Whole Body Vibration: A Valid Alternative Strategy to Exercise?
Abstract
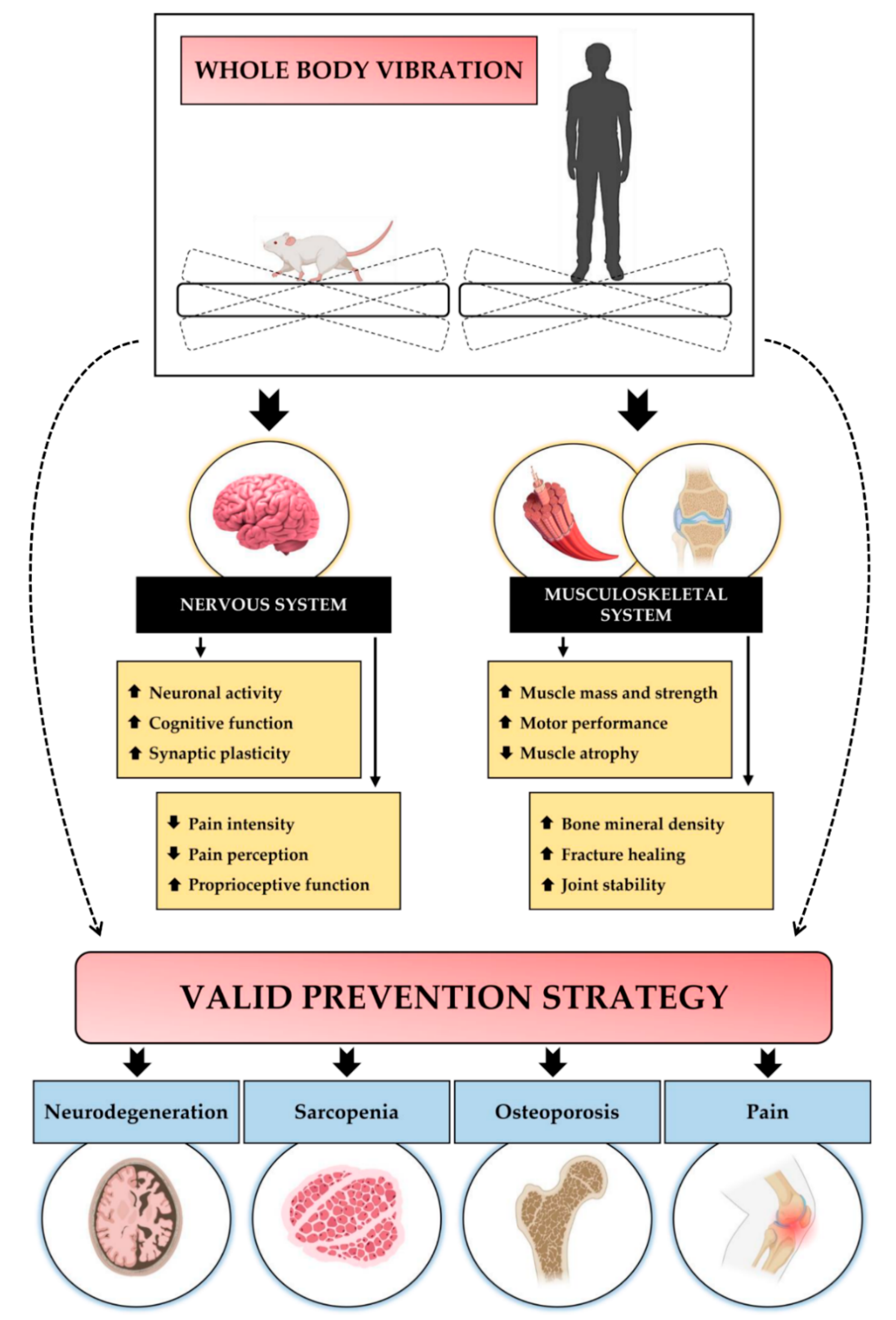
:1. Introduction
2. Literature Search Strategy
3. Physiological Adaptations to WBV
3.1. WBV Improves Cognitive Function and Counteracts Neurodegeneration
3.2. WBV Promotes Musculoskeletal Health
3.3. WBV Favours Pain Relief
4. Molecular Mediators Involved in WBV Effects
5. WBV vs. Exercise: Do We Have a Chance?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fethke, N.B.; Schall, M.C.; Merlino, L.A.; Chen, H.; Branch, C.A.; Ramaswamy, M. Whole-Body Vibration and Trunk Posture During Operation of Agricultural Machinery. Ann. Work Expo. Health 2018, 62, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Chadefaux, D.; Moorhead, A.P.; Marzaroli, P.; Marelli, S.; Marchetti, E.; Tarabini, M. Vibration transmissibility and apparent mass changes from vertical whole-body vibration exposure during stationary and propelled walking. Appl. Ergon. 2021, 90, 103283. [Google Scholar] [CrossRef] [PubMed]
- Krajnak, K. Health effects associated with occupational exposure to hand-arm or whole body vibration. J. Toxicol. Environ. Health B Crit. Rev. 2018, 21, 320–334. [Google Scholar] [CrossRef]
- Eger, T.; Thompson, A.; Leduc, M.; Krajnak, K.; Goggins, K.; Godwin, A.; House, R. Vibration induced white-feet: Overview and field study of vibration exposure and reported symptoms in workers. Work 2014, 47, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Bovenzi, M. A prospective cohort study of neck and shoulder pain in professional drivers. Ergonomics 2015, 58, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.J. Predicting and controlling risks from human exposures to vibration and mechanical shock: Flag waving and flag weaving. Ergonomics 2015, 58, 1063–1070. [Google Scholar] [CrossRef] [Green Version]
- Charles, L.E.; Ma, C.C.; Burchfiel, C.M.; Dong, R.G. Vibration and Ergonomic Exposures Associated with Musculoskeletal Disorders of the Shoulder and Neck. Saf. Health Work 2018, 9, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, M.; Burström, L.; Ekman, A.; Vilhelmsson, R. The association between whole body vibration exposure and musculoskeletal disorders in the Swedish work force is confounded by lifting and posture. J. Sound Vib. 2006, 298, 492–498. [Google Scholar] [CrossRef]
- Patterson, F.; Miralami, R.; Tansey, K.E.; Prabhu, R.K.; Priddy, L.B. Deleterious effects of whole-body vibration on the spine: A review of in vivo, ex vivo, and in vitro models. Anim. Model. Exp. Med. 2021, 4, 77–86. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Tancredi, V.; Iundusi, R.; Gasbarra, E.; Tarantino, U. Chronic Pain in Musculoskeletal Diseases: Do You Know Your Enemy? J. Clin. Med. 2022, 11, 2609. [Google Scholar] [CrossRef]
- Jalilian, H.; Zamanian, Z.; Gorjizadeh, O.; Riaei, S.; Monazzam, M.R.; Abdoli-Eramaki, M. Autonomic Nervous System Responses to Whole-Body Vibration and Mental Workload: A Pilot Study. Int. J. Occup. Environ. Med. 2019, 10, 174–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, Y. Adverse effects of whole-body vibration on gastric motility. Kurume Med. J. 2000, 47, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, S.; Dehghan, S.F.; Vaziri, M.H.; Gilani, M.A.S.; Ardakani, S.K. Assessment of semen quality of taxi drivers exposed to whole body vibration. J. Occup. Med. Toxicol. 2022, 17, 16. [Google Scholar] [CrossRef]
- Yilmaz, N.; Ila, K. Effect of vibration on the vestibular system in noisy and noise-free environments in heavy industry. Acta Otolaryngol. 2019, 139, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. The use of vibration as physical exercise and therapy. J. Funct. Morphol. Kinesiol. 2017, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Odano, I.; Maeyatsu, F.; Asari, M.; Yamaguchi, S.; Miura, T.; Taki, Y. Whole-body vibration exercise and training increase regional CBF in mild cognitive impairment with enhanced cognitive function. Ann. Nucl. Med. 2022, 36, 82–94. [Google Scholar] [CrossRef]
- Sierra-Guzmán, R.; Jiménez-Diaz, F.; Ramírez, C.; Esteban, P.; Abián-Vicén, J. Whole-Body-Vibration Training and Balance in Recreational Athletes with Chronic Ankle Instability. J. Athl. Train. 2018, 53, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Costantino, C.; Bertuletti, S.; Romiti, D. Efficacy of Whole-Body Vibration Board Training on Strength in Athletes After Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Study. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2018, 28, 339–349. [Google Scholar] [CrossRef]
- Shadloo, N.; Kamali, F.; Salehi Dehno, N. A comparison between whole-body vibration and conventional training on pain and performance in athletes with patellofemoral pain. J. Bodyw. Mov. Ther. 2021, 27, 661–666. [Google Scholar] [CrossRef]
- Costantino, C.; Gimigliano, R.; Olvirri, S.; Gimigliano, F. Whole body vibration in sport: A critical review. J. Sports Med. Phys. Fitness 2014, 54, 757–764. [Google Scholar]
- Roberts, R.E.; Bilgen, O.; Kineman, R.D.; Koh, T.J. Parameter-Dependency of Low-Intensity Vibration for Wound Healing in Diabetic Mice. Front. Bioeng. Biotechnol. 2021, 9, 654920. [Google Scholar] [CrossRef] [PubMed]
- Corbiere, T.F.; Weinheimer-Haus, E.M.; Judex, S.; Koh, T.J. Low-Intensity Vibration Improves Muscle Healing in a Mouse Model of Laceration Injury. J. Funct. Morphol. Kinesiol. 2018, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oroszi, T.; Geerts, E.; de Boer, S.F.; Schoemaker, R.G.; van der Zee, E.A.; Nyakas, C. Whole Body Vibration Improves Spatial Memory, Anxiety-Like Behavior, and Motor Performance in Aged Male and Female Rats. Front. Aging Neurosci. 2021, 13, 801828. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.-S.; Huang, L.; Chen, X.-H.; Wang, H.-B.; Sun, W.-S.; Huo, S.-C.; Li, Z.-Q.; Deng, W.-M. Effect of whole body vibration therapy on circulating serotonin levels in an ovariectomized rat model of osteoporosis. Iran. J. Basic Med. Sci. 2014, 17, 62–68. [Google Scholar] [PubMed]
- Nakamura, H.; Moroji, T.; Nohara, S.; Nakamura, H.; Okada, A. Activation of cerebral dopaminergic systems by noise and whole-body vibration. Environ. Res. 1992, 57, 10–18. [Google Scholar] [CrossRef]
- Vizzi, L.; Padua, E.; D’Amico, A.G.; Tancredi, V.; D’Arcangelo, G.; Cariati, I.; Scimeca, M.; Maugeri, G.; D’Agata, V.; Montorsi, M. Beneficial Effects of Physical Activity on Subjects with Neurodegenerative Disease. J. Funct. Morphol. Kinesiol. 2020, 5, 94. [Google Scholar] [CrossRef]
- Heesterbeek, M.; Jentsch, M.; Roemers, P.; Keijser, J.N.; Toth, K.; Nyakas, C.; Schoemaker, R.G.; van Heuvelen, M.J.G.; Van der Zee, E.A. Whole body vibration enhances choline acetyltransferase-immunoreactivity in cortex and amygdale. J. Neurol. Transl. Neurosci. 2017, 5, 1079. [Google Scholar]
- Van der Zee, E.A.; Riedel, G.; Rutgers, E.H.; De Vries, C.; Postema, F.; Venema, B.J. Enhanced neuronal activity in selective brain regions of mice induced by whole body stimulation. Fed. Eur. Neurosci. Soc. Abstr. 2010, 5, R2. [Google Scholar]
- Raval, A.P.; Schatz, M.; Bhattacharya, P.; d’Adesky, N.; Rundek, T.; Dietrich, W.D.; Bramlett, H.M. Whole Body Vibration Therapy after Ischemia Reduces Brain Damage in Reproductively Senescent Female Rats. Int. J. Mol. Sci. 2018, 19, 2749. [Google Scholar] [CrossRef] [Green Version]
- Peng, G.; Yang, L.; Wu, C.Y.; Zhang, L.L.; Wu, C.Y.; Li, F.; Shi, H.W.; Hou, J.; Zhang, L.M.; Ma, X.; et al. Whole body vibration training improves depression-like behaviors in a rat chronic restraint stress model. Neurochem. Int. 2021, 142, 104926. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Pallone, G.; Annino, G.; Tancredi, V.; D’Arcangelo, G. Modulation of Synaptic Plasticity by Vibratory Training in Young and Old Mice. Brain Sci. 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Cariati, I.; Bonanni, R.; Pallone, G.; Romagnoli, C.; Rinaldi, A.M.; Annino, G.; D’Arcangelo, G.; Tancredi, V. Whole Body Vibration Improves Brain and Musculoskeletal Health by Modulating the Expression of Tissue-Specific Markers: FNDC5 as a Key Regulator of Vibration Adaptations. Int. J. Mol. Sci. 2022, 23, 388. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, M.; Bonanni, R.; Cariati, I.; Frank, C.; D’Arcangelo, G. Amyloid Prefibrillar Oligomers: The Surprising Commonalities in Their Structure and Activity. Int. J. Mol. Sci. 2021, 22, 6435. [Google Scholar] [CrossRef] [PubMed]
- Regterschot, G.R.H.; Van Heuvelen, M.J.G.; Zeinstra, E.B.; Fuermaier, A.B.M.; Tucha, L.; Koerts, J.; Tucha, O.; Van Der Zee, E.A. Whole body vibration improves cognition in healthy young adults. PLoS ONE 2014, 9, e100506. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Cariati, I.; Masuelli, L.; Bei, R.; Tancredi, V.; Frank, C.; D’Arcangelo, G. Neurodegeneration in Niemann-Pick Type C Disease: An Updated Review on Pharmacological and Non-Pharmacological Approaches to Counteract Brain and Cognitive Impairment. Int. J. Mol. Sci. 2021, 22, 6600. [Google Scholar] [CrossRef]
- Boerema, A.S.; Heesterbeek, M.; Boersma, S.A.; Schoemaker, R.; de Vries, E.F.J.; van Heuvelen, M.J.G.; Van der Zee, E.A. Beneficial Effects of Whole Body Vibration on Brain Functions in Mice and Humans. Dose-Response 2018, 16, 1559325818811756. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Annino, G. Effects of Whole-Body Vibration on Motor Impairments in Patients with Neurological Disorders: A Systematic Review. Am. J. Phys. Med. Rehabil. 2019, 98, 1084–1098. [Google Scholar] [CrossRef]
- Annino, G.; Iellamo, F.; Palazzo, F.; Fusco, A.; Lombardo, M.; Campoli, F.; Padua, E. Acute changes in neuromuscular activity in vertical jump and flexibility after exposure to whole body vibration. Medicine 2017, 96, e7629. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, R.; Zheng, Y.; Xu, J.; Wu, Y.; Wang, X. Effect of Whole-Body Vibration Training on Muscle Activation for Individuals with Knee Osteoarthritis. BioMed Res. Int. 2021, 2021, 6671390. [Google Scholar] [CrossRef]
- Annino, G.; Alashram, A.R.; Alghwiri, A.A.; Romagnoli, C.; Messina, G.; Tancredi, V.; Padua, E.; Mercuri, N.B. Effect of segmental muscle vibration on upper extremity functional ability poststroke: A randomized controlled trial. Medicine 2019, 98, e14444. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Z.; Li, C.; Wang, Q. The effect of whole-body vibration exercise on postmenopausal women with osteoporosis: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e25606. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Goto, D. Effect of low-intensity whole-body vibration on bone defect repair and associated vascularization in mice. Med. Biol. Eng. Comput. 2017, 55, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Keijser, J.N.; van Heuvelen, M.J.G.; Nyakas, C.; Tóth, K.; Schoemaker, R.G.; Zeinstra, E.; van der Zee, E.A. Whole body vibration improves attention and motor performance in mice depending on the duration of the whole-body vibration session. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2017, 14, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cariati, I.; Bonanni, R.; Annino, G.; Scimeca, M.; Bonanno, E.; D’Arcangelo, G.; Tancredi, V. Dose-Response Effect of Vibratory Stimulus on Synaptic and Muscle Plasticity in a Middle-Aged Murine Model. Front. Physiol. 2021, 12, 678449. [Google Scholar] [CrossRef]
- Gnyubkin, V.; Guignandon, A.; Laroche, N.; Vanden-Bossche, A.; Malaval, L.; Vico, L. High-acceleration whole body vibration stimulates cortical bone accrual and increases bone mineral content in growing mice. J. Biomech. 2016, 49, 1899–1908. [Google Scholar] [CrossRef]
- Xie, L.; Jacobson, J.M.; Choi, E.S.; Busa, B.; Donahue, L.R.; Miller, L.M.; Rubin, C.T.; Judex, S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone 2006, 39, 1059–1066. [Google Scholar] [CrossRef]
- Vanleene, M.; Shefelbine, S.J. Therapeutic impact of low amplitude high frequency whole body vibrations on the osteogenesis imperfecta mouse bone. Bone 2013, 53, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Minematsu, A.; Nishii, Y.; Imagita, H.; Takeshita, D.; Sakata, S. Whole-body vibration can attenuate the deterioration of bone mass and trabecular bone microstructure in rats with spinal cord injury. Spinal Cord 2016, 54, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Tan, C.; Wu, Y.; Ding, Y.; Wang, H.; Chen, W.; Zhu, Y.; Ma, H.; Yang, H.; Liang, W.; et al. Whole-body vibration and resistance exercise prevent long-term hindlimb unloading-induced bone loss: Independent and interactive effects. Eur. J. Appl. Physiol. 2012, 112, 3743–3753. [Google Scholar] [CrossRef]
- Chen, G.-X.; Zheng, S.; Qin, S.; Zhong, Z.-M.; Wu, X.-H.; Huang, Z.-P.; Li, W.; Ding, R.-T.; Yu, H.; Chen, J.-T. Effect of low-magnitude whole-body vibration combined with alendronate in ovariectomized rats: A random controlled osteoporosis prevention study. PLoS ONE 2014, 9, e96181. [Google Scholar] [CrossRef]
- Ruan, X.-Y.; Jin, F.-Y.; Liu, Y.-L.; Peng, Z.-L.; Sun, Y.-G. Effects of vibration therapy on bone mineral density in postmenopausal women with osteoporosis. Chin. Med. J. 2008, 121, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- ElDeeb, A.M.; Abdel-Aziem, A.A. Effect of Whole-Body Vibration Exercise on Power Profile and Bone Mineral Density in Postmenopausal Women with Osteoporosis: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2020, 43, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Slatkovska, L.; Beyene, J.; Alibhai, S.M.H.; Wong, Q.; Sohail, Q.Z.; Cheung, A.M. Effect of whole-body vibration on calcaneal quantitative ultrasound measurements in postmenopausal women: A randomized controlled trial. Calcif. Tissue Int. 2014, 95, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.; Recker, R.; Cullen, D.; Ryaby, J.; McCabe, J.; McLeod, K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: A clinical trial assessing compliance, efficacy, and safety. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Marín-Cascales, E.; Alcaraz, P.E.; Ramos-Campo, D.J.; Martinez-Rodriguez, A.; Chung, L.H.; Rubio-Arias, J.Á. Whole-body vibration training and bone health in postmenopausal women: A systematic review and meta-analysis. Medicine 2018, 97, e11918. [Google Scholar] [CrossRef]
- Marazzi, S.; Kiper, P.; Palmer, K.; Agostini, M.; Turolla, A. Effects of vibratory stimulation on balance and gait in Parkinson’s disease: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2021, 57, 254–264. [Google Scholar] [CrossRef]
- Li, K.-Y.; Cho, Y.-J.; Chen, R.-S. The Effect of Whole-Body Vibration on Proprioception and Motor Function for Individuals with Moderate Parkinson Disease: A Single-Blind Randomized Controlled Trial. Occup. Ther. Int. 2021, 2021, 9441366. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef] [Green Version]
- Linek, P.; Noormohammadpour, P.; Mansournia, M.A.; Wolny, T.; Sikora, D. Morphological changes of the lateral abdominal muscles in adolescent soccer players with low back pain: A prospective cohort study. J. Sport Health Sci. 2020, 9, 614–619. [Google Scholar] [CrossRef]
- Ye, J.; Ng, G.; Yuen, K. Acute effects of whole-body vibration on trunk muscle functioning in young healthy adults. J. strength Cond. Res. 2014, 28, 2872–2879. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.-S.; Wang, X.-D.; Xie, B.; Li, Z.-H.; Chen, B.-L.; Wang, X.-Q.; Zhu, Y. Sling exercise for chronic low back pain: A systematic review and meta-analysis. PLoS ONE 2014, 9, e99307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, J.-A.; Abboud, J.; Dubois, J.-D.; Legault, E.; Descarreaux, M.; Henchoz, Y. Trunk neuromuscular responses to a single whole-body vibration session in patients with chronic low back pain: A cross-sectional study. J. Manip. Physiol. Ther. 2013, 36, 564–571. [Google Scholar] [CrossRef]
- Rittweger, J.; Just, K.; Kautzsch, K.; Reeg, P.; Felsenberg, D. Treatment of chronic lower back pain with lumbar extension and whole-body vibration exercise: A randomized controlled trial. Spine (Phila Pa 1976). 2002, 27, 1829–1834. [Google Scholar] [CrossRef]
- del Pozo-Cruz, B.; Hernández Mocholí, M.A.; Adsuar, J.C.; Parraca, J.A.; Muro, I.; Gusi, N. Effects of whole body vibration therapy on main outcome measures for chronic non-specific low back pain: A single-blind randomized controlled trial. J. Rehabil. Med. 2011, 43, 689–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinale, M.; Bosco, C. The use of vibration as an exercise intervention. Exerc. Sport Sci. Rev. 2003, 31, 3–7. [Google Scholar] [CrossRef]
- Burke, D.; Gandevia, S.C. The human muscle spindle and its fusimotor control. In Neural Control Of Movement; Springer: Boston, MA, USA, 1995; pp. 19–25. [Google Scholar]
- Belavý, D.L.; Armbrecht, G.; Gast, U.; Richardson, C.A.; Hides, J.A.; Felsenberg, D. Countermeasures against lumbar spine deconditioning in prolonged bed rest: Resistive exercise with and without whole body vibration. J. Appl. Physiol. 2010, 109, 1801–1811. [Google Scholar] [CrossRef] [Green Version]
- Stewart, V.H.; Saunders, D.H.; Greig, C.A. Responsiveness of muscle size and strength to physical training in very elderly people: A systematic review. Scand. J. Med. Sci. Sports 2014, 24, e1–e10. [Google Scholar] [CrossRef]
- Sonza, A. Human cutaneous mechanoreceptive afferents response after Whole Body Vibration: A literature review. Rev. Hosp. Univ. Pedro Ernesto 2018, 17, 35–38. [Google Scholar]
- Prager, J.P. What does the mechanism of spinal cord stimulation tell us about complex regional pain syndrome? Pain Med. 2010, 11, 1278–1283. [Google Scholar] [CrossRef] [Green Version]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Sonza, A.; Sanada, L.S.; de Oliveira, L.R.; Bernardo-Filho, M.; Sá-Caputo, D.D.C.D.; Zaro, M.A.; Achaval, M. Whole-body vibration mediates mechanical hypersensitivity through Aβ-fiber and C-fiber thermal sensation in a chronic pain model. Exp. Biol. Med. 2021, 246, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Pallone, G.; Palmieri, M.; Cariati, I.; Bei, R.; Masuelli, L.; D’Arcangelo, G.; Tancredi, V. Different continuous training modalities result in distinctive effects on muscle structure, plasticity and function. Biomed. Rep. 2020, 12, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, M.; Cariati, I.; Scimeca, M.; Pallone, G.; Bonanno, E.; Tancredi, V.; D’Arcangelo, G.; Frank, C. Effects of short-term aerobic exercise in a mouse model of Niemann-Pick type C disease on synaptic and muscle plasticity. Ann. Dell’istituto Super. Sanità 2019, 55, 330–337. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Tarantino, U.; D’Arcangelo, G.; Tancredi, V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. J. Funct. Morphol. Kinesiol. 2022, 7, 38. [Google Scholar] [CrossRef]
- von Bohlen Und Halbach, O.; von Bohlen Und Halbach, V. BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res. 2018, 373, 729–741. [Google Scholar] [CrossRef]
- Simão, A.P.; Mendonça, V.A.; Avelar, N.C.P.; da Fonseca, S.F.; Santos, J.M.; de Oliveira, A.C.C.; Tossige-Gomes, R.; Ribeiro, V.G.C.; Neves, C.D.C.; Balthazar, C.H.; et al. Whole Body Vibration Training on Muscle Strength and Brain-Derived Neurotrophic Factor Levels in Elderly Woman with Knee Osteoarthritis: A Randomized Clinical Trial Study. Front. Physiol. 2019, 10, 756. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, V.G.C.; Lacerda, A.C.R.; Santos, J.M.; Coelho-Oliveira, A.C.; Fonseca, S.F.; Prates, A.C.N.; Flor, J.; Garcia, B.C.C.; Tossige-Gomes, R.; Leite, H.R.; et al. Efficacy of Whole-Body Vibration Training on Brain-Derived Neurotrophic Factor, Clinical and Functional Outcomes, and Quality of Life in Women with Fibromyalgia Syndrome: A Randomized Controlled Trial. J. Healthc. Eng. 2021, 2021, 7593802. [Google Scholar] [CrossRef]
- Huang, D.; Yang, Z.; Wang, Z.; Wang, P.; Qu, Y. The macroscopic and microscopic effect of low-frequency whole-body vibration after cerebral ischemia in rats. Metab. Brain Dis. 2018, 33, 15–25. [Google Scholar] [CrossRef]
- Oberste, M.; Großheinrich, N.; Wunram, H.-L.; Graf, J.L.; Ziemendorff, A.; Meinhardt, A.; Fricke, O.; Mahabir, E.; Bender, S. Effects of a 6-week, whole-body vibration strength-training on depression symptoms, endocrinological and neurobiological parameters in adolescent inpatients experiencing a major depressive episode (the “Balancing Vibrations Study”): Study protocol for a randomized placebo-controlled trial. Trials 2018, 19, 347. [Google Scholar] [CrossRef]
- Zsuga, J.; Tajti, G.; Papp, C.; Juhasz, B.; Gesztelyi, R. FNDC5/irisin, a molecular target for boosting reward-related learning and motivation. Med. Hypotheses 2016, 90, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Cariati, I.; Bonanni, R.; Scimeca, M.; Rinaldi, A.M.; Marini, M.; Tarantino, U.; Tancredi, V. Exposure to Random Positioning Machine Alters the Mineralization Process and PTX3 Expression in the SAOS-2 Cell Line. Life 2022, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- LeBrasseur, N.K.; Schelhorn, T.M.; Bernardo, B.L.; Cosgrove, P.G.; Loria, P.M.; Brown, T.A. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 940–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Yu, K.; Liu, D.; Yang, J.; Tan, L.; Zhang, D. Irisin enhances osteogenic differentiation of mouse MC3T3-E1 cells via upregulating osteogenic genes. Exp. Ther. Med. 2021, 21, 580. [Google Scholar] [CrossRef]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagnani, F.; Giombini, A.; Di Cesare, A.; Pigozzi, F.; Di Salvo, V. The effects of a whole-body vibration program on muscle performance and flexibility in female athletes. Am. J. Phys. Med. Rehabil. 2006, 85, 956–962. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.; García-López, D.; González-Gallego, J.; Garatachea, N. Whole-body vibration training increases muscle strength and mass in older women: A randomized-controlled trial. Scand. J. Med. Sci. Sports 2010, 20, 200–207. [Google Scholar] [CrossRef]
- Verschueren, S.M.P.; Roelants, M.; Delecluse, C.; Swinnen, S.; Vanderschueren, D.; Boonen, S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: A randomized controlled pilot study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Vissers, D.; Verrijken, A.; Mertens, I.; Van Gils, C.; Van de Sompel, A.; Truijen, S.; Van Gaal, L. Effect of long-term whole body vibration training on visceral adipose tissue: A preliminary report. Obes. Facts 2010, 3, 93–100. [Google Scholar] [CrossRef]
- Delecluse, C.; Roelants, M.; Verschueren, S. Strength increase after whole-body vibration compared with resistance training. Med. Sci. Sports Exerc. 2003, 35, 1033–1041. [Google Scholar] [CrossRef]
- Osawa, Y.; Oguma, Y.; Ishii, N. The effects of whole-body vibration on muscle strength and power: A meta-analysis. J. Musculoskelet. Neuronal Interact. 2013, 13, 380–390. [Google Scholar]
- Berschin, G.; Sommer, B.; Behrens, A.; Sommer, H.-M. Whole Body Vibration Exercise Protocol versus a Standard Exercise Protocol after ACL Reconstruction: A Clinical Randomized Controlled Trial with Short Term Follow-Up. J. Sports Sci. Med. 2014, 13, 580–589. [Google Scholar] [PubMed]
- Cochrane, D.J.; Legg, S.J.; Hooker, M.J. The short-term effect of whole-body vibration training on vertical jump, sprint, and agility performance. J. Strength Cond. Res. 2004, 18, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Rogan, S.; de Bruin, E.D.; Radlinger, L.; Joehr, C.; Wyss, C.; Stuck, N.-J.; Bruelhart, Y.; de Bie, R.A.; Hilfiker, R. Effects of whole-body vibration on proxies of muscle strength in old adults: A systematic review and meta-analysis on the role of physical capacity level. Eur. Rev. Aging Phys. Act. 2015, 12, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwer, S.; Alghadir, A.; Zafar, H.; Al-Eisa, E. Effect of whole body vibration training on quadriceps muscle strength in individuals with knee osteoarthritis: A systematic review and meta-analysis. Physiotherapy 2016, 102, 145–151. [Google Scholar] [CrossRef]
- Rasti, E.; Rojhani-Shirazi, Z.; Ebrahimi, N.; Sobhan, M.R. Effects of whole body vibration with exercise therapy versus exercise therapy alone on flexibility, vertical jump height, agility and pain in athletes with patellofemoral pain: A randomized clinical trial. BMC Musculoskelet. Disord. 2020, 21, 705. [Google Scholar] [CrossRef]
- Gloeckl, R.; Schneeberger, T.; Leitl, D.; Reinold, T.; Nell, C.; Jarosch, I.; Kenn, K.; Koczulla, A.R. Whole-body vibration training versus conventional balance training in patients with severe COPD-a randomized, controlled trial. Respir. Res. 2021, 22, 138. [Google Scholar] [CrossRef]
- Guadarrama-Molina, E.; Barrón-Gámez, C.E.; Estrada-Bellmann, I.; Meléndez-Flores, J.D.; Ramírez-Castañeda, P.; Hernández-Suárez, R.M.G.; Menchaca-Pérez, M.; Salas-Fraire, O. Comparison of the effect of whole-body vibration therapy versus conventional therapy on functional balance of patients with Parkinson’s disease: Adding a mixed group. Acta Neurol. Belg. 2021, 121, 721–728. [Google Scholar] [CrossRef]
- van Heuvelen, M.J.G.; Rittweger, J.; Judex, S.; Sañudo, B.; Seixas, A.; Fuermaier, A.B.M.; Tucha, O.; Nyakas, C.; Marín, P.J.; Taiar, R.; et al. Reporting Guidelines for Whole-Body Vibration Studies in Humans, Animals and Cell Cultures: A Consensus Statement from an International Group of Experts. Biology 2021, 10, 965. [Google Scholar] [CrossRef]
| References | Experimental Groups | Objectives | WBV Parameters | WBV Effects |
|---|---|---|---|---|
| [29] | 15 female Sprague-Dawley rats (9–12 months) exposed to transient middle cerebral artery occlusion:
| Testing the efficacy of WBV in counteracting post-ischemic stroke fragility and brain damage in reproductively senescent female rats |
|
|
| [30] | 18 male rats (3 months):
| Examining the WBV effects on neuronal loss, synaptic protein and neurotrophin expression in a rat model of chronic restraint stress-induced depression |
|
|
| [31] | 32 male BALB/c mice 20 mice of 4 months:
| Assessing the WBV ability to modulate hippocampal synaptic plasticity |
|
|
| [32] | 20 male BALB/c mice:
| Investigate WBV-induced brain and musculoskeletal adaptations |
|
|
| [34] | 133 healthy young adults (20.5 ± 2.2.) | Studying the acute effects of passive WBV vibration on executive functions |
|
|
| [37] | 20 C57Bl/6J mice (15 weeks):
| Assessing the WBV impact on brain activity, arousal-induced activity, and executive functioning | For the animal model:
|
|
| [43] | 48 male C57BL/6 mice (13 weeks) with cortical perforation on the tibial bone:
| Investigating the WBV effects on vascularization during the early stages of fracture healing |
|
|
| [44] | 44 male CD1 mice (3 months):
| Assessing the WBV effects on attention and motor performance |
|
|
| [45] | 20 male BALB/c mice (12 months):
| Studying muscle adaptations to WBV in a middle-aged mouse model |
|
|
| [46] | 58 male mice (7 weeks):
| Identifying a WBV protocol that promotes bone growth in healthy young mice |
|
|
| [47] | 38 female BALB/cBxJ mice (8 weeks):
| Investigating the influence of WBV on trabecular and cortical formation and resorption processes in the growing skeleton |
|
|
| [48] |
| Studying the WBV effects on cortical and trabecular bone formation in young mice with osteogenesis imperfecta |
|
|
| [52] | 94 postmenopausal women with osteoporosis:
| Investigating the WBV effects on menopause-induced BMD decline and chronic back pain |
|
|
| [53] | 43 postmenopausal women:
| Studying the WBV impact on muscle work and BMD of the lumbar vertebrae and femur |
|
|
| [54] | 202 postmenopausal women:
| Examining the WBV effects on calcaneal QUS measurements |
|
|
| [55] | 64 postmenopausal women:
| Investigating the ability of WBV to inhibit bone loss |
|
|
| [58] | 29 patients with moderate PD:
| Studying the short-term effect of WBV on motor proprioceptive functions in patients with moderate PD |
|
|
| [64] | 50 patients with chronic low back pain:
| Comparing the WBV effects on chronic back pain versus LEX |
|
|
| [65] | 49 patients with non-specific low back pain:
| Testing the effectiveness of WBV in counteracting chronic non-specific low back pain |
|
|
| [73] | 60 male Wistar rats (3 months)
| Studying the WBV effects in a chronic pain model |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanni, R.; Cariati, I.; Romagnoli, C.; D’Arcangelo, G.; Annino, G.; Tancredi, V. Whole Body Vibration: A Valid Alternative Strategy to Exercise? J. Funct. Morphol. Kinesiol. 2022, 7, 99. https://doi.org/10.3390/jfmk7040099
Bonanni R, Cariati I, Romagnoli C, D’Arcangelo G, Annino G, Tancredi V. Whole Body Vibration: A Valid Alternative Strategy to Exercise? Journal of Functional Morphology and Kinesiology. 2022; 7(4):99. https://doi.org/10.3390/jfmk7040099
Chicago/Turabian StyleBonanni, Roberto, Ida Cariati, Cristian Romagnoli, Giovanna D’Arcangelo, Giuseppe Annino, and Virginia Tancredi. 2022. "Whole Body Vibration: A Valid Alternative Strategy to Exercise?" Journal of Functional Morphology and Kinesiology 7, no. 4: 99. https://doi.org/10.3390/jfmk7040099
APA StyleBonanni, R., Cariati, I., Romagnoli, C., D’Arcangelo, G., Annino, G., & Tancredi, V. (2022). Whole Body Vibration: A Valid Alternative Strategy to Exercise? Journal of Functional Morphology and Kinesiology, 7(4), 99. https://doi.org/10.3390/jfmk7040099







