asymmetry during walking gait. Gait Posture 2017, 52, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Seeley, M.K.; Umberger, B.R.; Shapiro, R. A test of the functional asymmetry hypothesis in walking. Gait Posture 2008, 28, 24–28. [Google Scholar] [CrossRef]
- Sadeghi, H.; Allard, P.; Duhaime, M. Functional gait asymmetry in able-bodied subjects. Hum. Mov. Sci. 1997, 16, 243–258. [Google Scholar] [CrossRef]
- Gregg, R.D.; Dhaher, Y.Y.; Degani, A.; Lynch, K.M. On the mechanics of functional asymmetry in bipedal walking. IEEE Trans. Biomed. Eng. 2012, 59, 1310–1318. [Google Scholar] [CrossRef]
- LaRoche, D.P.; Cook, S.B.; Mackala, K. Strength asymmetry increases gait asymmetry and variability in older women. Med. Sci. Sports Exerc. 2012, 44, 2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, H.; Yoshikawa, T.; Mimura, T.; Hara, T.; Nishimoto, K.; Fujimoto, S. Influence of lower-extremity muscle force, muscle mass and asymmetry in knee extension force on gait ability in community-dwelling elderly women. J. Phys. Ther. Sci. 2006, 18, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Burnett, D.R.; Campbell-Kyureghyan, N.H.; Cerrito, P.B.; Quesada, P.M. Symmetry of ground reaction forces and muscle activity in asymptomatic subjects during walking, sit-to-stand, and stand-to-sit tasks. J. Electromyogr. Kinesiol. 2011, 21, 610–615. [Google Scholar] [CrossRef]
- Rudroff, T.; Proessl, F. Effects of muscle function and limb loading asymmetries on gait and balance in people with multiple sclerosis. Front. Physiol. 2018, 9, 531. [Google Scholar] [CrossRef]
- Adegoke, B.; Olaniyi, O.; Akosile, C. Weight bearing asymmetry and functional ambulation performance in stroke survivors. Glob. J. Health Sci. 2012, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Yümin, E.T.; Şimşek, T.T.; Sertel, M.; Öztürk, A.; Yümin, M. The effect of functional mobility and balance on health-related quality of life (HRQoL) among elderly people living at home and those living in nursing home. Arch. Gerontol. Geriatr. 2011, 52, e180–e184. [Google Scholar] [CrossRef]
- Adedoyin, R.A.; Opayinka, A.J.; Oladokun, Z.O. Energy expenditure of stair climbing with elbow and axillary crutches. Physiotherapy 2002, 88, 47–51. [Google Scholar] [CrossRef]
- Annesley, A.L.; Almada-Norfleet, M.; Arnall, D.A.; Cornwall, M.W. Energy expenditure of ambulation using the Sure-Gait® crutch and the standard axillary crutch. Phys. Ther. 1990, 70, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Mcquade, K.J.; Finley, M.; Oliveira, A.S. Upper extremity joint stresses during walkerassisted ambulation in post-surgical patients. Braz. J. Phys. Ther. 2011, 15, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Muir-Hunter, S.; Montero-Odasso, M. The attentional demands of ambulating with an assistive device in older adults with Alzheimer’s disease. Gait Posture 2017, 54, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovaleski, J.E.; Heitman, R.J.; Gurchiek, L.R.; Erdmann, J.W.; Trundle, T.L. Reliability and effects of leg dominance on lower extremity isokinetic force and work using the closed chain rider system. J. Sport Rehabil. 1997, 6, 319–326. [Google Scholar] [CrossRef]
- Liang, S.; Xu, J.; Zhao, G. An investigation into the bilateral functional differences of the lower limb muscles in standing and walking. PeerJ 2016, 4, e2315. [Google Scholar] [CrossRef] [Green Version]
- Ounpuu, S.; Winter, D.A. Bilateral electromyographical analysis of the lower limbs during walking in normal adults. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 429–438. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M.; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2018. [Google Scholar]
- Stegeman, D.; Hermens, H. Standards for Surface Electromyography: The European Project Surface EMG for Non-Invasive Assessment of Muscles (SENIAM); Roessingh Research and Development: Enschede, The Netherlands, 2007. [Google Scholar]
- Van Melick, N.; Meddeler, B.M.; Hoogeboom, T.J.; Nijhuis-van der Sanden, M.W.; van Cingel, R.E. How to determine leg dominance: The agreement between self-reported and observed performance in healthy adults. PLoS ONE 2017, 12, e0189876. [Google Scholar] [CrossRef] [Green Version]
- Holder, C.G.; Haskvitz, E.M.; Weltman, A. The effects of assistive devices on the oxygen cost, cardiovascular stress, and perception of nonweight-bearing ambulation. J. Orthop. Sports Phys. Ther. 1993, 18, 537–542. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Hinton, C.A.; Cullen, K.E. Energy expenditure during ambulation with ortho crutches and axillary crutches. Phys. Ther. 1982, 62, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Thys, H.; Willems, P.; Saels, P. Energy cost, mechanical work and muscular efficiency in swing-through gait with elbow crutches. J. Biomech. 1996, 29, 1473–1482. [Google Scholar] [CrossRef]
- Bhambani, Y.; Clarkson, H. Acute physiologic and perceptual responses during three modes of ambulation: Walking, axillary crutch walking, and running. Arch. Phys. Med. Rehabil. 1989, 70, 445–450. [Google Scholar] [CrossRef]
- Opila, K.; Nicol, A.; Paul, J. Upper limb loadings of gait with crutches. J. Biomech. Eng. 1987, 109, 285–290. [Google Scholar] [CrossRef]
- Hotta, N.; Yamamoto, K.; Sato, K.; Katayama, K.; Fukuoka, Y.; Ishida, K. Ventilatory and circulatory responses at the onset of dominant and non-dominant limb exercise. Eur. J. Appl. Physiol. 2007, 101, 347–358. [Google Scholar] [CrossRef]
- Kadoguchi, T.; Horiuchi, M.; Kinugawa, S.; Okita, K. Heterogeneity in the vasodilatory function of individual extremities. Vascular 2020, 28, 87–95. [Google Scholar] [CrossRef]
- Jungmann, P.M.; Pfirrmann, C.; Federau, C. Characterization of lower limb muscle activation patterns during walking and running with Intravoxel Incoherent Motion (IVIM) MR perfusion imaging. Magn. Reson. Imaging 2019, 63, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Enoka, R. Maximum bilateral contractions are modified by neurally mediated interlimb effects. J. Appl. Physiol. 1991, 70, 306–316. [Google Scholar] [CrossRef]
- Dai, B.; Butler, R.J.; Garrett, W.E.; Queen, R.M. Anterior cruciate ligament reconstruction in adolescent patients: Limb asymmetry and functional knee bracing. Am. J. Sports Med. 2012, 40, 2756–2763. [Google Scholar] [CrossRef]
- Mansfield, A.; Danells, C.J.; Inness, E.; Mochizuki, G.; McIlroy, W.E. Between-limb synchronization for control of standing balance in individuals with stroke. Clin. Biomech. 2011, 26, 312–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, D.C.; Medola, F.O.; Bonfim, G.H.; Paschoarelli, L.C. Using a Pressure Mapping System to Evaluate Contact Pressure on Hands During Use of Axillary Crutches. In Proceedings of the AAATE Conference, Budapest, Hungary, 9–12 September 2015; pp. 432–439. [Google Scholar]
- Damholt, V.; Termansen, N. Asymmetry of plantar flexion strength in the foot. Acta Orthop. Scand. 1978, 49, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nüesch, C.; Valderrabano, V.; Huber, C.; von Tscharner, V.; Pagenstert, G. Gait patterns of asymmetric ankle osteoarthritis patients. Clin. Biomech. 2012, 27, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Coley, B.; Jolles, B.M.; Farron, A.; Pichonnaz, C.; Bassin, J.; Aminian, K. Estimating dominant upper-limb segments during daily activity. Gait Posture 2008, 27, 368–375. [Google Scholar] [CrossRef]
- Schwartz, C.; Tubez, F.; Wang, F.-C.; Croisier, J.-L.; Brüls, O.; Denoël, V.; Forthomme, B. Normalizing shoulder EMG: An optimal set of maximum isometric voluntary contraction tests considering reproducibility. J. Electromyogr. Kinesiol. 2017, 37, 1–8. [Google Scholar] [CrossRef] [Green Version]

| DOM | NDOM | |||||
|---|---|---|---|---|---|---|
| Muscle | 0.6 mph | 0.8 mph | 1.0 mph | 0.6 mph | 0.8 mph | 1.0 mph |
| Medial Gastrocnemius (MG) | 2.8 ± 0.7 | 2.7 ± 0.6 | 2.9 ± 0.9 | 4.0 ± 2.7 | 3.5 ± 1.4 | 3.2 ± 1.2 |
| Tibialis Anterior (TA) | 2.1 ± 0.8 | 2.1 ± 0.7 | 2.3 ± 0.8 | 2.1 ± 0.8 | 2.2 ± 0.7 | 2.2 ± 0.6 |
| Soleus (SOL) | 3.0 ± 1.4 | 2.9 ± 1.1 | 3.1 ± 1.1 | 3.3 ± 1.1 | 3.6 ± 1.5 | 2.9 ± 0.7 |
| DOM | NDOM | |||||
|---|---|---|---|---|---|---|
| Muscle | 0.6 mph | 0.8 mph | 1.0 mph | 0.6 mph | 0.8 mph | 1.0 mph |
| Ipsilateral Biceps Brachii (IBB) | 6.7 ± 3.5 *† | 7.8 ± 3.6 *† | 15.0 ± 9.4 | 7.9 ± 5.8 *† | 7.3 ± 4.4 *† | 15.8 ± 5.2 |
| Contralateral Biceps Brachii (CBB) | 14.7 ± 5.6 | 14.6 ± 6.4 | 15.1 ± 8.1 | 15.5 ± 6.8 | 15.1 ± 5.3 | 14.2 ± 4.5 |
| Ipsilateral Triceps Brachii (ITB) | 48.8 ± 20.8 | 32.8 ± 19.8 | 34.0 ± 17.9 | 56.7 ± 38.6 | 32.1 ± 20.2 | 35.9 ± 12.3 |
| Contralateral Triceps Brachii (CTB) | 58.8 ± 34.5 | 27.5 ± 16.3 # | 33.0 ± 15.0 # | 50.8 ± 43.4 | 24.4 ± 12.6 # | 24.4 ± 10.0 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellenfant, K.B.; Robbins, G.L.; Rogers, R.R.; Kopec, T.J.; Ballmann, C.G. Effects of Dominant and Nondominant Limb Immobilization on Muscle Activation and Physical Demand during Ambulation with Axillary Crutches. J. Funct. Morphol. Kinesiol. 2021, 6, 16. https://doi.org/10.3390/jfmk6010016
Bellenfant KB, Robbins GL, Rogers RR, Kopec TJ, Ballmann CG. Effects of Dominant and Nondominant Limb Immobilization on Muscle Activation and Physical Demand during Ambulation with Axillary Crutches. Journal of Functional Morphology and Kinesiology. 2021; 6(1):16. https://doi.org/10.3390/jfmk6010016
Chicago/Turabian StyleBellenfant, Kara B., Gracie L. Robbins, Rebecca R. Rogers, Thomas J. Kopec, and Christopher G. Ballmann. 2021. "Effects of Dominant and Nondominant Limb Immobilization on Muscle Activation and Physical Demand during Ambulation with Axillary Crutches" Journal of Functional Morphology and Kinesiology 6, no. 1: 16. https://doi.org/10.3390/jfmk6010016
APA StyleBellenfant, K. B., Robbins, G. L., Rogers, R. R., Kopec, T. J., & Ballmann, C. G. (2021). Effects of Dominant and Nondominant Limb Immobilization on Muscle Activation and Physical Demand during Ambulation with Axillary Crutches. Journal of Functional Morphology and Kinesiology, 6(1), 16. https://doi.org/10.3390/jfmk6010016






