Ex Vivo Systems to Study Chondrogenic Differentiation and Cartilage Integration
Abstract
:1. Introduction
Cartilage Defects and Healing Response
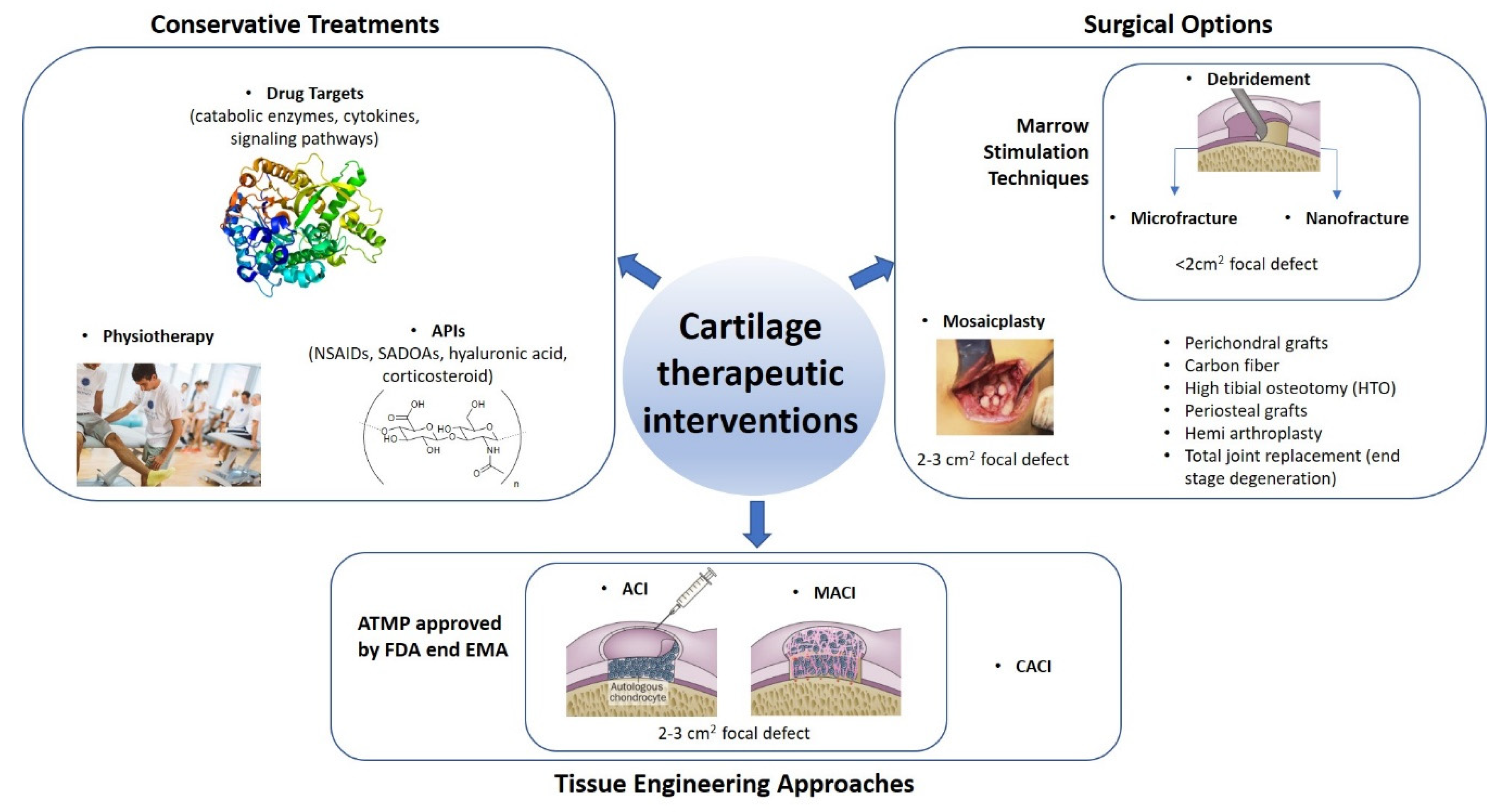
2. Therapeutic Interventions to Attempt Articular Cartilage Repair
3. Tissue Engineering of Osteochondral Implants
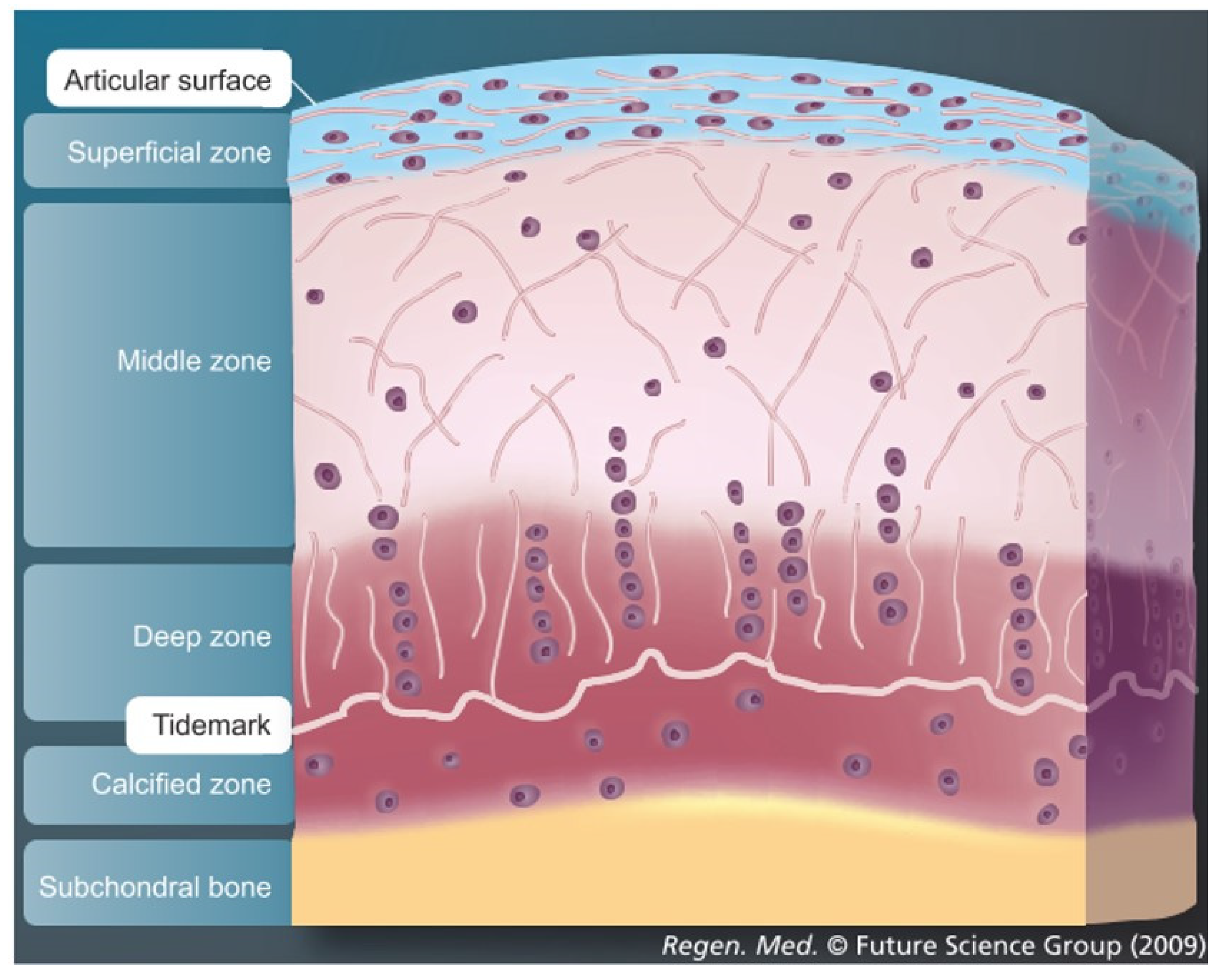
3.1. Importance of Reproducing the Zonal Organization of Articular Cartilage and Subchondral Bone
3.2. Biomaterials & Scaffolds
3.3. Chondro-Inductive and Osteo-Inductive Factor/Molecules/Signals
4. Pellet Culture: A Simple Cartilage Model
5. Cartilage Explant Culture and Cartilage Integration
6. Co-Culture Models
7. Microfluidics
8. Osteochondral Explant, Osteochondral Defect and Culture Models
9. Bioreactor Systems/Loading Devices Used for Osteochondral Applications
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoddart, M.J.; Grad, S.; Eglin, D.; Alini, M. Cells and biomaterials in cartilage tissue engineering. Regen. Med. 2009, 4, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.J.; Malda, J.; Sah, R.L.; Hutmacher, D.W. Tissue Engineering of Articular Cartilage with Biomimetic Zones. Tissue Eng. Part B Rev. 2009, 15, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.A.; Hinckel, B.B.; Bozynski, C.C.; Farr, J. Articular Cartilage: Structure and Restoration; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–24. [Google Scholar]
- Darling, E.M.; Athanasiou, K.A. Retaining Zonal Chondrocyte Phenotype by Means of Novel Growth Environments. Tissue Eng. 2005, 11, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Darling, E.M.; Hu, J.C.Y.; Athanasiou, K.A. Zonal and topographical differences in articular cartilage gene expression. J. Orthop. Res. 2004, 22, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.J.; Schumacher, B.L.; Schmidt, T.A.; Li, K.W.; Voegtline, M.S.; Masuda, K.; Thonar, E.-M.; Sah, R.L. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthr. Cartil. 2003, 11, 595–602. [Google Scholar] [CrossRef]
- Aydelotte, M.B.; Kuettner, K.E. Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connect. Tissue Res. 1988, 18, 205–222. [Google Scholar] [CrossRef]
- Aydelotte, M.B.; Greenhill, R.R.; Kuettner, K.E. Differences between sub-populations of cultured bovine articular chondro-cytes. II. Proteoglycan metabolism. Connect. Tissue Res. 1998, 18, 223–234. [Google Scholar] [CrossRef]
- Hodge, W.A.; Fijan, R.S.; Carlson, K.L.; Burgess, R.G.; Harris, W.H.; Mann, R.W. Contact pressures in the human hip joint measured in vivo. Proc. Natl. Acad. Sci. USA 1986, 83, 2879–2883. [Google Scholar] [CrossRef] [Green Version]
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721. [Google Scholar] [CrossRef]
- Hunziker, E.B. Biologic repair of articular cartilage. Defect models in experimental animals and matrix requirements. Clin. Orthop. Relat. Res. 1999, 367, S135–S146. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Rosenberg, L.C. Repair of partial-thickness defects in articular cartilage: Cell recruitment from the synovial membrane. J. Bone Jt. Surg. Am. 1996, 78, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage repair and transplantation. Arthritis Rheum. 1998, 41, 1331–1342. [Google Scholar] [CrossRef]
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res. 2001, 391, S26–S33. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A. Articular Cartilage: Injuries and Potential for Healing. J. Orthop. Sports Phys. Ther. 1998, 28, 192–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinert, A.F.; Ghivizzani, S.C.; Rethwilm, A.; Tuan, R.S.; Evans, C.H.; Nöth, U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res. Ther. 2007, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Hunziker, E. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Madry, H.; Grün, U.W.; Knutsen, G. Cartilage repair and joint preservation: Medical and surgical treatment options. Dtsch. Ärzteblatt Int. 2011, 108, 669. [Google Scholar]
- Browne, J.E.; Branch, T.P. Surgical Alternatives for Treatment of Articular Cartilage Lesions. J. Am. Acad. Orthop. Surg. 2000, 8, 180–189. [Google Scholar] [CrossRef]
- Marcacci, M.; Filardo, G.; Kon, E. Treatment of cartilage lesions: What works and why? Injury 2013, 44, S11–S15. [Google Scholar] [CrossRef]
- Steinmeyer, J.; Konttinen, Y.T. Oral treatment options for degenerative joint disease—Presence and future. Adv. Drug Deliv. Rev. 2006, 58, 168–211. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Giavaresi, G.; Torricelli, P.; Cavani, F.; Setti, S.; Cane, V.; Giardino, R. Pulsed electromagnetic fields reduce knee os-teoarthritic lesion progression in the aged Dunkin Hartley guinea pig. J. Orthop. Res. 2005, 23, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Drobnic, M.; Madry, H.; Jelic, M.; Van Dijk, N.; Della Villa, S. Non-surgical management of early knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Mandelbaum, B.; Buda, R.; Filardo, G.; Delcogliano, M.; Timoncini, A.; Fornasari, P.M.; Giannini, S.; Marcacci, M. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pa-thology: From early degeneration to osteoarthritis. Arthroscopy 2011, 27, 1490–1501. [Google Scholar] [CrossRef]
- Michael, J.W.-P.; Schlüter-Brust, K.U.; Eysel, P. The Epidemiology, Etiology, Diagnosis, and Treatment of Osteoarthritis of the Knee. Dtsch. Aerzteblatt Online 2010, 107, 152–162. [Google Scholar] [CrossRef]
- Pridie, K. A Method of resurfacing osteoarthric knee joints. J. Bone Jt. Surg. 1959, 41, 618–619. [Google Scholar]
- Steadman, J.R.; Rodkey, W.G.; Singleton, S.B.; Briggs, K.K. Microfracture technique forfull-thickness chondral defects: Technique and clinical results. Oper. Tech. Orthop. 1997, 7, 300–304. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Rodrigo, J.J. Microfracture: Surgical Technique and Rehabilitation to Treat Chondral Defects. Clin. Orthop. Relat. Res. 2001, 391, S362–S369. [Google Scholar] [CrossRef]
- Steadman, J.; Briggs, K.K.; Rodrigo, J.J.; Kocher, M.S.; Gill, T.J.; Rodkey, W.G. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 477–484. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Bursch, L.S.; Olson, E.J.; Havlas, V.; Carlson, C.S. Histologic and immunohistochemical characteristics of failed articular cartilage resurfacing procedures for osteochondritis of the knee: A case series. Am. J. Sports Med. 2008, 36, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Csaki, C.; Clutterbuck, A.L.; Rahmanzadeh, M.; Shakibaei, M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: Applications in cartilage repair and osteoarthritis therapy. Histol. Histopathol. 2009, 24, 347–366. [Google Scholar] [PubMed]
- Zedde, P.; Cudoni, S.; Giachetti, G.; Manunta, M.L.; Masala, G.; Brunetti, A.; Manunta, A.F. Subchondral bone remodeling: Comparing nanofracture with microfracture. An ovine in vivo study. Joints 2016, 4, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Matsusue, Y.; Yamamuro, T.; Hama, H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthrosc. J. Arthrosc. Relat. Surg. 1993, 9, 318–321. [Google Scholar] [CrossRef]
- Meyers, M.H.; Akeson, W.; Convery, F.R. Resurfacing of the knee with fresh osteochondral allograft. J. Bone Jt. Surg. Am. 1989, 71, 704–713. [Google Scholar] [CrossRef]
- Hangody, L.; Füles, P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: Ten years of experimental and clinical experience. J. Bone Jt. Surg. 2003, 85, 25–32. [Google Scholar] [CrossRef]
- Horas, U.; Pelinkovic, D.; Herr, G.; Aigner, T.; Schnettler, R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: A prospective, comparative trial. J. Bone Jt. Surg. 2003, 85, 185–192. [Google Scholar] [CrossRef]
- Matricali, G.; Dereymaeker, G.P.; Luyten, F.P. Donor site morbidity after articular cartilage repair procedures: A review. Acta Orthop. Belg. 2010, 76, 669. [Google Scholar]
- Amendola, A.; Bonasia, D. Results of high tibial osteotomy: Review of the literature. Int. Orthop. 2009, 34, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Alfredson, H.; Lorentzon, R. Superior results with continuous passive motion compared to active motion after periosteal transplantation A retrospective study of human patella cartilage defect treatment. Knee Surg. Sports Traumatol. Arthrosc. 1999, 7, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Angermann, P.; Riegels-Nielsen, P.; Pedersen, H. Osteochondritis dissecans of the femoral condyle treated with periosteal transplantation: Poor outcome in 14 patients followed for 6–9 years. Acta Orthop. Scand. 1998, 69, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Hoikkai, V.E.J.; Jaroma, H.J.; Ritsilä, V.A. Reconstruction of the patellar articulation with periosteal grafts: 4-year follow-up of 13 cases. Acta Orthop. Scand. 1990, 61, 36–39. [Google Scholar] [CrossRef]
- Niedermann, B.; Boe, S.; Lauritzen, J.; Rubak, J.M. Glued periosteal grafts in the knee. Acta Orthop. Scand. 1985, 56, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Lu, S.B.; Wang, J.F. Long-term clinical observation on the repair of large articular cartilage defects of the hip and the knee with free autogeneous periosteum. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi Zhongguo Xiufu Chongjian Waike Zazhi Chin. J. Reparative Reconstr. Surg. 2004, 18, 8–11. [Google Scholar]
- Hamilton, D.; Howie, C.; Burnett, R.; Simpson, A.; Patton, J. Dealing with the predicted increase in demand for revision total knee arthroplasty: Challenges, risks and opportunities. Bone Jt. J. 2015, 97, 723–728. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Brittberg, M. Cell carriers as the next generation of cell therapy for cartilage repair: A review of the matrix-induced autologous chondrocyte implantation procedure. Am. J. Sports Med. 2010, 38, 1259–1271. [Google Scholar] [CrossRef]
- Cherubino, P.; Grassi, F.; Bulgheroni, P.; Ronga, M. Autologous Chondrocyte Implantation Using a Bilayer Collagen Membrane: A Preliminary Report. J. Orthop. Surg. 2003, 11, 10–15. [Google Scholar] [CrossRef]
- Caron, M.M.; Emans, P.J.; Coolsen, M.M.; Voss, L.; Surtel, D.A.; Cremers, A.; van Rhijn, L.W.; Welting, T.J. Rediffer-entiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [Green Version]
- Bieback, K.; Kinzebach, S.; Karagianni, M. Translating Research into Clinical Scale Manufacturing of Mesenchymal Stromal Cells. Stem Cells Int. 2010, 2010, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Committee for Advanced Therapies (CAT); Schneider, C.K.; Salmikangas, P.; Jilma, B.; Flamion, B.; Todorova, L.R.; Paphitou, A.; Haunerova, I.; Maimets, T.; Trouvin, J.-H.; et al. Challenges with advanced therapy medicinal products and how to meet them. Nat. Rev. Drug Discov. 2010, 9, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Parekkadan, B.; Milwid, J.M. Mesenchymal Stem Cells as Therapeutics. Annu. Rev. Biomed. Eng. 2010, 12, 87–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Popp, F.; Verbeek, R.; Masoodi, M.; Nicolaou, A.; Baan, C.; Dahlke, M.-H. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int. Immunopharmacol. 2010, 10, 1496–1500. [Google Scholar] [CrossRef]
- Popp, F.; Eggenhofer, E.; Renner, P.; Slowik, P.; Lang, S.; Kaspar, H.; Geissler, E.K.; Piso, P.; Schlitt, H.J.; Dahlke, M. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl. Immunol. 2008, 20, 55–60. [Google Scholar] [CrossRef]
- Hara, Y.; Stolk, M.; Ringe, J.; Dehne, T.; Ladhoff, J.; Kotsch, K.; Reutzel-Selke, A.; Reinke, P.; Volk, H.-D.; Seifert, M. In vivo effect of bone marrow-derived mesenchymal stem cells in a rat kidney transplantation model with prolonged cold ischemia. Transpl. Int. 2011, 24, 1112–1123. [Google Scholar] [CrossRef]
- Lange, C.; Tögel, F.; Ittrich, H.; Clayton, F.; Nolte-Ernsting, C.; Zander, A.R.; Westenfelder, C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005, 68, 1613–1617. [Google Scholar] [CrossRef] [Green Version]
- Donizetti-Oliveira, C.; Semedo, P.; Burgos-Silva, M.; Cenedeze, M.A.; Malheiros, D.M.A.C.; Reis, M.A.; Pacheco-Silva, A.; Câmara, N.O.S. Adipose tissue-derived stem cell treatment prevents renal disease progression. Cell Transplant. 2012, 21, 1727–1741. [Google Scholar] [CrossRef] [Green Version]
- de Vries-van Melle, M.L.; Tihaya, M.S.; Kops, N.; Koevoet, W.; Murphy, J.M.; Verhaar, J.; Alini, M.; Eglin, D.; van Osch, G. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in a simulated osteochondral en-vironment is hydrogel dependent. Eur. Cell Mater. 2014, 27, 112–123. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Kim, K.-I.; Park, S.; Im, G.-I. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 2014, 35, 3571–3581. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002, 10, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejadnik, H.; Hui, J.H.; Feng Choong, E.P.; Tai, B.-C.; Lee, E.H. Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: An observational cohort study. Am. J. Sports Med. 2010, 38, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Holtzer, H.; Abbott, J.; Lash, J. THE LOSS OF PHENOTYPIC TRAITS BY DIFFERENTIATED CELLS IN VITRO, I. DEDIFFERENTIATION OF CARTILAGE CELLS. Proc. Natl. Acad. Sci. USA 1960, 46, 1533–1542. [Google Scholar] [CrossRef] [Green Version]
- von der Mark, K.; Gauss, V.; von der Mark, H.; Müller, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 1997, 267, 531. [Google Scholar] [CrossRef]
- Banfi, A.; Muraglia, A.; Dozin, B.; Mastrogiacomo, M.; Cancedda, R.; Quarto, R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp. Hematol. 2000, 28, 707–715. [Google Scholar] [CrossRef]
- Smith, J.R.; Pochampally, R.; Perry, A.; Hsu, S.-C.; Prockop, D.J. Isolation of a Highly Clonogenic and Multipotential Subfraction of Adult Stem Cells from Bone Marrow Stroma. Stem Cells 2004, 22, 823–831. [Google Scholar] [CrossRef]
- Fehrer, C.; Lepperdinger, G. Mesenchymal stem cell aging. Exp. Gerontol. 2005, 40, 926–930. [Google Scholar] [CrossRef]
- Kafienah, W.; Mistry, S.; Dickinson, S.C.; Sims, T.J.; Learmonth, I.; Hollander, A.P. Three-dimensional cartilage tissue engi-neering using adult stem cells from osteoarthritis patients. Arthritis Rheumatol. 2007, 56, 177–187. [Google Scholar] [CrossRef]
- Ringe, J.; Sittinger, M. Tissue engineering in the rheumatic diseases. Arthritis Res. Ther. 2009, 11, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for Interferon-γ in the Immunomodulatory Activity of Human Bone Marrow Mesenchymal Stem Cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Singer, N.G.; Caplan, A.I. Mesenchymal Stem Cells: Mechanisms of Inflammation. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 457–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liechty, K.W.; MacKenzie, T.C.; Shaaban, A.F.; Radu, A.; Moseley, A.B.; Deans, R.; Marshak, D.R.; Flake, A.W. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat. Med. 2000, 6, 1282–1286. [Google Scholar] [CrossRef]
- Wu, L.; Prins, H.-J.; Helder, M.N.; van Blitterswijk, C.A.; Karperien, M. Trophic effects of mesenchymal stem cells in chon-drocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng. Part A 2012, 18, 1542–1551. [Google Scholar] [CrossRef]
- Caplan, A. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Stoddart, M.J.; Bara, J.; Alini, M. Cells and secretome—Towards endogenous cell re-activation for cartilage repair. Adv. Drug Deliv. Rev. 2015, 84, 135–145. [Google Scholar] [CrossRef]
- Ochi, M.; Uchio, Y.; Tobita, M.; Kuriwaka, M. Current concepts in tissue engineering technique for repair of cartilage defect. Artif. Organs 2001, 25, 172–179. [Google Scholar] [CrossRef]
- Redman, S.N.; Oldfield, S.F.; Archer, C.W. Current strategies for articular cartilage repair. Eur. Cell Mater. 2005, 9, 23–32. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Madry, H.; Knutsen, G.; Van Dijk, N.; Seil, R.; Brittberg, M.; Kon, E. The subchondral bone in articular cartilage repair: Current problems in the surgical management. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 434–447. [Google Scholar] [CrossRef] [Green Version]
- Kon, E.; Mutini, A.; Arcangeli, E.; Delcogliano, M.; Filardo, G.; Aldini, N.N.; Pressato, D.; Quarto, R.; Zaffagnini, S.; Marcacci, M. Novel nanostructured scaffold for osteochondral regeneration: Pilot study in horses. J. Tissue Eng. Regen. Med. 2010, 4, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health A Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Madry, H.; Van Dijk, C.N.; Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 419–433. [Google Scholar] [CrossRef]
- Bryant, S.J.; Bender, R.J.; Durand, K.L.; Anseth, K.S. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: Engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol. Bioeng. 2004, 86, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Hahn, M.S.; Kim, I.; Nsiah, B.A.; West, J.L. Micropatterning of Poly(Ethylene Glycol) Diacrylate Hydrogels with Biomolecules to Regulate and Guide Endothelial Morphogenesis. Tissue Eng. Part A 2009, 15, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Sundararaghavan, H.G.; Burdick, J.A. Gradients with Depth in Electrospun Fibrous Scaffolds for Directed Cell Behavior. Biomacromolecules 2011, 12, 2344–2350. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Williams, C.G.; Kim, T.K.; Sun, D.; Malik, A.; Khan, M.; Leong, K.; Elisseeff, J.H. Designing zonal organization into tissue-engineered cartilage. Tissue Eng. 2007, 13, 405–414. [Google Scholar] [CrossRef]
- Tew, S.R.; Kwan, A.P.L.; Hann, A.; Thomson, B.M.; Archer, C.W. The reactions of articular cartilage to experimental wounding: Role of apoptosis. Arthritis Rheum. 2000, 43, 215–225. [Google Scholar] [CrossRef]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Prantl, L.; Kujat, R.; Nerlich, M.; Tuan, R.S.; Angele, P. Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of TGF-β isoforms and chondrogenic conditioning. Cells Tissues Organs 2010, 192, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wen, F.; Wu, Y.; Goh, G.S.H.; Ge, Z.; Tan, L.P.; Hui, J.H.P.; Yang, Z. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic com-pression. Biomaterials 2015, 38, 72–85. [Google Scholar] [CrossRef]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 7251–7256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhardt, A.; Lode, A.; Boxberger, S.; Pompe, W.; Gelinsky, M. Mineralised collagen—An artificial, extracellular bone ma-trix—improves osteogenic differentiation of bone marrow stromal cells. J. Mater. Sci. Mater. Med. 2008, 19, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Miot, S.; Barbero, A.; Jakob, M.; Wendt, D. Osteochondral tissue engineering. J. Biomech. 2007, 40, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; Celotti, G.; Landi, E.; Sandri, M.; Roveri, N.; Falini, G. Biologically inspired synthesis of bone-like composite: Self-assembled collagen fibers/hydroxyapatite nanocrystals. J. Biomed. Mater. Res. 2003, 67, 618–625. [Google Scholar] [CrossRef]
- Capito, R.; Spector, M. Scaffold-based articular cartilage repair—Future prospects wedding gene therapy and tissue engineering. IEEE Eng. Med. Boil. Mag. 2003, 22, 42–50. [Google Scholar] [CrossRef]
- Qu, D.; Li, J.; Huang, J.; Khadka, A.; Zuo, Y.; Wang, H.; Liu, Y.; Cheng, L. Ectopic osteochondral formation of biomimetic porous PVA-n-HA/PA6 bilayered scaffold and BMSCs construct in rabbit. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 96, 9–15. [Google Scholar] [CrossRef]
- Im, G.-I.; Ahn, J.-H.; Kim, S.-Y.; Choi, B.-S.; Lee, S.-W. A Hyaluronate–Atelocollagen/β-Tricalcium Phosphate–Hydroxyapatite Biphasic Scaffold for the Repair of Osteochondral Defects: A Porcine Study. Tissue Eng. Part A 2010, 16, 1189–1200. [Google Scholar] [CrossRef]
- Kon, E.; Delcogliano, M.; Filardo, G.; Busacca, M.; Di Martino, A.; Marcacci, M. Novel nano-composite multilayered bio-material for osteochondral regeneration: A pilot clinical trial. Am. J. Sports Med. 2011, 39, 1180–1190. [Google Scholar] [CrossRef]
- Kon, E.; Delcogliano, M.; Filardo, G.; Fini, M.; Giavaresi, G.; Francioli, S.; Martin, I.; Pressato, D.; Arcangeli, E.; Quarto, R.; et al. Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J. Orthop. Res. 2009, 28, 116–124. [Google Scholar] [CrossRef]
- Kon, E.; Delcogliano, M.; Filardo, G.; Pressato, D.; Busacca, M.; Grigolo, B.; Desando, G.; Marcacci, M. A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: Technique note and an early stability pilot clinical trial. Injury 2010, 41, 693–701. [Google Scholar] [CrossRef]
- Lien, S.-M.; Chien, C.-H.; Huang, T.-J. A novel osteochondral scaffold of ceramic–gelatin assembly for articular cartilage repair. Mater. Sci. Eng. C 2009, 29, 315–321. [Google Scholar] [CrossRef]
- Schett, G.; Stolina, M.; Dwyer, D.; Zack, D.; Uderhardt, S.; Krönke, G.; Kostenuik, P.; Feige, U. Tumor necrosis factor α and RANKL blockade cannot halt bony spur formation in experimental inflammatory arthritis. Arthritis Rheum. 2009, 60, 2644–2654. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, S.; Bradica, G.; Brekke, J.; Goldman, S.; Ieska, K.; Issack, P.; Bong, M.; Tian, H.; Gokhale, J.; Coutts, R.; et al. Regeneration of articular cartilage—Evaluation of osteochondral defect repair in the rabbit using multiphasic implants. Osteoarthr. Cartil. 2005, 13, 798–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, J.; Verdonk, P.; Widuchowski, J.; Snow, M.; Weiss, W.; Kruczyński, J. First Clinical Experience with INSTRUCT–A Single Surgery, Autologous Cell Based Technology for Cartilage Repair; European Society of Sports Traumatology, Knee Surgery and Arthroscopy Congress: Luxembourg, 2014; pp. 14–17. [Google Scholar]
- Thorp, B.H.; Anderson, I.; Jakowlew, S.B. Transforming growth factor-beta 1, -beta 2 and -beta 3 in cartilage and bone cells during endochondral ossification in the chick. Development 1992, 114, 907–911. [Google Scholar]
- Mauck, R.L.; Nicoll, S.B.; Seyhan, S.L.; Ateshian, G.A.; Hung, C.T. Synergistic Action of Growth Factors and Dynamic Loading for Articular Cartilage Tissue Engineering. Tissue Eng. 2003, 9, 597–611. [Google Scholar] [CrossRef]
- Byers, B.; Mauck, R.; Chiang, I.; Tuan, R. Temporal exposure of TGF-beta3 under serum-free conditions enhances biome-chanical and biochemical maturation of tissue-engineered cartilage. Trans. Orthop. Res. Soc. 2006, 31, 43. [Google Scholar]
- Tsuchiya, H.; Kitoh, H.; Sugiura, F.; Ishiguro, N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2003, 301, 338–343. [Google Scholar] [CrossRef]
- Miljkovic, N.; Cooper, G.; Marra, K.G. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthr. Cartil. 2008, 16, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Sekiya, I.; Colter, D.C.; Prockop, D.J. BMP-6 Enhances Chondrogenesis in a Subpopulation of Human Marrow Stromal Cells. Biochem. Biophys. Res. Commun. 2001, 284, 411–418. [Google Scholar] [CrossRef]
- Chia, S.-L.; Sawaji, Y.; Burleigh, A.; McLean, C.; Inglis, J.; Saklatvala, J.; Vincent, T.L. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009, 60, 2019–2027. [Google Scholar] [CrossRef]
- Madry, H.; Orth, P.; Kaul, G.; Zurakowski, D.; Menger, M.D.; Kohn, D.; Cucchiarini, M. Acceleration of articular cartilage repair by combined gene transfer of human insulin-like growth factor I and fibroblast growth factor-2 in vivo. Arch. Orthop. Trauma Surg. 2010, 130, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.B.; Chen, E.H.; Lynch, S.E. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Liming, B.; Stoker, A.M.; Marberry, K.M.; Ateshian, G.A.; Christopher, S.A.; Hung, C.T. Effects of Dexamethasone on the Functional Properties of Cartilage Explants during Long-Term Culture. Am. J. Sports Med. 2009, 38, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, H.A.; Halvorsen, Y.D.; Gimble, J.M.; Guilak, F. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 2003, 9, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, A.; Tyler, J.; Hardingham, T. Articular cartilage cultured with interleukin 1. Increased release of link protein, hyaluronate-binding region and other proteoglycan fragments. Biochem. J. 1986, 238, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Aydelotte, M.B.; Raiss, R.X.; Caterson, B.; Kuettner, K.E. Influence of interleukin-1 on the morphology and proteoglycan metabolism of cultured bovine articular chondrocytes. Connect. Tissue Res. 1992, 28, 143–159. [Google Scholar] [CrossRef]
- Lima, E.G.; Tan, A.R.; Tai, T.; Bian, L.; Stoker, A.M.; Ateshian, G.A.; Christopher, S.A.; Hung, C.T. Differences in Interleukin-1 Response Between Engineered and Native Cartilage. Tissue Eng. Part A 2008, 14, 1721–1730. [Google Scholar] [CrossRef]
- Hsiong, S.X.; Mooney, D.J. Regeneration of vascularized bone. Periodontology 2006, 41, 109–122. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef]
- Wang, X.; Wenk, E.; Zhang, X.; Meinel, L.; Vunjak-Novakovic, G.; Kaplan, D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J. Control. Release 2009, 134, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Dormer, N.H.; Singh, M.; Wang, L.; Berkland, C.J.; Detamore, M.S. Osteochondral interface tissue engineering using mac-roscopic gradients of bioactive signals. Ann. Biomed. Eng. 2010, 38, 2167–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-C.; Chang, Y.H.; Chuang, C.-K.; Lin, C.-Y.; Sung, L.-Y.; Wang, Y.-H.; Hu, Y.-C. The repair of osteochondral defects using baculovirus-mediated gene transfer with de-differentiated chondrocytes in bioreactor culture. Biomaterials 2009, 30, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H. Gene therapy for cartilage defects. J. Gene Med. 2005, 7, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Partridge, K.A.; Oreffo, R.O.C. Gene Delivery in Bone Tissue Engineering: Progress and Prospects Using Viral and Nonviral Strategies. Tissue Eng. 2004, 10, 295–307. [Google Scholar] [CrossRef]
- Neumann, A.J.; Schroeder, J.; Alini, M.; Archer, C.W.; Stoddart, M.J. Enhanced Adenovirus Transduction of hMSCs Using 3D Hydrogel Cell Carriers. Mol. Biotechnol. 2012, 53, 207–216. [Google Scholar] [CrossRef]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, Clearance, and Biocompatibility of Iron Oxide Magnetic Nanoparticles in Rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Phillips, M.A.; Gran, M.L.; Peppas, N.A. Targeted nanodelivery of drugs and diagnostics. Nano Today 2010, 5, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Bock, N.; Riminucci, A.; Dionigi, C.; Russo, A.; Tampieri, A.; Landi, E.; Goranov, V.; Marcacci, M.; Dediu, V. A novel route in bone tissue engineering: Magnetic biomimetic scaffolds. Acta Biomater. 2010, 6, 786–796. [Google Scholar] [CrossRef]
- Tampieri, A.; Landi, E.; Valentini, F.; Sandri, M.; D’Alessandro, T.; Dediu, V.; Marcacci, M. A conceptually new type of bio-hybrid scaffold for bone regeneration. Nanotechnology 2010, 22, 015104. [Google Scholar] [CrossRef]
- Fell, H.B. The histogenesis of cartilage and bone in the long bones of the embryonic fowl. J. Morphol. 1925, 40, 417–459. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitrochondrogenesis of bone marrow-derived mes-enchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.B.; Tuan, R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008, 58, 1377–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelttari, K.; Steck, E.; Richter, W. The use of mesenchymal stem cells for chondrogenesis. Injury 2008, 39, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2005, 97, 33–44. [Google Scholar] [CrossRef]
- Cancedda, R.; Castagnola, P.; Cancedda, F.D.; Dozin, B.; Quarto, R. Developmental control of chondrogenesis and osteo-genesis. Int. J. Dev. Biol. 2004, 44, 707–714. [Google Scholar]
- Mackie, E.J.; Tatarczuch, L.; Mirams, M. The skeleton: A multi-functional complex organ. The growth plate chondrocyte and endochondral ossification. J. Endocrinol. 2011, 211, 109–121. [Google Scholar] [CrossRef]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hyper-trophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef]
- Bernstein, P.; Sticht, C.; Jacobi, A.; Liebers, C.; Manthey, S.; Stiehler, M. Expression pattern differences between osteoarthritic chondrocytes and mesenchymal stem cells during chondrogenic differentiation. Osteoarthr. Cartil. 2010, 18, 1596–1607. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, S.; Katz, D.; Oswald, S.; Groneck, L.; Guilak, F. Formation of Osteochondral Organoids from Murine Induced Pluripotent Stem Cells. Tissue Eng. Part A 2020. [Google Scholar] [CrossRef]
- Chawla, S.; Berkelaar, M.H.M.; Dasen, B.; Halleux, C.; Guth-Gundel, S.; Kramer, I.; Ghosh, S.; Martin, I.; Barbero, A.; Oc-chetta, P. Blockage of bone morphogenetic protein signalling counteracts hypertrophy in a human osteoarthritic micro-cartilage model. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- Prosser, A.; Scotchford, C.; Roberts, G.; Grant, D.; Sottile, V. Integrated Multi-Assay Culture Model for Stem Cell Chon-drogenic Differentiation. Int. J. Mol. Sci. 2019, 20, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Su, P.; Xu, C.; Yang, J.; Yu, W.; Huang, D. Chondrogenic differentiation of human mesenchymal stem cells: A comparison between micromass and pellet culture systems. Biotechnol. Lett. 2010, 32, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Tare, R.S.; Howard, D.; Pound, J.C.; Roach, H.I.; Oreffo, R.O. Tissue engineering strategies for cartilage genera-tion—Micromass and three dimensional cultures using human chondrocytes and a continuous cell line. Biochem. Biophys. Res. Commun. 2005, 333, 609–621. [Google Scholar] [CrossRef]
- Murdoch, A.D.; Grady, L.M.; Ablett, M.P.; Katopodi, T.; Meadows, R.S.; Hardingham, T.E.; E Hardingham, T. Chondrogenic Differentiation of Human Bone Marrow Stem Cells in Transwell Cultures: Generation of Scaffold-Free Cartilage. Stem Cells 2007, 25, 2786–2796. [Google Scholar] [CrossRef]
- Ahsan, T.; Lottman, L.M.; Harwood, F.; Amiel, D.; Sah, R.L. Integrative Cartilage Repair: Inhibition by β-Aminopropionitrile. J. Bone Jt. Surg. Am. 2000, 82, 64. [Google Scholar] [CrossRef]
- Hunziker, E. Growth-factor-induced healing of partial-thickness defects in adult articular cartilage. Osteoarthr. Cartil. 2001, 9, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Aeschlimann, D.; Lyons, P.; Masterlark, T.; Hayashi, K.; Graf, B.; Vanderby, R. Repair of cartilage defects with autogenous osteochondral transplants (mosaicplasty) in a sheep model. In Proceedings of the 46th Annual Meeting, Orthopaedic Research Society, Orlando, FL, USA, 12–15 March 2000; p. 183. [Google Scholar]
- Hunziker, E.; Quinn, T.M. SURGICAL REMOVAL OF ARTICULAR CARTILAGE LEADS TO LOSS OF CHONDROCYTES FROM CARTILAGE BORDERING THE WOUND EDGE. J. Bone Jt. Surg. Am. 2003, 85, 85–92. [Google Scholar] [CrossRef]
- Archer, C.W.; Redman, S.; Khan, I.M.; Bishop, J.; Richardson, K. Enhancing tissue integration in cartilage repair procedures. J. Anat. 2006, 209, 481–493. [Google Scholar] [CrossRef]
- Giurea, A.; DiMicco, M.A.; Akeson, W.H.; Sah, R.L. Development-associated differences in integrative cartilage repair: Roles of biosynthesis and matrix. J. Orthop. Res. 2002, 20, 1274–1281. [Google Scholar] [CrossRef]
- Peretti, G.M.; Campo-Ruiz, V.; González, S.; Randolph, M.A.; Xu, J.W.; Morse, K.R.; Roses, R.E.; Yaremchuk, M.J. Tissue Engineered Cartilage Integration to Live and Devitalized Cartilage: A Study by Reflectance Mode Confocal Microscopy and Standard Histology. Connect. Tissue Res. 2006, 47, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.M.; Randolph, M.A.; Caruso, E.M.; Rossetti, F.; Zaleske, D.J. Bonding of cartilage matrices with cultured chon-drocytes: An experimental model. J. Orthop. Res. 1998, 16, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Iwai, R.; Fujiwara, M.; Wakitani, S.; Takagi, M. Ex vivo cartilage defect model for the evaluation of cartilage regeneration using mesenchymal stem cells. J. Biosci. Bioeng. 2011, 111, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Bravenboer, J.V.D.B.; Der Maur, C.D.I.; Bos, P.K.; Feenstra, L.; Verhaar, J.A.N.; Weinans, H.; Van Osch, G.J.V.M. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res. Ther. 2004, 6, R469–R476. [Google Scholar] [CrossRef] [Green Version]
- Vinardell, T.; Thorpe, S.D.; Buckley, C.; Kelly, D.J. Chondrogenesis and Integration of Mesenchymal Stem Cells Within an In Vitro Cartilage Defect Repair Model. Ann. Biomed. Eng. 2009, 37, 2556–2565. [Google Scholar] [CrossRef]
- Yodmuang, S.; Guo, H.; Brial, C.; Warren, R.F.; Torzilli, P.A.; Chen, T.; Maher, S.A. Effect of interface mechanical disconti-nuities on scaffold-cartilage integration. J. Orthop. Res. 2019, 37, 845–854. [Google Scholar] [CrossRef]
- Athens, A.A.; Makris, E.A.; Hu, J. Induced Collagen Cross-Links Enhance Cartilage Integration. PLoS ONE 2013, 8, e60719. [Google Scholar] [CrossRef] [Green Version]
- D’Lima, D.D.; Hashimoto, S.; Chen, P.C.; Lotz, M.K.; Colwell, C.W. Prevention of Chondrocyte Apoptosis. J. Bone Jt. Surg. Am. 2001, 83, 25–26. [Google Scholar] [CrossRef]
- Davidson, E.N.B.; Scharstuhl, A.; Vitters, E.L.; Van Der Kraan, P.M.; Berg, W.B.V.D. Reduced transforming growth factor-beta signaling in cartilage of old mice: Role in impaired repair capacity. Arthritis Res. Ther. 2005, 7, R1338–R1347. [Google Scholar] [CrossRef] [Green Version]
- Parsch, D.; Brümmendorf, T.H.; Richter, W.; Fellenberg, J. Replicative aging of human articular chondrocytes during ex vivo expansion. Arthritis Rheum. 2002, 46, 2911–2916. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. Human chondrocyte senescence and osteoarthritis. Biorheology 2002, 39, 145–152. [Google Scholar] [PubMed]
- Barbero, A.; Grogan, S.; Schäfer, D.; Heberer, M.; Mainil-Varlet, P.; Martin, I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthr. Cartil. 2004, 12, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tew, S.; Redman, S.; Kwan, A.; Walker, E.; Khan, I.; Dowthwaite, G.; Thomson, B.; Archer, C.W. Differences in repair re-sponses between immature and mature cartilage. Clin. Orthop. Relat. Res. 2001, 391, S142–S152. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264. [Google Scholar] [CrossRef]
- Tatebe, M.; Nakamura, R.; Kagami, H.; Okada, K.; Ueda, M. Differentiation of transplanted mesenchymal stem cells in a large osteochondral defect in rabbit. Cytotherapy 2005, 7, 520–530. [Google Scholar] [CrossRef]
- Englert, C.; McGowan, K.B.; Klein, T.; Giurea, A.; Schumacher, B.L.; Sah, R.L. Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum. 2005, 52, 1091–1099. [Google Scholar] [CrossRef]
- Schaefer, D.B.; Wendt, D.; Moretti, M.; Jakob, M.; Jay, G.D.; Heberer, M.; Martin, I. Lubricin reduces cartilage–cartilage in-tegration. Biorheology 2004, 41, 503–508. [Google Scholar]
- Jürgensen, K.; Aeschlimann, D.; Cavin, V.; Genge, M.; Hunziker, E.B. A new biological glue for cartilage-cartilage interfaces: Tissue transglutaminase. J. Bone Jt. Surg. Am. 1997, 79, 185–193. [Google Scholar] [CrossRef]
- Grande, D.; Pitman, M. The use of adhesives in chondrocyte transplantation surgery. Preliminary studies. Bull. Hosp. Jt. Dis. Orthop. Inst. 1988, 48, 140–148. [Google Scholar]
- Johnson, T.S.; Xu, J.-W.; Zaporojan, V.V.; Mesa, J.M.; Weinand, C.; Randolph, M.A.; Bonassar, L.J.; Winograd, J.M.; Yaremchuk, M.J. Integrative repair of cartilage with articular and nonarticular chondrocytes. Tissue Eng. 2004, 10, 1308–1315. [Google Scholar] [CrossRef]
- DiMicco, M.A.; Sah, R.L. Integrative cartilage repair: Adhesive strength is correlated with collagen deposition. J. Orthop. Res. 2001, 19, 1105–1112. [Google Scholar] [CrossRef]
- Englert, C.; Blunk, T.; Fierlbeck, J.; Kaiser, J.; Stosiek, W.; Angele, P.; Hammer, J.; Attur, M.G. Steroid hormones strongly support bovine articular cartilage integration in the absence of interleukin-1β. Arthritis Rheum. 2006, 54, 3890–3897. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Kapfinger, E.; Müller, M. Removal of proteoglycans from the surface of defects in articular cartilage tran-siently enhances coverage by repair cells. J. Bone Jt. Surg. 1998, 80, 144–150. [Google Scholar] [CrossRef]
- Qiu, W.; Murray, M.M.; Shortkroff, S.; Lee, C.R.; Martin, S.D.; Spector, M. Outgrowth of chondrocytes from human articular cartilage explants and expression of α-smooth muscle actin. Wound Repair Regen. 2000, 8, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Bentley, G.; Archer, C.W. Proteoglycan depletion alone is not sufficient to stimulate proteoglycan synthesis in cultured bovine cartilage explants. Osteoarthr. Cartil. 1994, 2, 175–185. [Google Scholar] [CrossRef]
- Seol, D.; Yu, Y.; Choe, H.; Jang, K.; Brouillette, M.J.; Zheng, H.; Lim, T.-H.; Buckwalter, J.A.; Martin, J.A. Effect of Short-Term Enzymatic Treatment on Cell Migration and Cartilage Regeneration: In Vitro Organ Culture of Bovine Articular Cartilage. Tissue Eng. Part A 2014, 20, 1807–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, P.; DeGroot, J.; Budde, M.; Verhaar, J.; Van Osch, G. Specific enzymatic treatment of bovine and human articular car-tilage: Implications for integrative cartilage repair. Arthritis Rheum. 2002, 46, 976–985. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Bentley, G.; Archer, C.W. The control of cell division in articular chondrocytes. Osteoarthr. Cartil. 1993, 1, 137–145. [Google Scholar] [CrossRef]
- Lee, M.C.; Sung, K.P.; Kurtis, M.S.; Akeson, W.H.; Sah, R.L. Adhesive force of chondrocytes to cartilage: Effects of chon-droitinase ABC. Clin. Orthop. Relat. Res. 2000, 370, 286–294. [Google Scholar] [CrossRef]
- Obradovic, B.; Martin, I.; Padera, R.F.; Treppo, S.; Freed, L.E.; Vunjak-Navakovic, G. Integration of engineered cartilage. J. Orthop. Res. 2001, 19, 1089–1097. [Google Scholar] [CrossRef]
- Silverman, R.P.; Bonasser, L.; Passaretti, D.; Randolph, M.A.; Yaremchuk, M.J. Adhesion of tissue-engineered cartilate to native cartilage. Plast. Reconstr. Surg. 2000, 105, 1393–1398. [Google Scholar] [PubMed]
- Hendriks, J.A.A.; Miclea, R.L.; Schotel, R.; De Bruijn, E.; Moroni, L.; Karperien, M.; Riesle, J.; Van Blitterswijk, C.A. Primary chondrocytes enhance cartilage tissue formation upon co-culture with a range of cell types. Soft Matter 2010, 6, 5080–5088. [Google Scholar] [CrossRef]
- De Windt, T.S.; Hendriks, J.A.; Zhao, X.; Vonk, L.A.; Creemers, L.B.; Dhert, W.J.; Randolph, M.A.; Saris, D.B.F. Concise Review: Unraveling Stem Cell Cocultures in Regenerative Medicine: Which Cell Interactions Steer Cartilage Regeneration and How? Stem Cells Transl. Med. 2014, 3, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Glueck, M.; Gardner, O.; Czekanska, E.; Alini, M.; Stoddart, M.J.; Salzmann, G.M.; Schmal, H. Induction of osteogenic dif-ferentiation in human mesenchymal stem cells by crosstalk with osteoblasts. BioRes. Open Access 2015, 4, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solchaga, L.A.; Cassiede, P.; Caplan, A.I. Different response to osteo-inductive agents in bone marrow-and perioste-um-derived cell preparations. Acta Orthop. Scand. 1998, 69, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, J.; Moroni, L.; Riesle, J.; De Wijn, J.; Van Blitterswijk, C. The effect of scaffold-cell entrapment capacity and physico-chemical properties on cartilage regeneration. Biomaterials 2013, 34, 4259–4265. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Chen, G.; Ushida, T.; Matsuno, T.; Tateishi, T. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Mater. Sci. Eng. C 2004, 24, 391–396. [Google Scholar] [CrossRef]
- Gruber, H.E.; Deepe, R.; Hoelscher, G.L.; Ingram, J.A.; Norton, H.J.; Scannell, B.; Loeffler, B.J.; Zinchenko, N.; Hanley, E.N.; Tapp, H. Human Adipose-Derived Mesenchymal Stem Cells: Direction to a Phenotype Sharing Similarities with the Disc, Gene Expression Profiling, and Coculture with Human Annulus Cells. Tissue Eng. Part A 2010, 16, 2843–2860. [Google Scholar] [CrossRef]
- Dahlin, R.L.; Meretoja, V.V.; Ni, M.; Kasper, F.K.; Mikos, A.G. Chondrogenic phenotype of articular chondrocytes in monoculture and co-culture with mesenchymal stem cells in flow perfusion. Tissue Eng. Part A 2014, 20, 2883–2891. [Google Scholar] [CrossRef] [Green Version]
- De Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.M.; Broek, M.P.H.V.D.; Nizak, R.; Van Rijen, M.H.P.; De Weger, R.A.; Dhert, W.J.A.; Saris, D.B.F. Allogeneic Mesenchymal Stem Cells Stimulate Cartilage Regeneration and Are Safe for Single-Stage Cartilage Repair in Humans upon Mixture with Recycled Autologous Chondrons. Stem Cells 2017, 35, 256–264. [Google Scholar] [CrossRef]
- Wu, L.; Leijten, J.C.; Georgi, N.; Post, J.N.; Van Blitterswijk, C.A.; Karperien, H.B.J. Trophic Effects of Mesenchymal Stem Cells Increase Chondrocyte Proliferation and Matrix Formation. Tissue Eng. Part A 2011, 17, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Adesida, A.; Zajac, P.; Mumme, M.; Riesle, J.; Martin, I.; Barbero, A. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J. Cell. Physiol. 2011, 227, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Q.; Xu, F.; Ye, Z.; Zhou, Y.; Tan, W.-S. Mesenchymal stem cells downregulate articular chondrocyte differen-tiation in noncontact coculture systems: Implications in cartilage tissue regeneration. Stem Cells Dev. 2013, 22, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.; Viegas, C.S.B.; Zubía, E.; Magalhães, J.; Ramos, A.; Carvalho, M.M.; Cruz, H.; Sousa, J.P.; Blanco, F.J.; Vermeer, C.; et al. Amentadione from the Alga Cystoseira usneoides as a Novel Osteoarthritis Protective Agent in an Ex Vivo Co-Culture OA Model. Mar. Drugs 2020, 18, 624. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nat. Cell Biol. 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Lopa, S.; Mondadori, C.; Mainardi, V.L.; Talò, G.; Costantini, M.; Candrian, C.; Święszkowski, W.; Moretti, M. Translational Application of Microfluidics and Bioprinting for Stem Cell-Based Cartilage Repair. Stem Cells Int. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Truong, V.X.; Thissen, H.; Frith, J.E.; Forsythe, J.S. Microfluidic Encapsulation of Human Mesenchymal Stem Cells for Articular Cartilage Tissue Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 8589–8601. [Google Scholar] [CrossRef]
- Rosser, J.; Bachmann, B.; Jordan, C.; Ribitsch, I.; Haltmayer, E.; Gueltekin, S.; Junttila, S.; Galik, B.; Gyenesei, A.; Haddadi, B.; et al. Microfluidic nutrient gradient-based three-dimensional chondrocyte cul-ture-on-a-chip as an in vitro equine arthritis model. Mater. Today Bio 2019, 4, 100023. [Google Scholar] [CrossRef]
- Bao, X.; Li, Z.; Liu, H.; Feng, K.; Yin, F.; Li, H.; Qin, J. Stimulation of chondrocytes and chondroinduced mesenchymal stem cells by osteoinduced mesenchymal stem cells under a fluid flow stimulus on an integrated microfluidic device. Mol. Med. Rep. 2017, 17, 2277–2288. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Erickson, A.; You, T.; Dudley, A.T.; Ryu, S. Pneumatic microfluidic cell compression device for high-throughput study of chondrocyte mechanobiology. Lab Chip 2018, 18, 2077–2086. [Google Scholar] [CrossRef]
- Paggi, C.A.; Venzac, B.; Karperien, M.; Leijten, J.C.; Le Gac, S. Monolithic microfluidic platform for exerting gradients of compression on cell-laden hydrogels, and application to a model of the articular cartilage. Sens. Actuators B Chem. 2020, 315, 127917. [Google Scholar] [CrossRef]
- Yin, L.; Wu, Y.; Yang, Z.; Tee, C.A.; Denslin, V.; Lai, Z.; Lim, C.T.; Lee, E.H.; Han, J. Microfluidic label-free selection of mes-enchymal stem cell subpopulation during culture expansion extends the chondrogenic potential in vitro. Lab Chip 2018, 18, 878–889. [Google Scholar] [CrossRef] [Green Version]
- Müller-Rath, R.; Gavénis, K.; Gravius, S.; Andereya, S.; Mumme, T.; Schneider, U. In vivo cultivation of human articular chondrocytes in a nude mouse-based contained defect organ culture model. BioMed. Mater. Eng. 2007, 17, 357–366. [Google Scholar]
- Schüller, G.C.; Tichy, B.; Majdisova, Z.; Jagersberger, T.; van Griensven, M.; Marlovits, S.; Redl, H. An in vivo mouse model for human cartilage regeneration. J. Tissue Eng. Regen. Med. 2008, 2, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; Cross, M.W.; McIlwraith, C.W. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Veter. Comp. Orthop. Traumatol. 2006, 19, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Simon, W.H. Scale effects in animal joints. I. articular cartilage thickness and compressive stress. Arthritis Rheum. 1970, 13, 244–255. [Google Scholar] [CrossRef]
- Sah, R.L.; Ratcliffe, A. Translational Models for Musculoskeletal Tissue Engineering and Regenerative Medicine. Tissue Eng. Part B Rev. 2010, 16, 1–3. [Google Scholar] [CrossRef]
- Ahern, B.; Parvizi, J.; Boston, R.; Schaer, T.P. Preclinical animal models in single site cartilage defect testing: A systematic review. Osteoarthr. Cartil. 2009, 17, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Muschler, G.F.; Raut, V.P.; Patterson, T.E.; Wenke, J.C.; Hollinger, J.O. The Design and Use of Animal Models for Translational Research in Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part B Rev. 2010, 16, 123–145. [Google Scholar] [CrossRef] [Green Version]
- Specchia, N.; Gigante, A.; Falciglia, F.; Greco, F. Fetal chondral homografts in the repair of articular cartilage defects. Bull. Hosp. Jt. Dis. 1996, 54, 230–235. [Google Scholar]
- Ferguson, J.W.; Luyk, N.H.; Parr, N.C. A potential role for costo-chondral grafting in adults with mandibular condylar destruction secondary to rheumatoid arthritis—A case report. J. Cranio-Maxillofac. Surg. 1993, 21, 15–18. [Google Scholar] [CrossRef]
- Törnqvist, E.; Annas, A.; Granath, B.; Jalkesten, E.; Cotgreave, I.; Öberg, M. Strategic focus on 3R principles reveals major reductions in the use of animals in pharmaceutical toxicity testing. PLoS ONE 2014, 9, e101638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flecknell, P. Replacement, reduction and refinement. Altex 2002, 19, 73–78. [Google Scholar] [PubMed]
- Secretan, C.; Bagnall, K.; Jomha, N.M. Effects of introducing cultured human chondrocytes into a human articular cartilage explant model. Cell Tissue Res. 2009, 339, 421–427. [Google Scholar] [CrossRef]
- Melle, M.L.D.V.-V.; Mandl, E.W.; Kops, N.; Koevoet, W.J.; Verhaar, J.A.N.; Van Osch, G.J. An Osteochondral Culture Model to Study Mechanisms Involved in Articular Cartilage Repair. Tissue Eng. Part C Methods 2012, 18, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Espregueira-Mendes, J.; Andrade, R.; Monteiro, A.; Pereira, H.F.C.; Da Silva, M.V.; Oliveira, J.M.; Reis, R.L. Mosaicplasty Using Grafts From the Upper Tibiofibular Joint. Arthrosc. Tech. 2017, 6, e1979–e1987. [Google Scholar] [CrossRef] [Green Version]
- Vainieri, M.L.; Wahl, D.; Alini, M.; Van Osch, G.J.V.M.; Grad, S. Mechanically stimulated osteochondral organ culture for evaluation of biomaterials in cartilage repair studies. Acta Biomater. 2018, 81, 256–266. [Google Scholar] [CrossRef]
- Botter, S.; Van Osch, G.; Waarsing, J.; Van Der Linden, J.; Verhaar, J.A.N.; Pols, H.; Van Leeuwen, J.; Weinans, H. Cartilage damage pattern in relation to subchondral plate thickness in a collagenase-induced model of osteoarthritis. Osteoarthr. Cartil. 2008, 16, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Botter, S.; Glasson, S.; Hopkins, B.; Clockaerts, S.; Weinans, H.; Van Leeuwen, J.; Van Osch, G. ADAMTS5-/- mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: Implications for a link between cartilage and subchondral bone changes. Osteoarthr. Cartil. 2009, 17, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Sniekers, Y.; van Osch, G.; Ederveen, A.; Inzunza, J.; Gustafsson, J.-Å.; van Leeuwen, J.; Weinans, H. Development of oste-oarthritic features in estrogen receptor knockout mice. Osteoarthr. Cartil. 2009, 17, 1356–1361. [Google Scholar] [CrossRef] [Green Version]
- Sniekers, Y.H.; Weinans, H.; Van Osch, G.J.; Van Leeuwen, J. Oestrogen is important for maintenance of cartilage and subchondral bone in a murine model of knee osteoarthritis. Arthritis Res. 2010, 12, R182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intema, F.; Thomas, T.; Anderson, D.; Elkins, J.; Brown, T.; Amendola, A.; Lafeber, F.; Saltzman, C. Subchondral bone re-modeling is related to clinical improvement after joint distraction in the treatment of ankle osteoarthritis. Osteoarthr. Cartil. 2011, 19, 668–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Hu, H.; Tian, F.; Song, H.; Zhang, Y. Enhancement of subchondral bone quality by alendronate administration for the reduction of cartilage degeneration in the early phase of experimental osteoarthritis. Z. Gesamte Exp. Med. 2011, 11, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; Morisset, S.; Ho, C.P.; Rodkey, W.G.; Steadman, J.R.; Mcllwraith, C.W. Effects of Calcified Cartilage on Healing of Chondral Defects Treated with Microfracture in Horses. Am. J. Sports Med. 2006, 34, 1824–1831. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Oxford, J.T.; Southwood, L.; Trotter, G.W.; Rodkey, W.G.; Steadman, J.R.; Goodnight, J.L.; McIlwraith, C.W. Early Events in Cartilage Repair After Subchondral Bone Microfracture. Clin. Orthop. Relat. Res. 2003, 407, 215–227. [Google Scholar] [CrossRef]
- Leyh, M.; Seitz, A.; Dürselen, L.; Schaumburger, J.; Ignatius, A.; Grifka, J.; Grässel, S. Subchondral bone influences chon-drogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. Arthritis Res. Ther. 2014, 16, 453. [Google Scholar] [CrossRef] [Green Version]
- Melle, M.L.D.V.; Narcisi, R.; Kops, N.; Koevoet, W.J.; Bos, P.K.; Murphy, J.M.; Verhaar, J.A.; Van Der Kraan, P.M.; Van Osch, G.J. Chondrogenesis of Mesenchymal Stem Cells in an Osteochondral Environment Is Mediated by the Subchondral Bone. Tissue Eng. Part A 2014, 20, 23–33. [Google Scholar] [CrossRef]
- Bos, P.; Van Osch, G.; Frenz, D.; Verhaar, J.A.N.; Verwoerd-Verhoef, H. Growth factor expression in cartilage wound healing: Temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthr. Cartil. 2001, 9, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Bos, P.; Verhaar, J.; van Osch, G. Age-related differences in articular cartilage wound healing: A potential role for trans-forming growth factor β1 in adult cartilage repair. Adv. Exp. Med. Biol. 2006, 585, 297–309. [Google Scholar]
- Pfander, D.; Gelse, K. Hypoxia and osteoarthritis: How chondrocytes survive hypoxic environments. Curr. Opin. Rheumatol. 2007, 19, 457–462. [Google Scholar] [CrossRef]
- Bakay, A.; Csönge, L.; Papp, G.; Fekete, L. Osteochondral resurfacing of the knee joint with allograft. Int. Orthop. 1998, 22, 277–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, R.; Davis, A.M.; Allan, D.; Langer, F.; Czitrom, A.; Gross, A. Fresh osteochondral allografts for advanced giant cell tumors at the knee. J. Arthroplast. 1994, 9, 603–609. [Google Scholar] [CrossRef]
- Chu, C.R.; Convery, F.R.; Akeson, W.H.; Meyers, M.; Amiel, D. Articular cartilage transplantation. Clinical results in the knee. Clin. Orthop. Relat. Res. 1999, 360, 159–168. [Google Scholar] [CrossRef]
- Garrett, J.C. Treatment of osteochondral defects of the distal femur with fresh osteochondral allografts: A preliminary report. Arthrosc. J. Arthrosc. Relat. Surg. 1986, 2, 222–226. [Google Scholar] [CrossRef]
- Marco, F.; Lopez-Oliva, F.; Fedz-Arroyo, J.M.F.; De Pedro, J.A.; Perez, A.J.; Leon, C.; Lopez-Duran, L. Osteochondral allografts for osteochondritis dissecans and osteonecrosis of the femoral condyles. Int. Orthop. 1993, 17, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; West, M.; Urovitz, E. The immunogenicity of allograft knee joint transplants. Clin. Orthop. Relat. Res. 1978, 132, 155–162. [Google Scholar]
- Kleuskens, M.W.A.; Van Donkelaar, C.C.; Kock, L.M.; Janssen, R.P.A.; Ito, K. An ex vivo human osteochondral culture model. J. Orthop. Res. 2020. [Google Scholar] [CrossRef]
- Linn, F.C.; Sokoloff, L. Movement and composition of interstitial fluid of cartilage. Arthritis Rheum. 2007, 8, 481–494. [Google Scholar] [CrossRef]
- Frank, E.H.; Grodzinsky, A.J. Cartilage electromechanics—I. Electrokinetic transduction and the effects of electrolyte pH and ionic strength. J. Biomech. 1987, 20, 615–627. [Google Scholar] [CrossRef]
- Maroudas, A. Physicochemical properties of articular cartilage. Adult Articul. Cartil. 1979, 2, 215–290. [Google Scholar]
- Maroudas, A.; Bullough, P.; Swanson, S.A.V.; Freeman, M.A.R. THE PERMEABILITY OF ARTICULAR CARTILAGE. J. Bone Jt. Surg. Br. 1968, 50, 166–177. [Google Scholar] [CrossRef]
- Mow, V.; Ateshian, G.; Ratcliffe, A. Anatomic form and biomechanical properties of articular cartilage of the knee joint. In Biology and Biomechanics of the Traumatized Synovial Joint: The Knee as a Model, 2nd ed.; Finerman, G.A.M., Noyes, F.R., Eds.; American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 1992. [Google Scholar]
- Mow, V.C.; Holmes, M.H.; Lai, W.M. Fluid transport and mechanical properties of articular cartilage: A review. J. Biomech. 1984, 17, 377–394. [Google Scholar] [CrossRef]
- Mow, V.; Rosenwasser, M. Articular cartilage: Biomechanics. Injury and repair of the musculoskeletal soft tissues. In Injury and Repair of the Musculoskeletal Soft Tissues; Woo, S.L.-Y., Buckwalter, J.A., Eds.; AAOS: Park Ridge, IL, USA, 1988; Volume 1, pp. 427–463. [Google Scholar]
- Buckwalter, J.; Mankin, H.J. Articular cartilage: Tissue design and chondrocyte-matrix interactions. Instr. Course Lect. 1998, 47, 477–486. [Google Scholar] [PubMed]
- Wall, M.; Butler, D.; El Haj, A.; Bodle, J.C.; Loboa, E.G.; Banes, A.J. Key developments that impacted the field of mechano-biology and mechanotransduction. J. Orthop. Res. 2018, 36, 605–619. [Google Scholar] [PubMed]
- Palmoski, M.J.; Colyer, R.A.; Brandt, K.D. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 1980, 23, 325–334. [Google Scholar] [CrossRef]
- Palmoski, M.J.; Brandt, K.D. Running Inhibits the Reversal of Atrophic Changes in Canine Knee Cartilage After Removal of a Leg Cast. Arthritis Rheum. 1981, 24, 1329–1337. [Google Scholar] [CrossRef]
- Foster, N.C.; Henstock, J.R.; Reinwald, Y.; El Haj, A.J. Dynamic 3D culture: Models of chondrogenesis and endochondral ossification. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.B.; Wong, D.D.; Chao, P.-H.G.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional Tissue Engineering of Articular Cartilage Through Dynamic Loading of Chondrocyte-Seeded Agarose Gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Bader, D.L. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J. Orthop. Res. 1997, 15, 181–188. [Google Scholar] [CrossRef]
- Lomas, C.; Tang, X.D.; Chanalaris, A.; Saklatvala, J.; Vincent, T.L. Cyclic mechanical load causes global translational arrest in articular chondrocytes: A process which is partially dependent upon PKR phosphorylation. Eur. Cell Mater 2011, 22, 178–189. [Google Scholar] [CrossRef]
- Williams, D.F. The Williams Dictionary of Biomaterials; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Wolff, J. The Law of Bone Remodeling; Springer: Berlin/Heildelberg, Germany, 1986. [Google Scholar]
- Nagel, T.; Kelly, D.J. Mechanically induced structural changes during dynamic compression of engineered cartilaginous constructs can potentially explain increases in bulk mechanical properties. J. R. Soc. Interface 2011, 9, 777–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, J.; Schrobback, K.; Hutmacher, D.; Klein, T. Dynamic compression improves biosynthesis of human zonal chondrocytes from osteoarthritis patients. Osteoarthr. Cartil. 2012, 20, 906–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.W.; Mauck, R.L.; Statman, L.Y.; Lin, E.Y.; Ateshian, G.A.; Hung, C.T. Dynamic deformational loading results in se-lective application of mechanical stimulation in a layered, tissue-engineered cartilage construct. Biorheology 2006, 43, 497–507. [Google Scholar] [PubMed]
- Li, K.W.; Klein, T.; Chawla, K.; Nugent, G.E.; Bae, W.C.; Sah, R.L. In Vitro Physical Stimulation of Tissue-Engineered and Native Cartilage. Cartil. Osteoarthr. 2004, 100, 325–352. [Google Scholar] [CrossRef]
- Heath, C.A.; Magari, S.R. Mini-review: Mechanical factors affecting cartilage regeneration in vitro. Biotechnol. Bioeng. 2000, 50, 430–437. [Google Scholar] [CrossRef]
- Daly, A.C.; Sathy, B.N.; Kelly, D.J. Engineering large cartilage tissues using dynamic bioreactor culture at defined oxygen conditions. J. Tissue Eng. 2018, 9, 2041731417753718. [Google Scholar] [CrossRef] [Green Version]
- Meinert, C.; Schrobback, K.; Hutmacher, D.W.; Klein, T. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Nazempour, A.; Van Wie, B.J. A flow perfusion bioreactor with controlled mechanical stimulation: Application in cartilage tissue engineering and beyond. J. Stem Cell Ther. Transplant. 2018, 2, 15–34. [Google Scholar]
- Jonnalagadda, U.S.; Hill, M.; Messaoudi, W.; Cook, R.B.; Oreffo, R.O.C.; Glynne-Jones, P.; Tare, R.S. Acoustically modulated biomechanical stimulation for human cartilage tissue engineering. Lab Chip 2018, 18, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.S.; Eckert, H.; Hess, R.; Gelinsky, M.; Rancourt, D.; Krawetz, R.; Cuniberti, G.; Scharnweber, D. Developing a cus-tomized perfusion bioreactor prototype with controlled positional variability in oxygen partial pressure for bone and car-tilage tissue engineering. Tissue Eng. Part C Methods 2017, 23, 286–297. [Google Scholar] [CrossRef]
- Grad, S.; Eglin, D.; Alini, M.; Stoddart, M.J. Physical Stimulation of Chondrogenic Cells In Vitro: A Review. Clin. Orthop. Relat. Res. 2011, 469, 2764–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, A.; Van de Velde, S.K.; Kozanek, M.; Gill, T.J.; Grodzinsky, A.J.; Rubash, H.E.; Li, G. In-vivo time-dependent ar-ticular cartilage contact behavior of the tibiofemoral joint. Osteoarthr. Cartil. 2010, 18, 909–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozanek, M.; Hosseini, A.; Liu, F.; Van De Velde, S.K.; Gill, T.J.; Rubash, H.E.; Li, G. Tibiofemoral kinematics and condylar motion during the stance phase of gait. J. Biomech. 2009, 42, 1877–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sucosky, P.; Osorio, D.F.; Brown, J.B.; Neitzel, G.P. Fluid mechanics of a spinner-flask bioreactor. Biotechnol. Bioeng. 2003, 85, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Begley, C.M.; Kleis, S.J. The fluid dynamic and shear environment in the NASA/JSC rotating-wall perfused-vessel biore-actor. Biotechnol. Bioeng. 2000, 70, 32–40. [Google Scholar] [CrossRef]
- Galban, C.J.; Locke, B.R. Effects of spatial variation of cells and nutrient and product concentrations coupled with product inhibition on cell growth in a polymer scaffold. Biotechnol. Bioeng. 1999, 64, 633–643. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Obradovic, B.; Martin, I.; Freed, L. Bioreactor studies of native and tissue engineered cartilage. Biorheol. 2002, 39, 259–268. [Google Scholar]
- Marsano, A.; Wendt, D.; Quinn, T.; Sims, T.; Farhadi, J.; Jakob, M.; Heberer, M.; Martin, I. Bi-zonal cartilaginous tissues en-gineered in a rotary cell culture system. Biorheology 2006, 43, 553–560. [Google Scholar]
- Sikavitsas, V.I.; Bancroft, G.N.; Holtorf, H.L.; Jansen, J.A.; Mikos, A.G. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc. Natl. Acad. Sci. USA 2003, 100, 14683–14688. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.A.; Sondh, I.S.; Litte, S.R.; Zunino, P.; Gottardi, R. Design and validation of an osteochondral bioreactor for the screening of treatments for osteoarthritis. Biomed. Microdevices 2018, 20, 18. [Google Scholar] [CrossRef] [Green Version]
- Reinwald, Y.; Leonard, K.H.; Henstock, J.R.; Whiteley, J.P.; Osborne, J.M.; Waters, S.L.; Lévesque, P.; El Haj, A.J. Evaluation of the growth environment of a hydrostatic force bioreactor for preconditioning of tissue-engineered constructs. Tissue Eng. Part C Methods 2015, 21, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyland, J.; Wiegandt, K.; Goepfert, C.; Nagel-Heyer, S.; Ilinich, E.; Schumacher, U.; Pörtner, R. Redifferentiation of chon-drocytes and cartilage formation under intermittent hydrostatic pressure. Biotechnol. Lett. 2006, 28, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S. A novel method for assessing effects of hydrostatic fluid pressure on intracellular calcium: A study with bovine articular chondrocytes. Am. J. Physiol. Physiol. 2005, 288, C329–C337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, G.M.; Chan, E.F.; Temple-Wong, M.M.; Bae, W.C.; Masuda, K.; Bugbee, W.D.; Sah, R.L. Shape, loading, and motion in the bioengineering design, fabrication, and testing of personalized synovial joints. J. Biomech. 2010, 43, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, D.L.; Goldstein, S.A.; Guldberg, R.E.; Guo, X.E.; Kamm, R.D.; Laurencin, C.T.; McIntire, L.V.; Mow, V.C.; Nerem, R.M.; Sah, R.L.; et al. The Impact of Biomechanics in Tissue Engineering and Regenerative Medicine. Tissue Eng. Part B: Rev. 2009, 15, 477–484. [Google Scholar] [CrossRef]
- Jin, M.; Frank, E.H.; Quinn, T.M.; Hunziker, E.B.; Grodzinsky, A.J. Tissue Shear Deformation Stimulates Proteoglycan and Protein Biosynthesis in Bovine Cartilage Explants. Arch. Biochem. Biophys. 2001, 395, 41–48. [Google Scholar] [CrossRef]
- Sah, R.L.-Y.; Kim, Y.-J.; Doong, J.-Y.H.; Grodzinsky, A.J.; Plass, A.H.K.; Sandy, J.D. Biosynthetic response of cartilage explants to dynamic compression. J. Orthop. Res. 1989, 7, 619–636. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Gluzband, Y.; Grodzinsky, A.J.; Hunziker, E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J. Cell Sci. 1995, 108, 1497–1508. [Google Scholar]
- Nugent-Derfus, G.; Takara, T.; O’Neill, J.; Cahill, S.; Gortz, S.; Pong, T.; Inoue, H.; Aneloski, N.; Wang, W.; Vega, K.; et al. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthr. Cartil. 2007, 15, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Czichos, H.; Dowson, D. Tribology: A systems approach to the Science and Technology of friction, lubrication and wear. Tribol. Int. 1978, 11, 259–260. [Google Scholar] [CrossRef]
- Wimmer, M.A.; Grad, S.; Kaup, T.; Hänni, M.; Schneider, E.; Gogolewski, S.; Alini, M. Tribology approach to the engineering and study of articular cartilage. Tissue Eng. 2004, 10, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Vainieri, M.L.; Alini, M.; Yayon, A.; van Osch, G.; Grad, S. Mechanical Stress Inhibits Early Stages of Endogenous Cell Mi-gration: A Pilot Study in an Ex Vivo Osteochondral Model. Polymers 2020, 12, 1754. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, M.J.; Ettinger, L.; Häuselmann, H.J. Enhanced matrix synthesis in de novo, scaffold free cartilage-like tissue subjected to compression and shear. Biotechnol. Bioeng. 2006, 95, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.V.; Wang, Q.G.; Kuiper, N.J.; El Haj, A.J.; Thomas, C.R.; Zhang, Z. Biomechanical properties of single chondro-cytes and chondrons determined by micromanipulation and finite-element modelling. J. R. Soc. Interface 2010, 7, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of Mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Allori, A.C.; Sailon, A.M.; Pan, J.H.; Warren, S.M. Biological Basis of Bone Formation, Remodeling, and Repair—Part III: Biomechanical Forces. Tissue Eng. Part B Rev. 2008, 14, 285–293. [Google Scholar] [CrossRef]
- Fahy, N.; Alini, M.; Stoddart, M. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J. Orthop. Res. 2018, 36, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Schatti, O.; Grad, S.; Goldhahn, J.; Salzmann, G.; Li, Z.; Alini, M.; Stoddart, M. A combination of shear and dynamic com-pression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur. Cell Mater. 2011, 22, b97. [Google Scholar]
- Huang, A.H.; Farrell, M.J.; Kim, M.; Mauck, R.L. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur. Cell Mater. 2010, 19, 72–85. [Google Scholar] [CrossRef]
- Thorpe, S.; Buckley, C.; Vinardell, T.; O’Brien, F.J.; Campbell, V.; Kelly, D.J. Dynamic compression can inhibit chondrogenesis of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2008, 377, 458–462. [Google Scholar] [CrossRef]
- Kupcsik, L.; Stoddart, M.J.; Li, Z.; Benneker, L.M.; Alini, M. Improving Chondrogenesis: Potential and Limitations ofSOX9Gene Transfer and Mechanical Stimulation for Cartilage Tissue Engineering. Tissue Eng. Part A 2010, 16, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kupcsik, L.; Yao, S.-J.; Alini, M.; Stoddart, M.J. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-β pathway. J. Cell. Mol. Med. 2009, 14, 1338–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, O.; Fahy, N.; Alini, M.; Stoddart, M.J. Differences in human mesenchymal stem cell secretomes during chondrogenic induction. Eur. Cells Mater. 2016, 31, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Gardner, O.F.; Fahy, N.; Alini, M.; Stoddart, M.J. Joint mimicking mechanical load activates TGFβ1 in fibrin-poly (es-ter-urethane) scaffolds seeded with mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 2663–2666. [Google Scholar] [CrossRef] [PubMed]
- Grad, S.; Loparic, M.; Peter, R.; Stolz, M.; Aebi, U.; Alini, M. Sliding motion modulates stiffness and friction coefficient at the surface of tissue engineered cartilage. Osteoarthr. Cartil. 2012, 20, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Balazs, E.A. The physical properties of synovial fluid and the special role of hyaluronic acid. Disord. Knee 1974, 2, 61–74. [Google Scholar]
- Funck-Brentano, T.; Cohen-Solal, M. Crosstalk between cartilage and bone: When bone cytokines matter. Cytokine Growth Factor Rev. 2011, 22, 91–97. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaco, G.; El Haj, A.J.; Alini, M.; Stoddart, M.J. Ex Vivo Systems to Study Chondrogenic Differentiation and Cartilage Integration. J. Funct. Morphol. Kinesiol. 2021, 6, 6. https://doi.org/10.3390/jfmk6010006
Monaco G, El Haj AJ, Alini M, Stoddart MJ. Ex Vivo Systems to Study Chondrogenic Differentiation and Cartilage Integration. Journal of Functional Morphology and Kinesiology. 2021; 6(1):6. https://doi.org/10.3390/jfmk6010006
Chicago/Turabian StyleMonaco, Graziana, Alicia J. El Haj, Mauro Alini, and Martin J. Stoddart. 2021. "Ex Vivo Systems to Study Chondrogenic Differentiation and Cartilage Integration" Journal of Functional Morphology and Kinesiology 6, no. 1: 6. https://doi.org/10.3390/jfmk6010006
APA StyleMonaco, G., El Haj, A. J., Alini, M., & Stoddart, M. J. (2021). Ex Vivo Systems to Study Chondrogenic Differentiation and Cartilage Integration. Journal of Functional Morphology and Kinesiology, 6(1), 6. https://doi.org/10.3390/jfmk6010006





