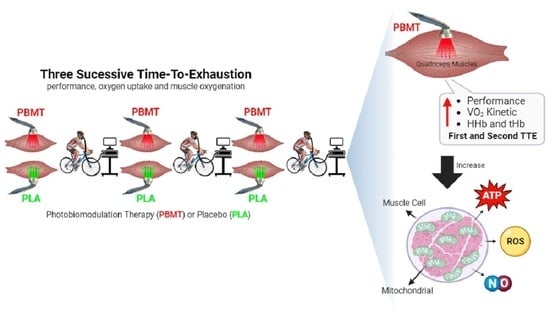
Effects of Photobiomodulation Therapy on Performance in Successive Time-to-Exhaustion Cycling Tests: A Randomized Double-Blinded Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
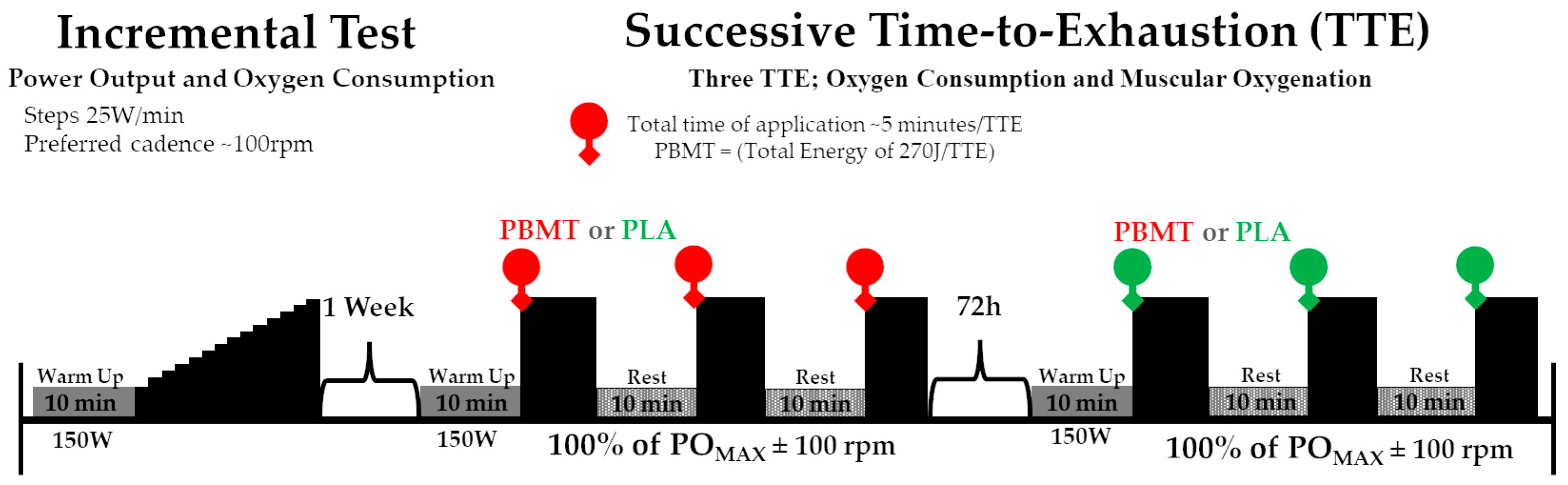
2.1. Experimental Design
2.2. Participants
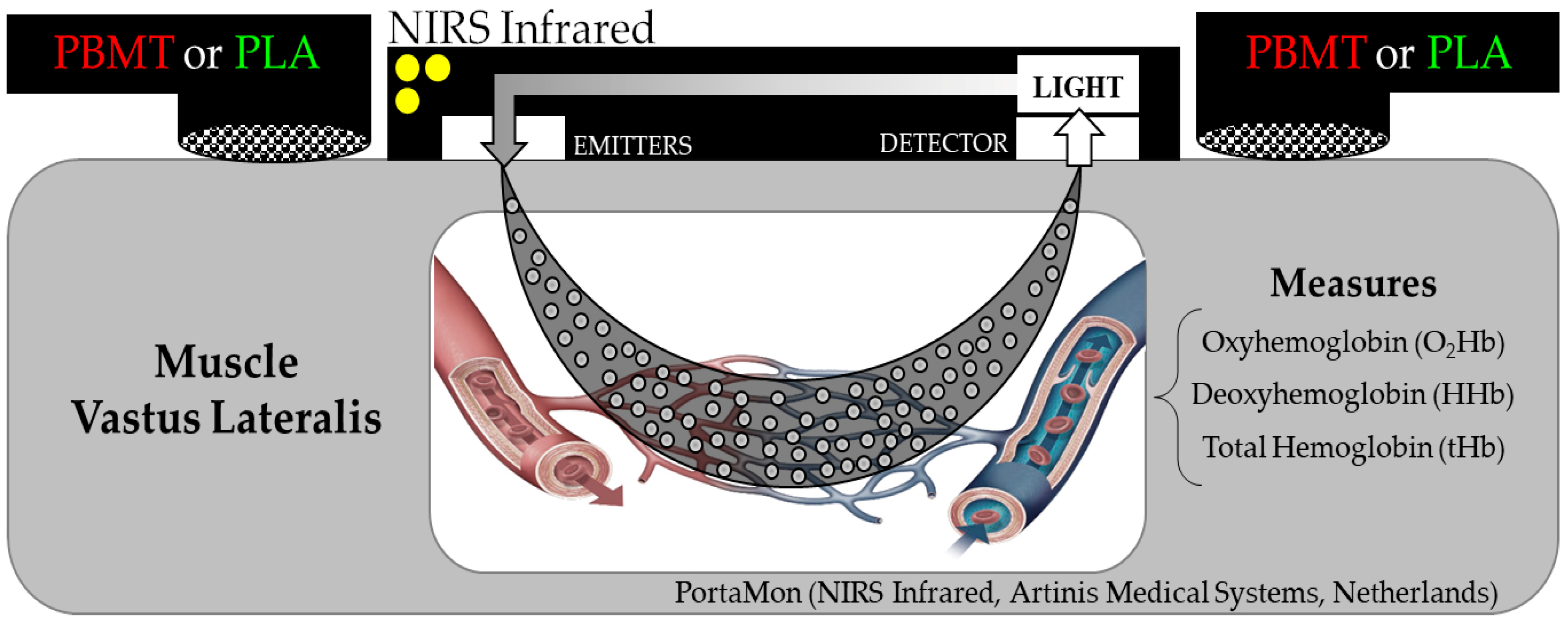
2.3. Procedures
2.4. Exhaustive Severe-Intensity Cycling Test Exercise
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations
4.2. Practical Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gastin, P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R., Jr.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Abbiss, C.R.; Laursen, P.B. Models to explain fatigue during prolonged endurance cycling. Sports Med. 2005, 35, 865–898. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Hopkins, W.G. Skeletal muscle glycogen content at rest and during endurance exercise in humans: A meta-analysis. Sports Med. 2018, 48, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.; Wilborn, C.; La Bounty, P.; Taylor, L.; Nelson, M.T.; Greenwood, M.; Ziegenfuss, T.N.; Lopez, H.L.; Hoffman, J.R.; Stout, J.R.; et al. International society of sports nutrition position stand: Energy drinks. J. Int. Soc. Sports Nutr. 2013, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, C.; Huang, Y.Y.; Hamblin, M.R. Photobiomodulation in human muscle tissue: An advantage in sports performance? J. Biophotonics 2016, 9, 1273–1299. [Google Scholar] [CrossRef]
- Dellagrana, R.A.; Rossato, M.; Sakugawa, R.L.; Baroni, B.M.; Diefenthaeler, F. Photobiomodulation therapy on physiological and performance parameters during running tests: Dose-response effects. J. Strength Cond. Res. 2018, 32, 2807–2815. [Google Scholar] [CrossRef]
- De Marchi, T.; Leal Junior, E.C.; Bortoli, C.; Tomazoni, S.S.; Lopes-Martins, R.A.; Salvador, M. Low-level laser therapy (LLLT) in human progressive-intensity running: Effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med. Sci. 2012, 27, 231–236. [Google Scholar] [CrossRef]
- Lanferdini, F.J.; Silva, E.S.; Boeno, F.P.; Sonda, F.C.; Rosa, R.G.; Quevedo, R.; Baroni, B.M.; Reischak-Oliveira, A.; Vaz, M.A.; Peyre-Tartaruga, L.A. Effect of photobiomodulation therapy on performance and running economy in runners: A randomized double-blinded placebo-controlled trial. J. Sports Sci. 2021, 39, 1348–1355. [Google Scholar] [CrossRef]
- Lanferdini, F.J.; Bini, R.R.; Baroni, B.M.; Klein, K.D.; Carpes, F.P.; Vaz, M.A. Improvement of performance and reduction of fatigue with low-level laser therapy in competitive cyclists. Int. J. Sports Physiol. Perform. 2018, 13, 14–22. [Google Scholar] [CrossRef]
- Ferraresi, C.; de Sousa, M.V.; Huang, Y.Y.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Time response of increases in ATP and muscle resistance to fatigue after low-level laser (light) therapy (LLLT) in mice. Lasers Med. Sci. 2015, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Lanferdini, F.J.; Kruger, R.L.; Baroni, B.M.; Lazzari, C.; Figueiredo, P.; Reischak-Oliveira, A.; Vaz, M.A. Low-level laser therapy improves the VO2 kinetics in competitive cyclists. Lasers Med. Sci. 2018, 33, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, F.; Soni, S.S.; Gonzalez-Lima, F.; Liu, H. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci. Rep. 2016, 6, 30540. [Google Scholar] [CrossRef] [PubMed]
- Tomazoni, S.S.; Machado, C.; De Marchi, T.; Casalechi, H.L.; Bjordal, J.M.; de Carvalho, P.T.C.; Leal-Junior, E.C.P. Infrared low-level laser therapy (photobiomodulation therapy) before intense progressive running test of high-level soccer players: Effects on functional, muscle damage, inflammatory, and oxidative stress markers-a randomized controlled trial. Oxid. Med. Cell. Longev. 2019, 16, 6239058. [Google Scholar] [CrossRef]
- da Silva Alves, M.A.; Pinfildi, C.E.; Neto, L.N.; Lourenco, R.P.; de Azevedo, P.H.; Dourado, V.Z. Acute effects of low-level laser therapy on physiologic and electromyographic responses to the cardiopulmonary exercise testing in healthy untrained adults. Lasers Med. Sci. 2014, 29, 1945–1951. [Google Scholar] [CrossRef]
- Arena, R.; Humphrey, R.; Peberdy, M.A. Measurement of oxygen consumption on-kinetics during exercise: Implications for patients with heart failure. J. Card. Fail. 2001, 7, 302–310. [Google Scholar] [CrossRef]
- Beltrame, T.; Ferraresi, C.; Parizotto, N.A.; Bagnato, V.S.; Hughson, R.L. Light-emitting diode therapy (photobiomodulation) effects on oxygen uptake and cardiac output dynamics during moderate exercise transitions: A randomized, crossover, double-blind, and placebo-controlled study. Lasers Med. Sci. 2018, 33, 1065–1071. [Google Scholar] [CrossRef]
- Jones, B.; Hamilton, D.K.; Cooper, C.E. Muscle oxygen changes following Sprint Interval Cycling training in elite field hockey players. PLoS ONE 2015, 10, e0120338. [Google Scholar] [CrossRef]
- DiMenna, F.J.; Bailey, S.J.; Jones, A.M. Influence of body position on muscle deoxy[Hb+Mb] during ramp cycle exercise. Respir. Physiol. Neurobiol. 2010, 173, 138–145. [Google Scholar] [CrossRef]
- Mortensen, S.P.; Saltin, B. Regulation of the skeletal muscle blood flow in humans. Exp. Physiol. 2014, 99, 1552–1558. [Google Scholar] [CrossRef]
- Spires, S.; Lai, N.; Zhou, H.; Saidel, G.M. Hemoglobin and myoglobin contributions to skeletal muscle oxygenation in response to exercise. Adv. Exp. Med. Biol. 2011, 701, 347–352. [Google Scholar] [PubMed]
- Fernandes, R.J.; de Jesus, K.; Baldari, C.; de Jesus, K.; Sousa, A.C.; Vilas-Boas, J.P.; Guidetti, L. Different VO2max time-averaging intervals in swimming. Int. J. Sports Med. 2012, 33, 1010–1015. [Google Scholar] [PubMed]
- Coyle, E.F.; Feltner, M.E.; Kautz, S.A.; Hamilton, M.T.; Montain, S.J.; Baylor, A.M.; Abraham, L.D.; Petrek, G.W. Physiological and biomechanical factors associated with elite endurance cycling performance. Med. Sci. Sport. Exerc. 1991, 23, 93–107. [Google Scholar] [CrossRef]
- Diefenthaeler, F.; Coyle, E.F.; Bini, R.B.; Carpes, F.P.; Vaz, M.A. Muscle activity and pedal force profile of triathletes during cycling to exhaustion. Sports Biomech. 2012, 11, 10–19. [Google Scholar] [CrossRef]
- Kordi, M.; Folland, J.; Goodall, S.; Haralabidis, N.; Maden-Wilkinson, T.; Sarika Patel, T.; Leeder, J.; Barratt, P.; Howatson, G. Mechanical and morphological determinants of peak power output in elite cyclists. Scan. J. Med. Sci. Sport. 2020, 30, 227–237. [Google Scholar] [CrossRef]
- Sousa, A.; Ribeiro, J.; Sousa, M.; Vilas-Boas, J.P.; Fernandes, R.J. Influence of prior exercise on VO2 kinetics subsequent exhaustive rowing performance. PLoS ONE 2014, 9, e84208. [Google Scholar] [CrossRef]
- Whipp, B.J.; Casaburi, R. Characterizing O2 uptake response kinetics during exercise. Int J Sports Med. 1982, 3, 97–99. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Malta Ede, S.; De Poli, R.A.; Brisola, G.M.; Milioni, F.; Miyagi, W.E.; Machado, F.A.; Zagatto, A.M. Acute LED irradiation does not change the anaerobic capacity and time to exhaustion during a high-intensity running effort: A double-blind, crossover, and placebo-controlled study: Effects of LED irradiation on anaerobic capacity and performance in running. Lasers Med. Sci. 2016, 31, 1473–1480. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose Response 2011, 9, 602–618. [Google Scholar] [CrossRef]
- Ferraresi, C.; Hamblin, M.R.; Parizotto, N.A. Low-level laser (light) therapy (LLLT) on muscle tissue: Performance, fatigue and repair benefited by the power of light. Photonics Lasers Med. 2012, 1, 267–286. [Google Scholar] [CrossRef]
- Bathini, M.; Raghushaker, C.R.; Mahato, K.K. The molecular mechanisms of action of photobiomodulation against neurodegenerative diseases: A systematic review. Cell. Mol. Neurobiol. 2022, 42, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS. Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Moskvin, S.V.; Khadartsev, A.A. Methods of effective low-level laser therapy in the treatment of patients with bronchial asthma (literature review). Biomedicine 2020, 28, 1–20. [Google Scholar]
- Gerbino, A.; Ward, S.A.; Whipp, B.J. Effects of prior exercise on pulmonary gas-exchange kinetics during high-intensity exercise in humans. J. Appl. Physiol. 1996, 80, 99–107. [Google Scholar] [CrossRef]
- Silveira, P.C.; Silva, L.A.; Fraga, D.B.; Freitas, T.P.; Streck, L.M.; Pinho, R. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J. Photochem. Photobiol. B 2009, 95, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Ihsan, M.; Watson, G.; Lipski, M.; Abbiss, C.R. Influence of postexercise cooling on muscle oxygenation and blood volume changes. Med. Sci. Sports Exerc. 2013, 45, 876–882. [Google Scholar] [CrossRef]
- Fukuoka, Y.; Poole, D.C.; Barstow, T.J.; Kondo, N.; Nishiwaki, M.; Okushima, D.; Koga, S. Reduction of V̇O2 slow component by priming exercise: Novel mechanistic insights from time-resolved near-infrared spectroscopy. Physiol. Rep. 2015, 3, e12432. [Google Scholar] [CrossRef]
- Dutra, Y.M.; Malta, E.S.; Elias, A.S.; Broatch, J.R.; Zagatto, A.M. Deconstructing the ergogenic effects of photobiomodulation: A systematic review and meta-analysis of its efficacy in improving mode-specific exercise performance in humans. Sports Med. 2022, 52, 2733–2757. [Google Scholar] [CrossRef]
- Lamarra, N.; Whipp, B.J.; Ward, S.A.; Wasserman, K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J. Appl. Physiol. 1987, 62, 2003–2012. [Google Scholar] [CrossRef]
- Jones, A.M.; Burnley, M. Oxygen uptake kinetics: An underappreciated determinant of exercise performance. Int. J. Sports Physiol. Perform. 2009, 4, 524–532. [Google Scholar] [CrossRef]
- Elmer, S.J.; Barratt, P.R.; Korff, T.; Martin, J.C. Joint-specific power production during submaximal and maximal cycling. Med. Sci. Sport. Exerc. 2011, 43, 1940–1947. [Google Scholar] [CrossRef] [PubMed]




| PBMT | PLA | PBMT vs. PLA | |||||
|---|---|---|---|---|---|---|---|
| Test 1 | p-Value | ES | |||||
| Baseline (mL·min−1) | 531 ± 59 | 549 ± 52 | 0.888 | 0.27 | |||
| Time Delay (s) | 6.0 ± 2.7 | 5.5 ± 2.5 | 0.226 | 0.14 | |||
| Tau (s) | 13.9 ± 3.6 | 17.4 ± 4.3 | 0.007 | 0.79 | |||
| Amplitude of VO2 (mL·min−1) | 3803 ± 343 | 3825 ± 401 | 0.737 | 0.10 | |||
| O2 Deficit (mL·min−1) | 783 ± 167 | 921 ± 171 | 0.005 | 0.82 | |||
| Test 2 | |||||||
| Baseline (mL·min−1) | 635 ± 100 | 631 ± 91 | 0.888 | 0.04 | |||
| Time Delay (s) | 6.0 ± 1.8 | 5.1 ± 2.1 | 0.226 | 0.43 | |||
| Tau (s) | 12.5 ± 2.2 | 14.2 ± 3.3 | 1.000 | 0.48 | |||
| Amplitude of VO2 (mL·min−1) | 3808 ± 360 | 3826 ± 366 | 0.737 | 0.05 | |||
| O2 Deficit (mL·min−1) | 659 ± 119 | 724 ± 94 | 1.000 | 0.48 | |||
| Test 3 | |||||||
| Baseline (mL·min−1) | 618 ± 79 | 613 ± 102 | 0.888 | 0.05 | |||
| Time Delay (s) | 5.5 ± 2.3 | 5.2 ± 2.3 | 0.226 | 0.11 | |||
| Tau (s) | 12.0 ± 2.4 | 13.4 ± 2.0 | 1.000 | 0.52 | |||
| Amplitude of VO2 (mL·min−1) | 3743 ± 295 | 3758 ± 243 | 0.737 | 0.03 | |||
| O2 Deficit (mL·min−1) | 630 ± 108 | 670 ± 130 | 1.000 | 0.27 | |||
| Test 1 vs. 2 | Test 1 vs. 3 | Test 2 vs. 3 | |||||
| p-value | ES | p-value | ES | p-value | ES | ||
| Baseline (mL·min−1) | PBMT | 0.001 | 1.23 | 0.001 | 1.20 | 1.000 | 0.21 |
| PLA | 0.001 | 1.22 | 0.001 | 0.76 | 1.000 | 0.43 | |
| Time Delay (s) | PBMT | 0.685 | 0.01 | 0.685 | 0.17 | 0.685 | 0.22 |
| PLA | 0.685 | 0.16 | 0.685 | 0.09 | 0.685 | 0.06 | |
| Tau (s) | PBMT | 1.000 | 0.43 | 0.544 | 0.68 | 1.000 | 0.20 |
| PLA | 0.008 | 0.74 | 0.001 | 0.79 | 1.000 | 0.32 | |
| Amplitude of VO2 (mL·min−1) | PBMT | 0.730 | 0.01 | 0.730 | 0.11 | 0.730 | 0.13 |
| PLA | 0.730 | 0.00 | 0.730 | 0.16 | 0.730 | 0.21 | |
| O2 Deficit (mL.min−1) | PBMT | 0.003 | 0.96 | 0.001 | 1.39 | 1.000 | 0.28 |
| PLA | 0.001 | 1.57 | 0.001 | 1.48 | 1.000 | 0.50 | |
| PBMT | PLA | PBMT vs. PLA | |||||
|---|---|---|---|---|---|---|---|
| Test 1 | p-Value | ES | |||||
| Baseline (µM) | −2.5 ± 3.2 | −4.4 ± 3.7 | 0.385 | 0.37 | |||
| Time Delay (s) | 5.2 ± 1.7 | 5.9 ± 1.7 | 0.218 | 0.46 | |||
| Tau (s) | 9.4 ± 2.4 | 11.8 ± 2.3 | 0.039 | 1.04 | |||
| Amplitude (µM) | 14.6 ± 3.4 | 11.9 ± 1.6 | 0.049 | 0.75 | |||
| Test 2 | |||||||
| Baseline (µM) | −2.0 ± 2.6 | −3.3 ± 5.0 | 0.385 | 0.21 | |||
| Time Delay (s) | 5.2 ± 1.0 | 5.2 ± 1.7 | 0.218 | 0.03 | |||
| Tau (s) | 10.4 ± 2.3 | 11.9 ± 4.7 | 1.000 | 0.42 | |||
| Amplitude (µM) | 13.9 ± 3.7 | 11.9 ± 3.2 | 0.293 | 0.57 | |||
| Test 3 | |||||||
| Baseline (µM) | −1.8 ± 3.2 | −2.5 ± 6.0 | 0.385 | 0.10 | |||
| Time Delay (s) | 5.2 ± 1.8 | 5.8 ± 2.8 | 0.218 | 0.19 | |||
| Tau (s) | 12.0 ± 4.1 | 12.2 ± 4.9 | 1.000 | 0.08 | |||
| Amplitude (µM) | 12.6 ± 3.7 | 11.2 ± 4.2 | 0.694 | 0.35 | |||
| Test 1 vs. 2 | Test 1 vs. 3 | Test 2 vs. 3 | |||||
| p-value | ES | p-value | ES | p-value | ES | ||
| Baseline (µM) | PBMT | 0.979 | 0.28 | 0.870 | 0.32 | 0.999 | 0.14 |
| PLA | 0.628 | 0.44 | 0.083 | 0.49 | 0.847 | 0.24 | |
| Time Delay (s) | PBMT | 0.724 | 0.02 | 0.724 | 0.00 | 0.724 | 0.03 |
| PLA | 0.724 | 0.36 | 0.724 | 0.02 | 0.724 | 0.19 | |
| Tau (s) | PBMT | 0.314 | 0.42 | 0.314 | 0.57 | 0.314 | 0.37 |
| PLA | 0.314 | 0.02 | 0.314 | 0.08 | 0.314 | 0.07 | |
| Amplitude (µM) | PBMT | 0.068 | 0.33 | 0.068 | 0.75 | 0.068 | 0.76 |
| PLA | 0.068 | 0.01 | 0.068 | 0.18 | 0.068 | 0.18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanferdini, F.J.; Baroni, B.M.; Lazzari, C.D.; Sakugawa, R.L.; Dellagrana, R.A.; Diefenthaeler, F.; Caputo, F.; Vaz, M.A. Effects of Photobiomodulation Therapy on Performance in Successive Time-to-Exhaustion Cycling Tests: A Randomized Double-Blinded Placebo-Controlled Trial. J. Funct. Morphol. Kinesiol. 2023, 8, 144. https://doi.org/10.3390/jfmk8040144
Lanferdini FJ, Baroni BM, Lazzari CD, Sakugawa RL, Dellagrana RA, Diefenthaeler F, Caputo F, Vaz MA. Effects of Photobiomodulation Therapy on Performance in Successive Time-to-Exhaustion Cycling Tests: A Randomized Double-Blinded Placebo-Controlled Trial. Journal of Functional Morphology and Kinesiology. 2023; 8(4):144. https://doi.org/10.3390/jfmk8040144
Chicago/Turabian StyleLanferdini, Fábio Juner, Bruno Manfredini Baroni, Caetano Decian Lazzari, Raphael Luiz Sakugawa, Rodolfo André Dellagrana, Fernando Diefenthaeler, Fabrizio Caputo, and Marco Aurélio Vaz. 2023. "Effects of Photobiomodulation Therapy on Performance in Successive Time-to-Exhaustion Cycling Tests: A Randomized Double-Blinded Placebo-Controlled Trial" Journal of Functional Morphology and Kinesiology 8, no. 4: 144. https://doi.org/10.3390/jfmk8040144
APA StyleLanferdini, F. J., Baroni, B. M., Lazzari, C. D., Sakugawa, R. L., Dellagrana, R. A., Diefenthaeler, F., Caputo, F., & Vaz, M. A. (2023). Effects of Photobiomodulation Therapy on Performance in Successive Time-to-Exhaustion Cycling Tests: A Randomized Double-Blinded Placebo-Controlled Trial. Journal of Functional Morphology and Kinesiology, 8(4), 144. https://doi.org/10.3390/jfmk8040144