Sedentary Behaviour Impairs Skeletal Muscle Repair Modulating the Inflammatory Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Tissue Collection and Processing for Light Microscopy
2.3. Histomorphometry, Fibrosis, and Immunohistochemistry Analysis
2.4. Statistical Analysis
3. Results
3.1. Body Weight and Voluntary Physical Activity
3.2. Histomorphometry, Fibrosis, and Immunohistochemistry Analysis
3.3. First Day Post-Injury
3.4. Seventh Day Post-Injury
3.5. 15th Day Post-Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thyfault, J.P.; Du, M.; Kraus, W.E.; Levine, J.A.; Booth, F.W. Physiology of sedentary behavior and its relationship to health outcomes. Med. Sci. Sports Exerc. 2015, 47, 1301. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Tarp, J.; Fagerland, M.W.; Johannessen, J.S.; Hansen, B.H.; Jefferis, B.J.; Whincup, P.H.; Diaz, K.M.; Hooker, S.; Howard, V.J.; et al. Joint associations of accelerometer-measured physical activity and sedentary time with all-cause mortality: A harmonised meta-analysis in more than 44 000 middle-aged and older individuals. Br. J. Sports Med. 2020, 54, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143. [Google Scholar] [CrossRef]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Sass, F.A.; Fuchs, M.; Pumberger, M.; Geissler, S.; Duda, G.N.; Perka, C.; Schmidt-Bleek, K. Immunology Guides Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2018, 19, 835. [Google Scholar] [CrossRef]
- Chang, N.C.; Rudnicki, M.A. Satellite Cells: The architects of skeletal muscle. Curr. Top. Dev. Biol. 2014, 107, 161–181. [Google Scholar] [CrossRef]
- Saclier, M.; Yacoub-Youssef, H.; Mackey, A.L.; Arnold, L.; Ardjoune, H.; Magnan, M.; Sailhan, F.; Chelly, J.; Pavlath, G.K.; Mounier, R.; et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 2013, 31, 384–396. [Google Scholar] [CrossRef]
- Wang, H.; Melton, D.W.; Porter, L.; Sarwar, Z.U.; McManus, L.M.; Shireman, P.K. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am. J. Pathol. 2014, 184, 1167–1184. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Serrano, A.L. Macrophages decide between regeneration and fibrosis in muscle. Trends Endocrinol. Metab. 2015, 26, 449–450. [Google Scholar] [CrossRef]
- Teixeira, E.; Duarte, J.A. Skeletal muscle loading changes its regenerative capacity. Sports Med. 2016, 46, 783–792. [Google Scholar] [CrossRef] [PubMed]
- da Silva Rossato, J.; Krause, M.; Fernandes, A.J.M.; Fernandes, J.R.; Seibt, I.L.; Rech, A.; Homem de Bittencourt, P.I. Role of alpha-and beta-adrenoreceptors in rat monocyte/macrophage function at rest and acute exercise. J. Physiol. Biochem. 2014, 70, 363–374. [Google Scholar] [CrossRef]
- Silveira, L.S.; Antunes, B.D.M.M.; Minari, A.L.A.; Dos Santos, R.V.T.; Neto, J.C.R.; Lira, F.S. Macrophage polarization: Implications on metabolic diseases and the role of exercise. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 115–132. [Google Scholar] [CrossRef]
- Fonseca, H.; Powers, S.K.; Goncalves, D.; Santos, A.; Mota, M.P.; Duarte, J.A. Physical inactivity is a major contributor to ovariectomy-induced sarcopenia. Int. J. Sports Med. 2012, 33, 268–278. [Google Scholar] [CrossRef]
- Harriss, D.J.; Macsween, A.; Atkinson, G. Standards for Ethics in Sport and Exercise Science Research: 2018 Update. Int. J. Sports Med. 2017, 38, 1126–1131. [Google Scholar] [CrossRef]
- Sweat, F.; Puchter, H.; Rosenthal, S.I. Sirius red F3BA as a stain for connective tissue. Arch. Pathol. 1964, 78, 69–72. [Google Scholar]
- Garcia, J.; Costa, V.M.; Carvalho, A.T.; Silvestre, R.; Duarte, J.A.; Dourado, D.F.; Arbo, M.D.; Baltazar, T.; Dinis-Oliveira, R.J.; Baptista, P.; et al. A breakthrough on Amanita phalloides poisoning: An effective antidotal effect by polymyxin B. Arch. Pathol. 2015, 89, 2305–2323. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Zissler, A.; Steinbacher, P.; Zimmermann, R.; Pittner, S.; Stoiber, W.; Bathke, A.C.; Sänger, A.M. Extracorporeal shock wave therapy accelerates regeneration after acute skeletal muscle injury. Am. J. Sports Med. 2017, 45, 676–684. [Google Scholar] [CrossRef]
- Askling, C.M.; Tengvar, M.; Thorstensson, A. Acute hamstring injuries in Swedish elite football: A prospective randomised controlled clinical trial comparing two rehabilitation protocols. Br. J. Sports Med. 2013, 47, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Askling, C.M.; Tengvar, M.; Tarassova, O.; Thorstensson, A. Acute hamstring injuries in Swedish elite sprinters and jumpers: A prospective randomised controlled clinical trial comparing two rehabilitation protocols. Br. J. Sports Med. 2014, 48, 532–539. [Google Scholar] [CrossRef]
- Kjær, M.; Langberg, H.; Heinemeier, K.; Bayer, M.L.; Hansen, M.; Holm, L.; Doessing, S.; Kongsgaard, M.; Krogsgaard, M.R.; Magnusson, S.P. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand. J. Med. Sci. Sports 2009, 19, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Ra, Y.J.; Lee, K.M.; Lee, J.Y.; Ghil, S.H. Therapeutic effect of passive mobilization exercise on improvement of muscle regeneration and prevention of fibrosis after laceration injury of rat. Arch. Phys. Med. Rehabil. 2006, 87, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Vandercappellen, E.J.; Koster, A.; Savelberg, H.H.; Eussen, S.J.; Dagnelie, P.C.; Schaper, N.C.; Schram, M.T.; van der Kallen, C.J.; van Greevenbroek, M.M.; Wesselius, A.; et al. Sedentary behaviour and physical activity are associated with biomarkers of endothelial dysfunction and low-grade inflammation—relevance for (pre) diabetes: The Maastricht Study. Diabetologia 2022, 65, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Collao, N.; Rada, I.; Francaux, M.; Deldicque, L.; Zbinden-Foncea, H. Anti-Inflammatory Effect of Exercise Mediated by Toll-Like Receptor Regulation in Innate Immune Cells—A Review: Anti-inflammatory effect of exercise mediated by Toll-like receptor regulation in innate immune cells. Int. Rev. Immunol. 2020, 39, 39–52. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Sorensen, J.R.; Kaluhiokalani, J.P.; Hafen, P.S.; Deyhle, M.R.; Parcell, A.C.; Hyldahl, R.D. An altered response in macrophage phenotype following damage in aged human skeletal muscle: Implications for skeletal muscle repair. FASEB J. 2019, 33, 10353–10368. [Google Scholar] [CrossRef]
- Runhaar, J.; Bierma-Zeinstra, S.M.A. Should exercise therapy for chronic musculoskeletal conditions focus on the anti-inflammatory effects of exercise? Br. J. Sports Med. 2017, 51, 762–763. [Google Scholar] [CrossRef]
- Mahdy, M.A. Skeletal muscle fibrosis: An overview. Cell Tissue Res. 2019, 375, 575–588. [Google Scholar] [CrossRef]
- Huang, E.; Peng, N.; Xiao, F.; Hu, D.; Wang, X.; Lu, L. The roles of immune cells in the pathogenesis of fibrosis. Int. J. Mol. Sci. 2020, 21, 5203. [Google Scholar] [CrossRef]
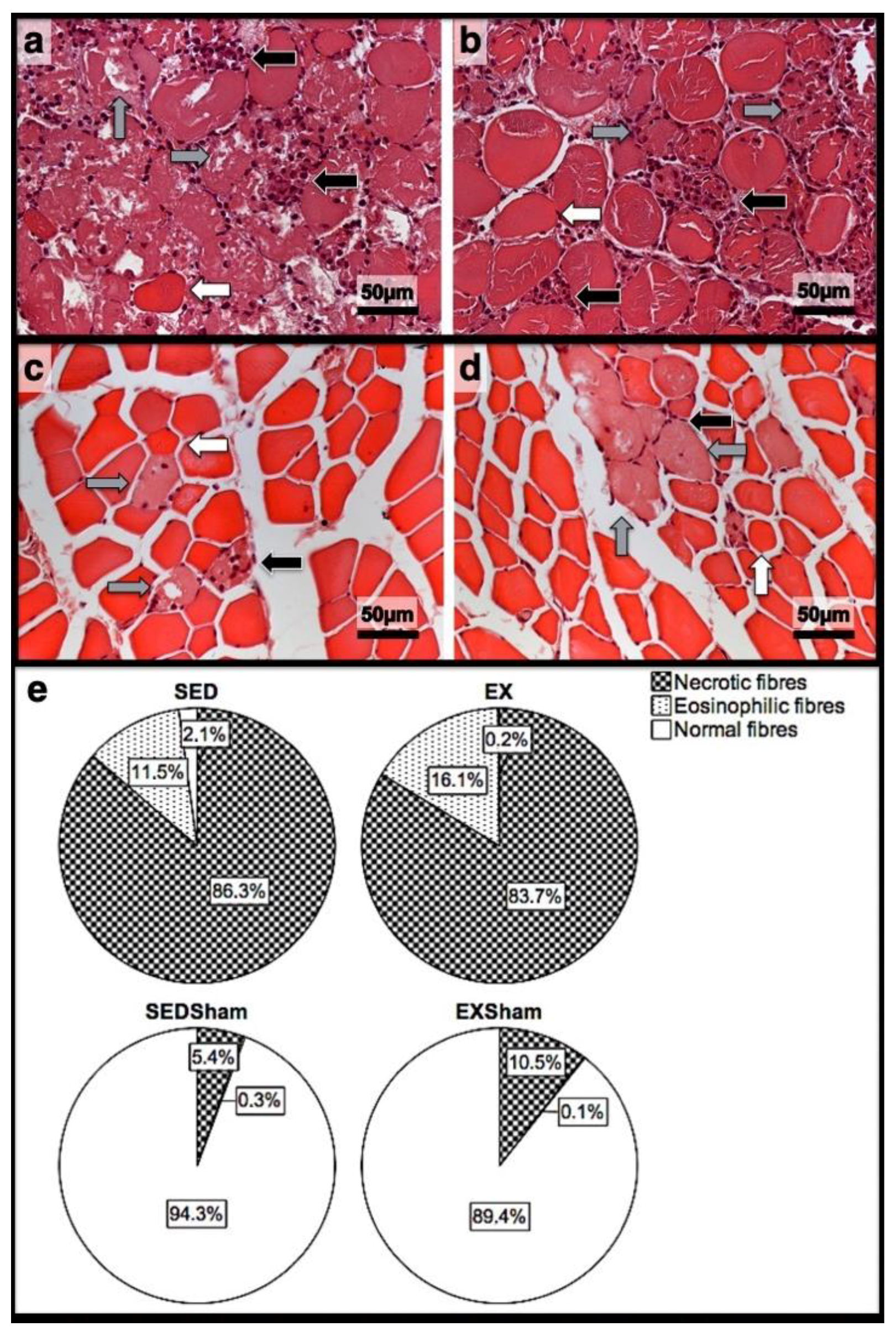

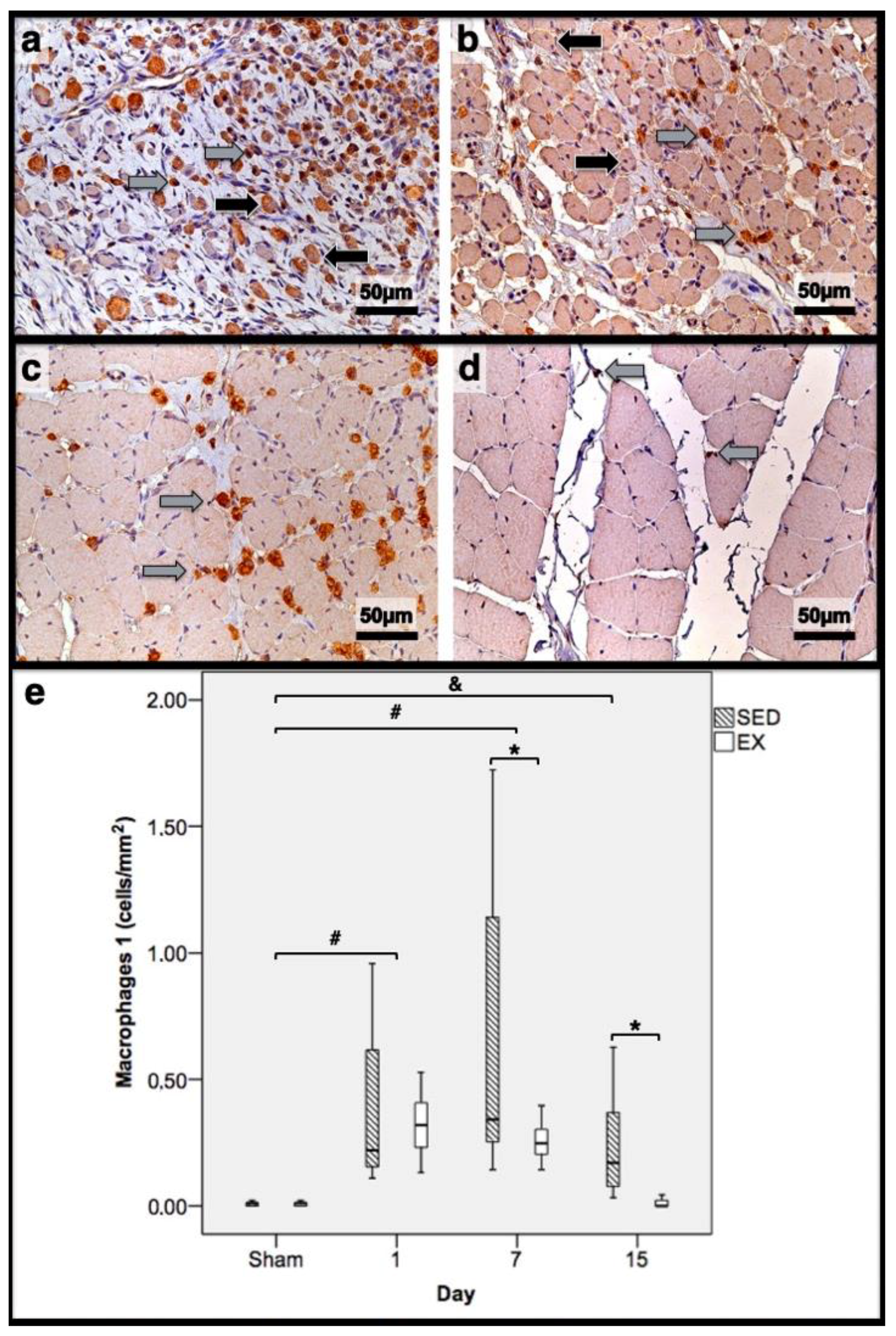
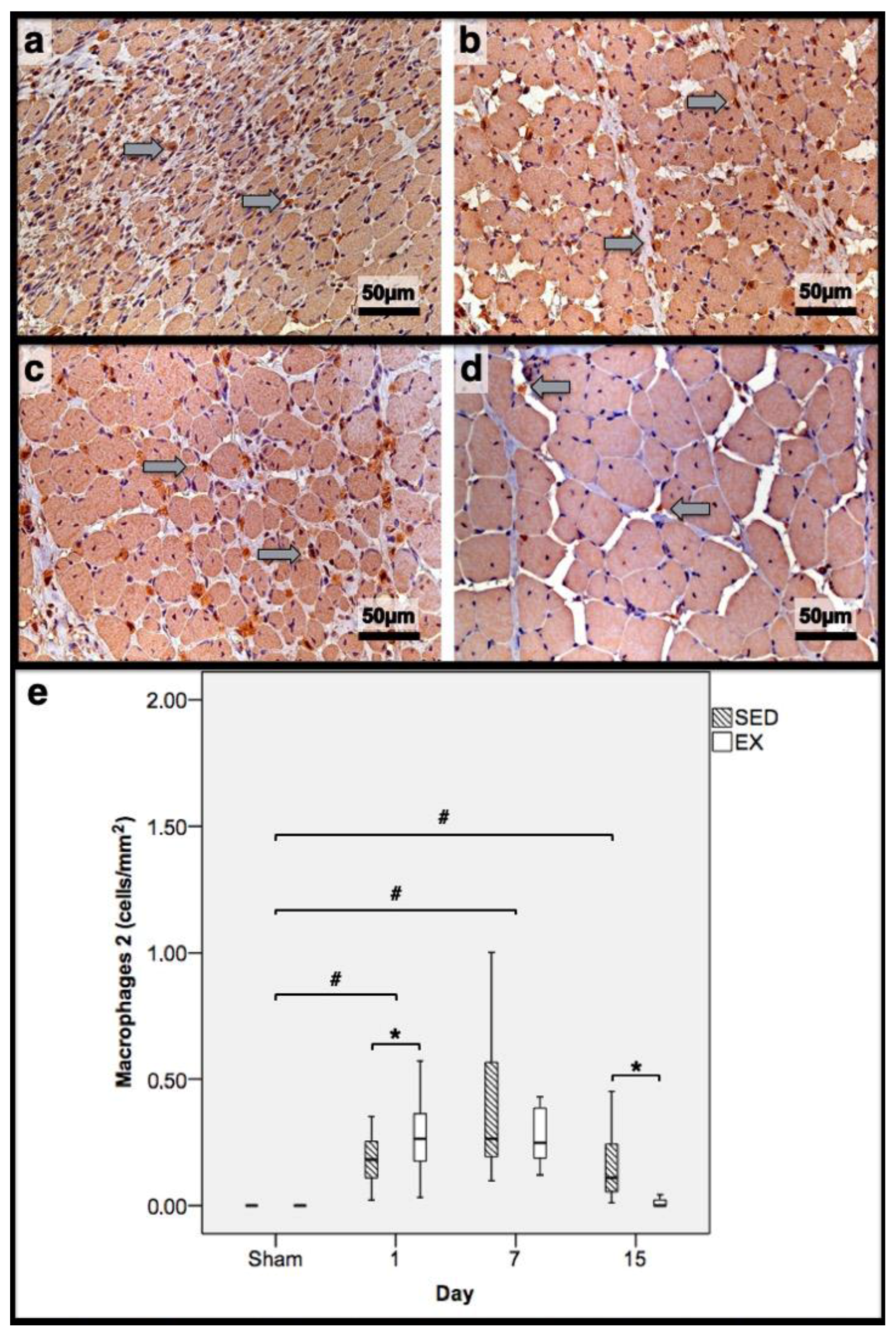

| Histomorphometry | Immunohistochemistry (Cells/mm2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Necrotic Fibres (%) | Myotubes (%) | Fibrosis (mm2) | Macrophages 1 | Macrophages 2 | Neutrophils | |||||||
| Day/Group | SED | EX | SED | EX | SED | EX | SED | EX | SED | EX | SED | EX |
| 1st dpi | 86.3 | 83.7 | 0.0 | 0.0 | 11.7 | 11.1 | 0.22 | 0.32 | 0.18 * | 0.26 | 0.31 * | 0.20 |
| 7th dpi | 0.0 | 0.0 | 58.4 * | 69.3 | 36.1 * | 24.9 | 0.34 * | 0.24 | 0.26 | 0.25 | 0.81 * | 0.58 |
| 15th dpi | 0.0 | 0.0 | 46.0 * | 10.2 | 17.9 * | 8.4 | 0.17 * | 0.00 | 0.11 * | 0.00 | 0.03 * | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, E.; Garcia, J.; Bovolini, A.; Carvalho, A.; Pacheco, J.; Duarte, J.A. Sedentary Behaviour Impairs Skeletal Muscle Repair Modulating the Inflammatory Response. J. Funct. Morphol. Kinesiol. 2022, 7, 76. https://doi.org/10.3390/jfmk7040076
Teixeira E, Garcia J, Bovolini A, Carvalho A, Pacheco J, Duarte JA. Sedentary Behaviour Impairs Skeletal Muscle Repair Modulating the Inflammatory Response. Journal of Functional Morphology and Kinesiology. 2022; 7(4):76. https://doi.org/10.3390/jfmk7040076
Chicago/Turabian StyleTeixeira, Eduardo, Juliana Garcia, António Bovolini, Ana Carvalho, Júlio Pacheco, and José A. Duarte. 2022. "Sedentary Behaviour Impairs Skeletal Muscle Repair Modulating the Inflammatory Response" Journal of Functional Morphology and Kinesiology 7, no. 4: 76. https://doi.org/10.3390/jfmk7040076
APA StyleTeixeira, E., Garcia, J., Bovolini, A., Carvalho, A., Pacheco, J., & Duarte, J. A. (2022). Sedentary Behaviour Impairs Skeletal Muscle Repair Modulating the Inflammatory Response. Journal of Functional Morphology and Kinesiology, 7(4), 76. https://doi.org/10.3390/jfmk7040076







