EMG Amplitude–Force Relationship of Lumbar Back Muscles during Isometric Submaximal Tasks in Healthy Inactive, Endurance and Strength-Trained Subjects
Abstract
1. Introduction
2. Methods
2.1. Participants
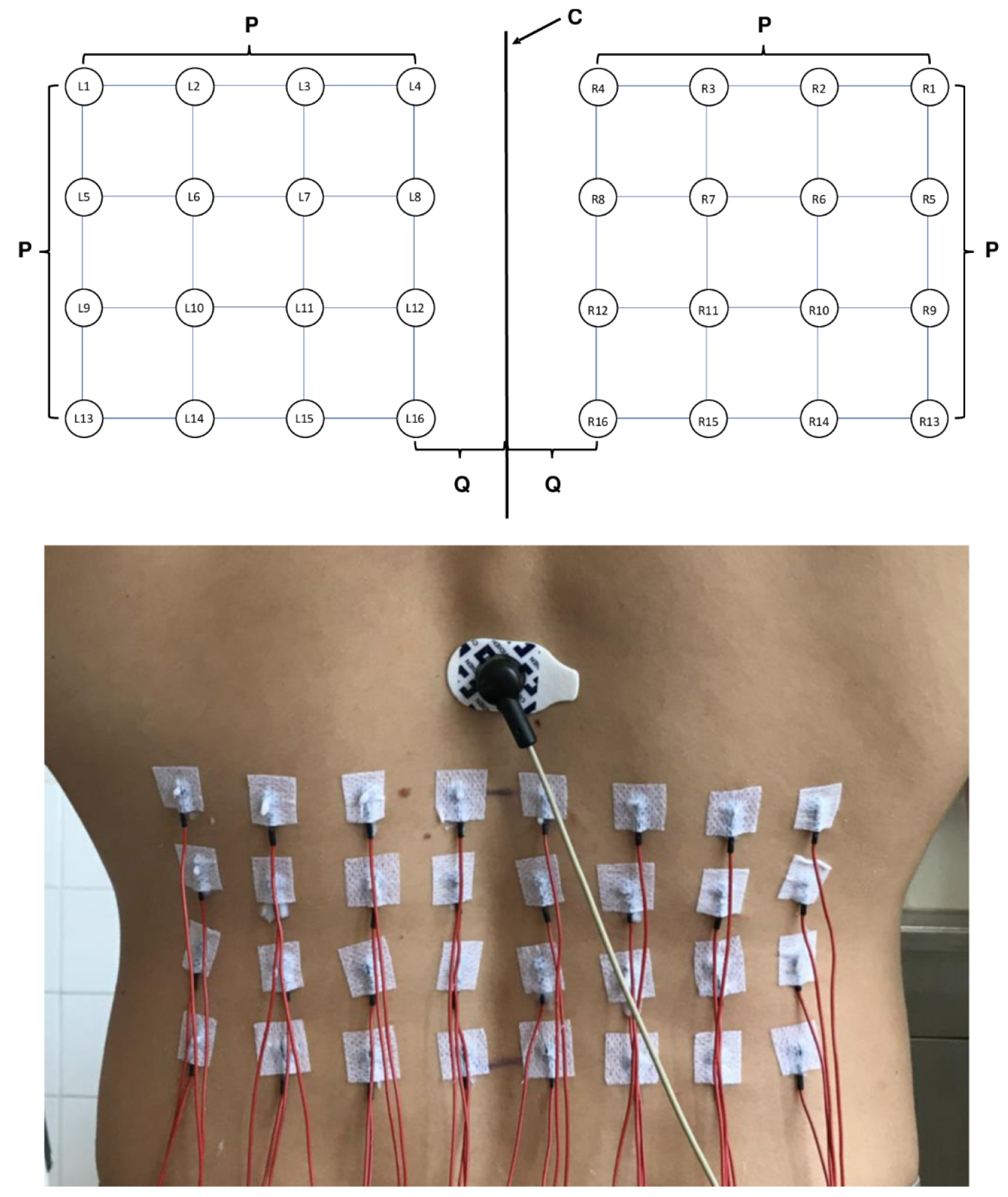
2.2. Device and Investigation
2.3. Surface EMG Measurement and Analysis
2.4. Statistical Analysis
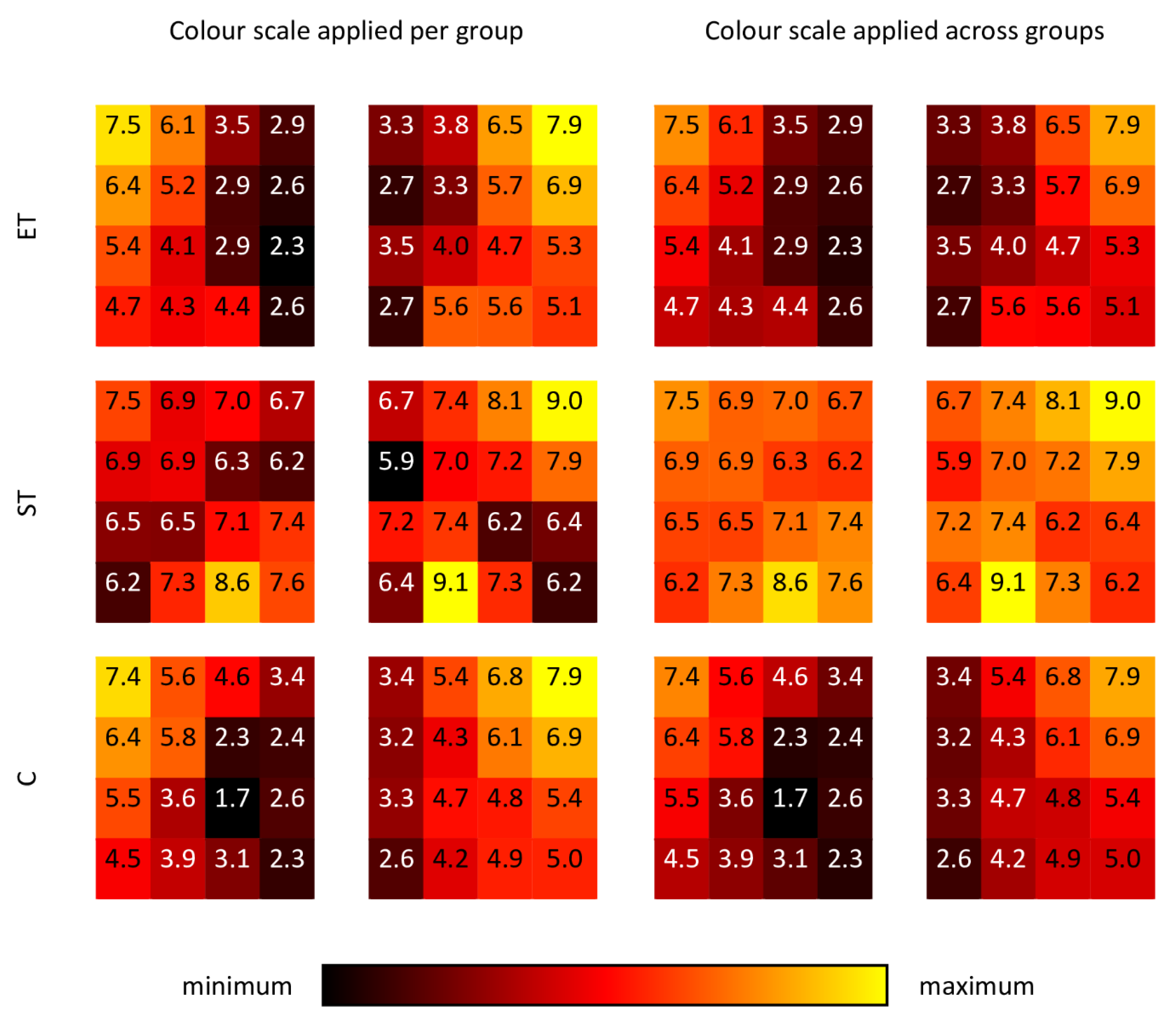
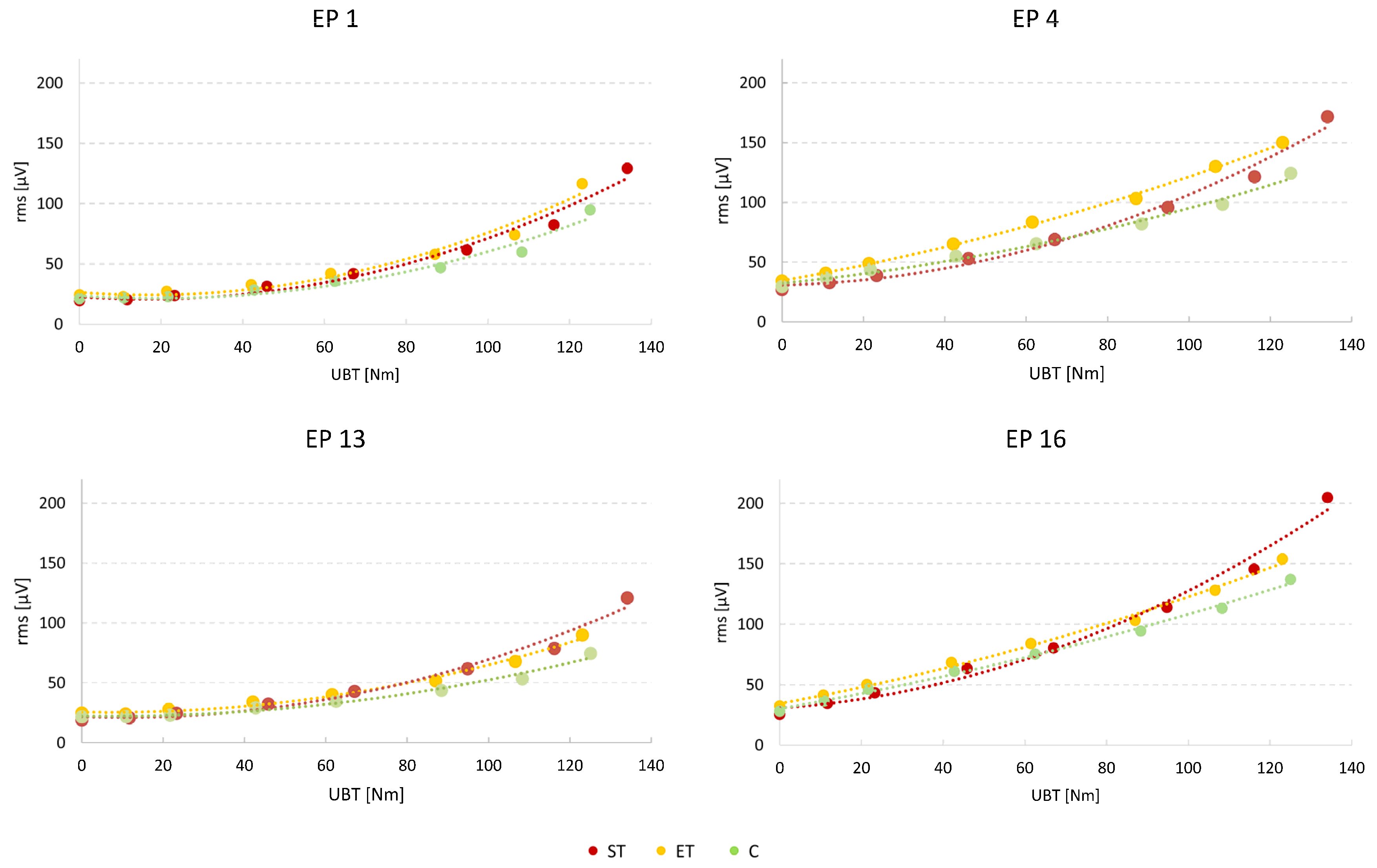
3. Results
4. Discussion
4.1. Recruitment Strategies
4.2. Muscle Fiber Composition
4.3. Training Associated Effects
4.4. Clinical Implications
4.5. Limitations
5. Conclusions/Suggestions for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gandevia, S.C. Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Luttmann, A.; Jäger, M.; Laurig, W. Electromyographical indication of muscular fatigue in occupational field studies. Int. J. Ind. Ergon. 2000, 25, 645–660. [Google Scholar] [CrossRef]
- Anders, C.; Wenzel, B. Activation characteristics of trunk muscles during cyclic upper body pertubations. Acta Physiol. 2006, 186, 245. [Google Scholar]
- Graven-Nielsen, T.; Svensson, P.; Arendt-Nielsen, L. Effects of experimental muscle pain on muscle activity and co-ordination during static and dynamic motor function. Electromyogr. Motor C 1997, 105, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Van Dieën, J.H.; Cholewicki, J.; Radebold, A. Trunk Muscle Recruitment Patterns in Patients with Low Back Pain Enhance the Stability of the Lumbar Spine. Spine 2003, 28, 834–841. [Google Scholar] [CrossRef]
- Chapman, A.R.; Vicenzino, B.; Blanch, P.; Hodges, P.W. Patterns of leg muscle recruitment vary between novice and highly trained cyclists. J. Electromyogr. Kinesiol. 2008, 18, 359–371. [Google Scholar] [CrossRef]
- Fuglevand, A.J.; Winter, D.A.; Patla, A.E. Models of recruitment and rate coding organization in motor-unit pools. J. Neurophysiol. 1993, 70, 2470–2488. [Google Scholar] [CrossRef]
- Urquhart, D.M.; Hodges, P.W.; Allen, T.J.; Story, I.H. Abdominal muscle recruitment during a range of voluntary exercises. Man. Ther. 2005, 10, 144–153. [Google Scholar] [CrossRef]
- Lippold, O.C.J. The relation between integrated action potentials in a human muscle and its isometric tension. J. Physiol. 1952, 117, 492–499. [Google Scholar] [CrossRef]
- Bernshtein, V.M. Statistical parameters of the electrical signal of a muscle model. Biofizika 1967, 12, 693–703. [Google Scholar]
- Bouisset, S. EMG and Muscle Force in Normal Motor Activities. In New Developments in Electromyography and Clinical Neurophysiology; Desmedt, J.E., Ed.; Karger: Basel, Switzerland, 1973; Volume 1, pp. 547–583. [Google Scholar]
- Lawrence, J.H.; De Luca, C.J. Myoelectric signal versus force relationship in different human muscles. J. Appl. Physiol. 1983, 54, 1653–1659. [Google Scholar] [CrossRef]
- Solomonow, M.; Baratta, R.; Shoji, H.; D’Ambrosia, R. The EMG-force relationships of skeletal muscle; dependence on contraction rate, and motor units control strategy. Electromyogr. Clin. Neurophysiol. 1990, 30, 141–152. [Google Scholar]
- Johnson, M.A.; Polgar, J.; Weightman, D.; Appleton, D. Data on the distribution of fibre types in thirty-six human muscles: An autopsy study. J. Neurol. Sci. 1973, 18, 111–129. [Google Scholar] [CrossRef]
- Gollnick, P.D.; Armstrong, R.B.; Saubert, C.W.; Piehl, K.; Saltin, B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J. Appl. Physiol. 1972, 33, 312–319. [Google Scholar] [CrossRef]
- Gollnick, P.D.; Armstrong, R.B.; Saltin, B.; Saubert, C.W.; Sembrowich, W.L.; Shepherd, R.E. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J. Appl. Physiol. 1973, 34, 107–111. [Google Scholar] [CrossRef]
- Häggmark, T.; Thorstensson, A. Fibre types in human abdominal muscles. Acta Physiol. Scand. 1979, 107, 319–325. [Google Scholar] [CrossRef]
- Mannion, A.F. Fibre type characteristics and function of the human paraspinal muscles: Normal values and changes in association with low back pain. J. Electromyogr. Kinesiol. 1999, 9, 363–377. [Google Scholar] [CrossRef]
- Thorstensson, A.; Carlson, H. Fibre types in human lumbar back muscles. Acta Physiol. Scand. 1987, 131, 195–202. [Google Scholar] [CrossRef]
- Anders, C.; Brose, G.; Hofmann, G.O.; Scholle, H.-C. Evaluation of the EMG–force relationship of trunk muscles during whole body tilt. J. Biomech. 2008, 41, 333–339. [Google Scholar] [CrossRef]
- Huebner, A.; Faenger, B.; Scholle, H.-C.; Anders, C. Re-evaluation of the amplitude–force relationship of trunk muscles. J. Biomech. 2015, 48, 1198–1205. [Google Scholar] [CrossRef]
- Anders, C.; Brose, G.; Hofmann, G.O.; Scholle, H.-C. Gender specific activation patterns of trunk muscles during whole body tilt. Eur. J. Appl. Physiol. 2007, 101, 195–205. [Google Scholar] [CrossRef]
- Simoneau, J.A.; Bouchard, C. Genetic determinism of fiber type proportion in human skeletal muscle. FASEB J. 1995, 9, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Comerford, M.J.; Mottram, S.L. Movement and stability dysfunction—contemporary developments. Man. Ther. 2001, 6, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, A. Stability of the lumbar spine: A study in mechanical engineering. Acta Orthop. 1989, 60, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Anders, C. Influence of different control strategies on muscle activation patterns in trunk muscles. Physiol. Rep. 2014, 2, e12229. [Google Scholar] [CrossRef]
- Anders, C.; Steiniger, B. Main force directions of trunk muscles: A pilot study in healthy male subjects. Hum. Mov. Sci. 2018, 60, 214–224. [Google Scholar] [CrossRef]
- Pfeifle, C.; Edel, M.; Schleifenbaum, S.; Kühnapfel, A.; Heyde, C.-E. The reliability of a restraint sensor system for the computer-supported detection of spinal stabilizing muscle deficiencies. BMC Musculoskelet. Disord. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Mörl, F.; Anders, C.; Grassme, R. An easy and robust method for ECG artifact elimination of SEMG signals. In Proceedings of the XVII Congress of the International Society of Electrophysiology and Kinesiology Aalborg, Aalborg, Denmark, 16–19 June 2010. [Google Scholar]
- De Luca, C.J.; LeFever, R.S.; McCue, M.P.; Xenakis, A.P. Behaviour of human motor units in different muscles during linearly varying contractions. J. Physiol. 1982, 329, 113–128. [Google Scholar] [CrossRef]
- Kukulka, C.G.; Clamann, H.P. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res. 1981, 219, 45–55. [Google Scholar] [CrossRef]
- Gibbons, S.G.T.; Comerford, M.J. Strength versus stability: Part 1: Concept and terms. Orth. Div. Rev. 2001, 43, 21–27. [Google Scholar]
- Woods, J.J.; Bigland-Ritchie, B. Linear and non-linear surface EMG/force relationships in human muscles. An anatomical/functional argument for the existence of both. Am. J. Phys. Med. 1983, 62, 287–299. [Google Scholar]
- Farina, D.; Fosci, M.; Merletti, R. Motor unit recruitment strategies investigated by surface EMG variables. J. Appl. Physiol. 2002, 92, 235–247. [Google Scholar] [CrossRef]
- Milner-Brown, H.S.; Stein, R.B.; Yemm, R. The orderly recruitment of human motor units during voluntary isometric contractions. J. Physiol. 1973, 230, 359–370. [Google Scholar] [CrossRef]
- Goldspink, G.; Scutt, A.; Loughna, P.T.; Wells, D.J.; Jaenicke, T.; Gerlach, G.F. Gene expression in skeletal muscle in response to stretch and force generation. Am. J. Physiol. 1992, 262, R356–R363. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Patton, J.F.; Gordon, S.E.; Harman, E.A.; Deschenes, M.R.; Reynolds, K.; Newton, R.U.; Triplett, N.T.; Dziados, J.E. Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J. Appl. Physiol. 1995, 78, 976–989. [Google Scholar] [CrossRef]
- Putman, C.T.; Xu, X.; Gillies, E.; MacLean, I.M.; Bell, G.J. Effects of strength, endurance and combined training on myosin heavy chain content and fibre-type distribution in humans. Eur. J. Appl. Physiol. 2004, 92, 376–384. [Google Scholar] [CrossRef]
- Merletti, R.; De Luca, C.J.; Sathyan, D. Electrically evoked myoelectric signals in back muscles: Effect of side dominance. J. Appl. Physiol. 1994, 77, 2104–2114. [Google Scholar] [CrossRef]
- Pranata, A.; Perraton, L.; El-Ansary, D.; Clark, R.; Fortin, K.; Dettmann, T.; Brandham, R.; Bryant, A. Lumbar extensor muscle force control is associated with disability in people with chronic low back pain. Clin. Biomech. 2017, 46, 46–51. [Google Scholar] [CrossRef]
- Panjabi, M.M. A hypothesis of chronic back pain: Ligament subfailure injuries lead to muscle control dysfunction. Eur. Spine J. 2005, 15, 668–676. [Google Scholar] [CrossRef]
- Da Silva, R.A., Jr.; Arsenault, A.B.; Gravel, D.; Larivière, C.; de Oliveira, E., Jr. Back muscle strength and fatigue in healthy and chronic low back pain subjects: A comparative study of 3 assessment protocols. Arch. Phys. Med. Rehabil. 2005, 86, 722–729. [Google Scholar] [CrossRef]
- Anders, C.; Kankaanpää, M.; Airaksinen, O.; Scholle, H.C.; Hänninen, O. Coordination of lumbar back muscles of dynamic loading. Man. Med. 1998, 36, 61–65. [Google Scholar] [CrossRef]
- Hildebrandt, J.; Pfingsten, M.; Saur, P.; Jansen, J. Prediction of success from a multidisciplinary treatment pro-gram for chronic low back pain. Spine 1997, 22, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.; Schönau, T. Spatiotemporal characteristics of lower back muscle fatigue during a ten minutes endurance test at 50% upper body weight in healthy inactive, endurance, and strength trained subjects. PLoS ONE 2022, 17, e0273856. [Google Scholar] [CrossRef] [PubMed]
- Mohr, M.; Schön, T.; Von Tscharner, V.; Nigg, B.M. Intermuscular Coherence Between Surface EMG Signals Is Higher for Monopolar Compared to Bipolar Electrode Configurations. Front. Physiol. 2018, 9, 566. [Google Scholar] [CrossRef]

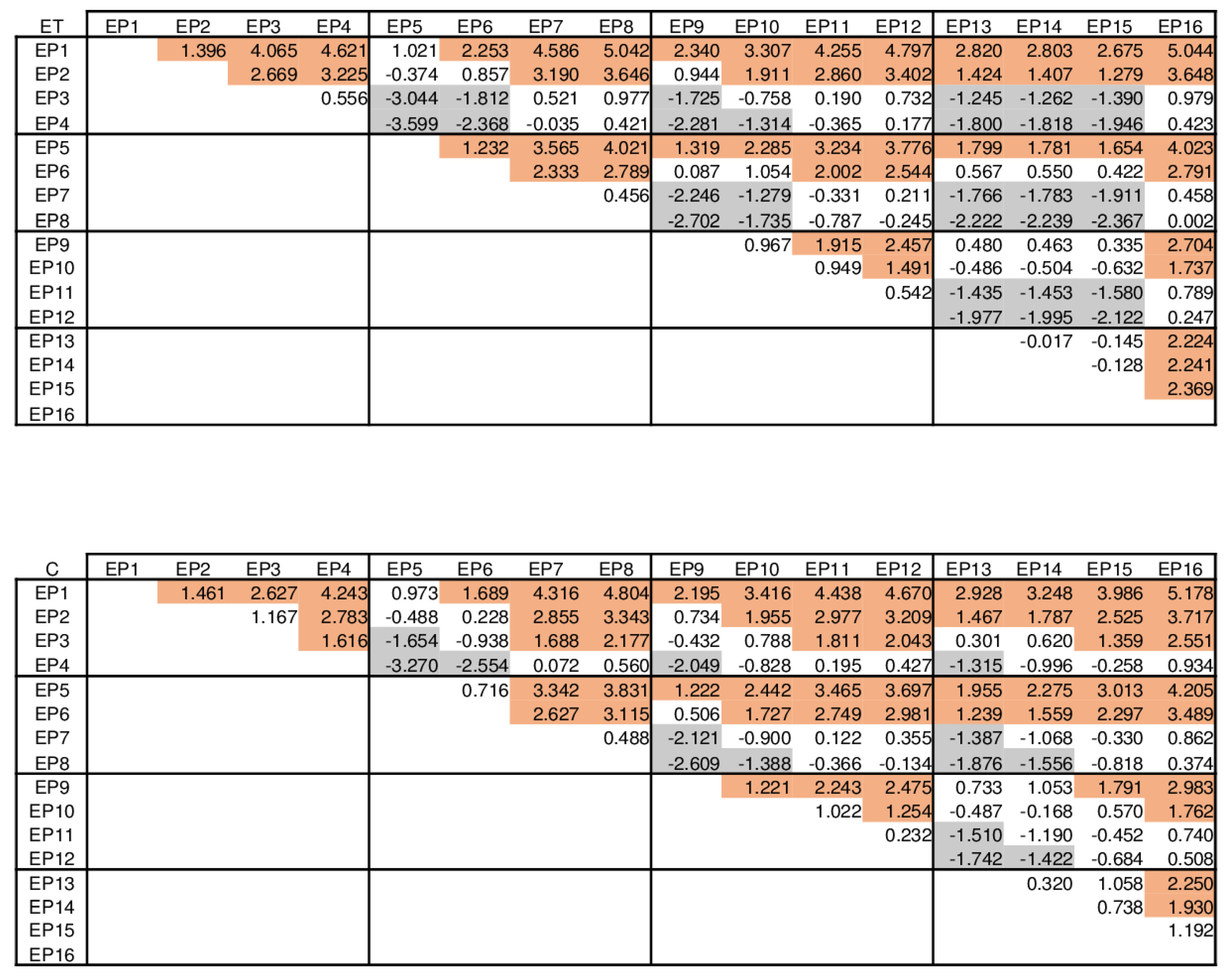
| ET vs. ST | C vs. ST | ET vs. C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | |||||||
| Mean Diff. | Lower Border | Upper Border | Mean Diff. | Lower Border | Upper Border | Mean Diff. | Lower Border | Upper Border | |
| EP1 | −0.580 | −4.388 | 3.228 | −0.652 | −4.629 | 3.326 | 0.072 | −3.906 | 4.049 |
| EP2 | −1.168 | −4.976 | 2.641 | −1.304 | −5.282 | 2.673 | 0.136 | −3.841 | 4.114 |
| EP3 | −3.543 | −7.351 | 0.265 | −2.176 | −6.154 | 1.801 | −1.366 | −5.344 | 2.611 |
| EP4 | −3.611 | −7.419 | 0.197 | −3.305 | −7.283 | 0.672 | −0.306 | −4.283 | 3.672 |
| EP5 | −0.683 | −4.491 | 3.126 | −0.706 | −4.683 | 3.272 | 0.023 | −3.954 | 4.001 |
| EP6 | −1.598 | −5.406 | 2.210 | −1.106 | −5.083 | 2.872 | −0.493 | −4.470 | 3.485 |
| EP7 | −3.546 | −7.354 | 0.262 | −3.347 | −7.325 | 0.630 | −0.199 | −4.176 | 3.779 |
| EP8 | −3.393 | −7.201 | 0.416 | −3.226 | −7.204 | 0.751 | −0.167 | −4.144 | 3.811 |
| EP9 | −1.064 | −4.872 | 2.744 | −0.990 | −4.968 | 2.987 | −0.073 | −4.051 | 3.904 |
| EP10 | −1.940 | −5.748 | 1.868 | −2.120 | −6.098 | 1.857 | 0.181 | −3.797 | 4.158 |
| EP11 | −3.807 | −7.615 | 0.001 | −4.061 | −8.039 | −0.084 | 0.254 | −3.723 | 4.232 |
| EP12 | −4.373 | −8.182 | −0.565 | −4.318 | −8.295 | −0.340 | −0056 | −4.033 | 3.922 |
| EP13 | −1.286 | −5.094 | 2.522 | −1.465 | −5.443 | 2.512 | 0.179 | −3.798 | 4.157 |
| EP14 | −2.405 | −6.213 | 1.403 | −2.922 | −6.899 | 1.056 | 0.517 | −3.461 | 4.494 |
| EP15 | −3.823 | −7.631 | −0.015 | −5.205 | −9.183 | −1.228 | 1.382 | −2.595 | 5.360 |
| EP16 | −4.341 | −8.150 | −0.533 | −4.547 | −8.524 | −0.569 | 0.206 | −3.772 | 4.183 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönau, T.; Anders, C. EMG Amplitude–Force Relationship of Lumbar Back Muscles during Isometric Submaximal Tasks in Healthy Inactive, Endurance and Strength-Trained Subjects. J. Funct. Morphol. Kinesiol. 2023, 8, 29. https://doi.org/10.3390/jfmk8010029
Schönau T, Anders C. EMG Amplitude–Force Relationship of Lumbar Back Muscles during Isometric Submaximal Tasks in Healthy Inactive, Endurance and Strength-Trained Subjects. Journal of Functional Morphology and Kinesiology. 2023; 8(1):29. https://doi.org/10.3390/jfmk8010029
Chicago/Turabian StyleSchönau, Tim, and Christoph Anders. 2023. "EMG Amplitude–Force Relationship of Lumbar Back Muscles during Isometric Submaximal Tasks in Healthy Inactive, Endurance and Strength-Trained Subjects" Journal of Functional Morphology and Kinesiology 8, no. 1: 29. https://doi.org/10.3390/jfmk8010029
APA StyleSchönau, T., & Anders, C. (2023). EMG Amplitude–Force Relationship of Lumbar Back Muscles during Isometric Submaximal Tasks in Healthy Inactive, Endurance and Strength-Trained Subjects. Journal of Functional Morphology and Kinesiology, 8(1), 29. https://doi.org/10.3390/jfmk8010029






