Experimental Ultrasound Approach for Studying Knee Intra-Articular Femur–Tibia Movements under Different Loads
Abstract
:1. Introduction
- Finding optimal physical exercises related to individual sportsman fitness status.
- Joint rehabilitation.
- The modeling and design of artificial joints supporting human movements.
- The creation of general-purpose technical devices (robots, mobile lever systems in lifting equipment, etc.).
- Intra-articular biomechanical processes via mathematical modeling.
- Information regarding intra-articular cartilage deformation under different loads.
1.1. Mechanical Response to Physical Loads
1.1.1. Composition and Structure of Cartilage
1.1.2. Mechanical Cartilage’s Response under Physical Loading with Different Profiles
1.2. Methods for Joint Biomechanical Characterization
2. Materials and Methods
- At rest, femur–tibia angle of 140°, leg on the floor (black);
- Own weight stretched, femur–tibia angle of 140°, leg in the air (red);
- Straight, femur–tibia angle of 140°, leg without extra load (green);
- Straight, femur–tibia angle of 140°, leg with 4 kg extra load (yellow);
- Straight, femur–tibia angle of 140°, leg with 8 kg extra load (blue).
3. Results and Discussion
- The extra load levels.
- The biomechanical properties of the knee joint components—femur, tibia, fibula, and patella cartilage deformability, knee joint ligament and tendon viscoelasticity, considering that these are unique to each person and depend on various factors (age, gender, height, level of training, etc.).
- Amount and viscosity of synovial fluid.
- The lower limb pose when increasing extra load.
- Age, sex, and weight.
4. Limitations of This Study
5. Conclusions
- A first-time description of the experimental model stage 1 data with graphs.
- A first-time description of the model’s final accuracy, as shown in Table 2.
- First time data separation for stages 2 and 3.
- A description of the experimental model data, as shown in Table 1 (stages 2 and 3).
- Increase loading weight.
- Enlarge participant groups.
- Compare men and women.
- A study of cartilage deformation under stretch loading.
- Identify the influence of isometric stretching on knee hemorheology [51].
- The development/design of exercises in order to separately divide and estimate the contribution to the load of the joint cavity from stretching only and in combination with other loads.
- The development of practically applicable mechanical trainers for joint fitness.
- The creation of an experimental in vivo ultrasonography model for the evaluation of the change in the distance between the bony cartilaginous surfaces inside the ankle joint under different vertical external loads.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamill, J.; Knutzen, K.M. Biomechanical Basis of Human Movement; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of osteoarthritis: Risk factors, regulatory pathways in chondrocytes, and experimental models. Biology 2020, 9, 194. [Google Scholar] [CrossRef]
- Eschweiler, J.; Li, J.; Quack, V.; Rath, B.; Baroncini, A.; Hildebrand, F.; Migliorini, F. Anatomy, biomechanics, and loads of the wrist joint. Life 2022, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Orejel Bustos, A.; Belluscio, V.; Camomilla, V.; Lucangeli, L.; Rizzo, F.; Sciarra, T.; Martelli, F.; Giacomozzi, C. Overuse-Related Injuries of the Musculoskeletal System: Systematic Review and Quantitative Synthesis of Injuries, Locations, Risk Factors and Assessment Techniques. Sensors 2021, 21, 2438. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Gray-Gaillard, E.F.; Elisseeff, J.H. Cellular senescence in mus-culoskeletal homeostasis, diseases, and regeneration. Bone Res. 2021, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Ranchev, S.; Ivanov, I.M.; Yotov, I.; Stoytchev, S. Studies on a Paradox in The Work of The Musculoskeletal System in Isometric Tretching. J. Appl. Sports Sci. 2020, 2, 80–90. [Google Scholar]
- Lu, X.L.; Mow, V.C. Biomechanics of Articular Cartilage and Determination of Material Properties. Med. Sci. Sports Exerc. 2008, 40, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Stoytchev, S.; Ivanov, I.; Ranchev, S.; Iotov, I. A review of the biomechanics of synovial joints with emphasize to static stretching exercise. Ser. Biomech. 2021, 35, 3–20. [Google Scholar]
- Edwards, J. Physical characteristics of articular cartilage. Proc. Inst. Mech. Eng. 1967, 181, 16–24. [Google Scholar]
- Hayes, W.C.; Mockros, L.F. Viscoelastic properties of human articular cartilage. J. Appl. Physiol. 1971, 31, 562–568. [Google Scholar] [CrossRef]
- Wouters, K.; Puers, R.; Gasik, M. Modelling of poro-visco-elastic biological systems. J. Phys. Conf. Ser. 2015, 633, 012134. [Google Scholar]
- Nia, H.T.; Bozchalooi, I.S.; Li, Y.; Han, L.; Hung, H.H.; Frank, E.; Youcef-Toumi, K.; Ortiz, C.; Grodzinsky, A. High-bandwidth AFM-based rheology reveals that cartilage is most sensitive to high loading rates at early stages of impairment. Biophys. J. 2013, 104, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Cotofana, S.; Eckstein, F.; Wirth, W.; Souza, R.B.; Li, X.; Wyman, B.; Majumdar, S. In vivo measures of cartilage deformation: Patterns in healthy and osteoarthritic female knees using 3T MR imaging. Eur. Radiol. 2011, 21, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Herberhold, C.; Faber, S.; Stammberger, T.; Steinlechner, M.; Putz, R.; Englmeier, K.H.; Eckstein, F. In situ measurement of articular cartilage deformation in intact femoropatellar joints under static loading. J. Biomech. 1999, 32, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F.; Hudelmaier, M.; Putz, R. The effects of exercise on human articular cartilage. J. Anat. 2006, 206, 491–512. [Google Scholar] [CrossRef]
- Kubo, K.; Kanehisa, H.; Fukunaga, T. Is passive stiffness in human muscles related to the elasticity of tendon structures? Eur. J. Appl. Physiol. 2001, 85, 226–232. [Google Scholar] [CrossRef]
- Kubo, K.; Kanehisa, H.; Fukunaga, T. Effects of resistance and stretching training programmes on the viscoelastic properties of human tendon structures in vivo. J. Physiol. 2002, 538, 219–226. [Google Scholar] [CrossRef]
- Ranchev, S.; Ivanov, I.; Iotov, I.; Stoytchev, S. On the biomechanical processes in human knee joint during active isometric stretching. Ser. Biomech. 2019, 33, 56–61. [Google Scholar]
- Brandon, S.C.E.; Smith, C.R.; Thelen, D.G. Simulation of Soft Tissue Loading from Observed Movement Dynamics. In Handbook of Human Motion; Wolf, M.S.I., Ed.; Springer International Publishing: Berlin, Germany, 2017. [Google Scholar]
- Raikova, R.; Prilutsky, B. Sensitivity of predicted muscle forces to parameters of optimization-based human leg model revealed by analytical and numerical analyses. J. Biomech. 2001, 34, 1243–1255. [Google Scholar] [CrossRef]
- Lloyd, D.G.; Besier, T.F. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J. Biomech. 2003, 36, 765–776. [Google Scholar] [CrossRef]
- Kim, H.J.; Fernandez, J.W.; Akbarshahi, M.; Walter, J.P.; Fregly, B.J.; Pandy, M.G. Evaluation of predicted knee-joint muscle forces during gait using an instrumented knee implant. J. Orthop. Res. 2009, 27, 1326–1331. [Google Scholar] [CrossRef]
- Shelburne, K.B.; Torry, M.R.; Pandy, M.G. Muscle, ligament, and joint-contact forces at the knee during walking. Med. Sci. Sports Exerc. 2005, 37, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, K.B.; Pandy, M.G. A musculoskeletal model of the knee for evaluating ligament forces during isometric contractions. J. Biomech. 1997, 30, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Richard, R.N. Individual Muscle Contributions to the Axial Knee Joint Contact Force during Normal Walking. J. Biomech. 2010, 43, 2780–2784. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Manal, K.T.; Rudolph, K.S. Knee joint loading during gait in healthy controls and individuals with knee osteoarthritis. Osteoarthr. Cartil. 2012, 21, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Koehle, M.; Hull, M. The Effect of Knee Model on Estimates of Muscle and Joint Forces in Recumbent Pedaling. J. Biomech. Eng. 2010, 132, 011007. [Google Scholar] [CrossRef] [PubMed]
- Dumas, R.; Moissenet, F.; Gasparutto, X.; Cheze, L. Influence of joint models on lower-limb musculo-tendon forces and three-dimensional joint reaction forces during gait. Proc. Inst. Mech. Eng. H 2012, 226, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Raikova, R. A general approach for modelling and mathematical investigation of the human upper limb. J. Biomech. 1992, 25, 857–867. [Google Scholar] [CrossRef]
- Zhu, J.; Li, B.; Qiu, L.; Liu, H.; Zhang, M.; Wang, Y.; Du, G. A measurement method of knee joint space width by ultrasound: A large multicenter study. Quant. Imaging Med. Surg. 2020, 10, 979. [Google Scholar] [CrossRef]
- Razek, A.A.K.A.; Fouda, N.S.; Elmetwaley, N.; Elbogdady, E. Sonography of the knee joint. J. Ultrasound 2009, 12, 53–60. [Google Scholar] [CrossRef]
- Okano, T.; Filippucci, E.; Di Carlo, M.; Draghessi, A.; Carotti, M.; Salaffi, F.; Wright, G.; Grassi, W. Ultrasonographic evaluation of joint damage in knee osteoarthritis: Feature-specific comparisons with conventional radiography. Rheumatology 2016, 55, 2040–2049. [Google Scholar] [CrossRef]
- Riecke, B.F.; Christensen, R.; Torp-Pedersen, S.; Boesen, M.; Gudbergsen, H.; Bliddal, H. An ultrasound score for knee osteoarthritis: A cross-sectional validation study. Osteoarthr. Cartil. 2014, 22, 1675–1691. [Google Scholar] [CrossRef] [PubMed]
- Bevers, K.; Zweers, M.C.; van den Ende, C.H.; Martens, H.A.; Mahler, E.; Bijlsma, J.W.J.; den Broeder, A.A. Ultrasonographic analysis in knee osteoarthritis: Evaluation of inter-observer reliability. Clin. Exp. Rheumatol.-Incl Suppl. 2012, 30, 673. [Google Scholar]
- Bevers, K.; Bijlsma, J.W.; Vriezekolk, J.E.; van den Ende, C.H.; den Broeder, A.A. Ultrasonographic features in symptomatic osteoarthritis of the knee and relation with pain. Rheumatology 2014, 53, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Giokits-Kakavouli, G.; Karokis, D.; Raftakis, I.; Siagkri, C. Ultrasound of the knee in Rheumatology: Pitfalls, what is new? Use of US in Rheumatology. Mediterr. J. Rheumatol. 2016, 27, 151–160. [Google Scholar] [CrossRef]
- Shimozaki, K.; Nakase, J.; Asai, K.; Yoshimizu, R.; Kimura, M.; Kanayama, T.; Kitagawa, T.; Tsuchiya, H. Usefulness of ultrasonography for dynamic evaluation of medial meniscus hoop function in early knee osteoarthritis. Sci. Rep. 2021, 11, 20091. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, S.; Shan, X.; Yoshida, K.; Yakura, T.; Naito, M.; Kawakami, Y. Site dependent elastic property of human iliotibial band and the effect of hip and knee joint angle configuration. J. Biomech. 2020, 109, 109919. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Chadwick, N.; von Beck, K.; Goswami, P.; Soliman, S.B.; Patel, A.; McGill, K.C. Ultrasound-guided joint interventions of the lower extremity. Skelet. Radiol. 2023, 52, 911–921. [Google Scholar] [CrossRef]
- Patel, Y.C.; Ahir, B.K. Study of intraarticular proximal tibia fractures treated with plating. Int. J. Orthop. Sci. 2020, 6, 216–219. [Google Scholar] [CrossRef]
- Raikova, R.; Ivanov, I.; Hristov, O.; Markova, N.; Trenev, l.; Angelova, S. Detailed Investigation of Knee Biomechanics during Posture Maintenance while Applying Different Static Loadings on the Spine. Int. J. Bioautom. 2023, 27, 83–98. [Google Scholar] [CrossRef]
- Zvetkova, E.; Koytchev, E.; Ivanov, I.; Ranchev, S.; Antonov, A. Biomechanical, Healing and Therapeutic Effects of Stretching: A Comprehensive Review. Appl. Sci. 2023, 13, 8596. [Google Scholar] [CrossRef]
- Alshami, A.; Elsayed, M.; Ali, E.; Eltoukhy, A.E.; Zayed, T. Harnessing the Power of ChatGPT for Automating Systematic Review Process: Methodology, Case Study, Limitations, and Future Directions. Systems 2023, 11, 351. [Google Scholar] [CrossRef]
- Romano, A.; Staber, D.; Grimm, A.; Kronlage, C.; Marquetand, J. Limitations of muscle ultrasound shear wave elastography for clinical routine—Positioning and muscle selection. Sensors 2021, 21, 8490. [Google Scholar] [CrossRef]
- Leitner, C.; Hager, P.A.; Penasso, H.; Tilp, M.; Benini, L.; Peham, C.; Baumgartner, C. Ultrasound as a tool to study muscle–tendon functions during locomotion: A systematic review of applications. Sensors 2019, 19, 4316. [Google Scholar] [CrossRef] [PubMed]
- Kronlage, C.; Grimm, A.; Romano, A.; Stahl, J.H.; Martin, P.; Winter, N.; Marquetand, J. Muscle ultrasound shear wave elastography as a non-invasive biomarker in myotonia. Diagnostics 2021, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Buda, N.; Cylwik, J.; Mróz, K.; Rudzińska, R.; Dubik, P.; Malczewska, A.; Oraczewska, A.; Skoczyński, S.; Suska, A.; Górecki, T.; et al. Lung Ultrasound Examination in Patients with SARS-CoV-2 Infection: Multicenter Study. J. Clin. Med. 2021, 10, 3255. [Google Scholar] [CrossRef] [PubMed]
- Kettelkamp, D.B.; Jacobs, A.W. Tibiofemoral Contact Area—Determination and Implications. J. Bone Joint Surg. Am. 1972, 54, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Shiramizu, K.; Vizesi, F.; Bruce, W.; Herrmann, S.; Walsh, W.R. Tibiofemoral contact areas and pressures in six high flexion knees. Int. Orthop. 2009, 33, 403–406. [Google Scholar] [CrossRef]
- Fukubayashi, Т.; Kurosawa, H. The Contact Area and Pressure Distribution Pattern of the Knee: A Study of Normal and Osteoarthrotic Knee Joints. Acta Orthop. Scand. 1980, 51, 871–879. [Google Scholar] [CrossRef]
- Ivanov, I. Hemorheological Alterations and Physical Activity. Appl. Sci. 2022, 12, 10374. [Google Scholar] [CrossRef]
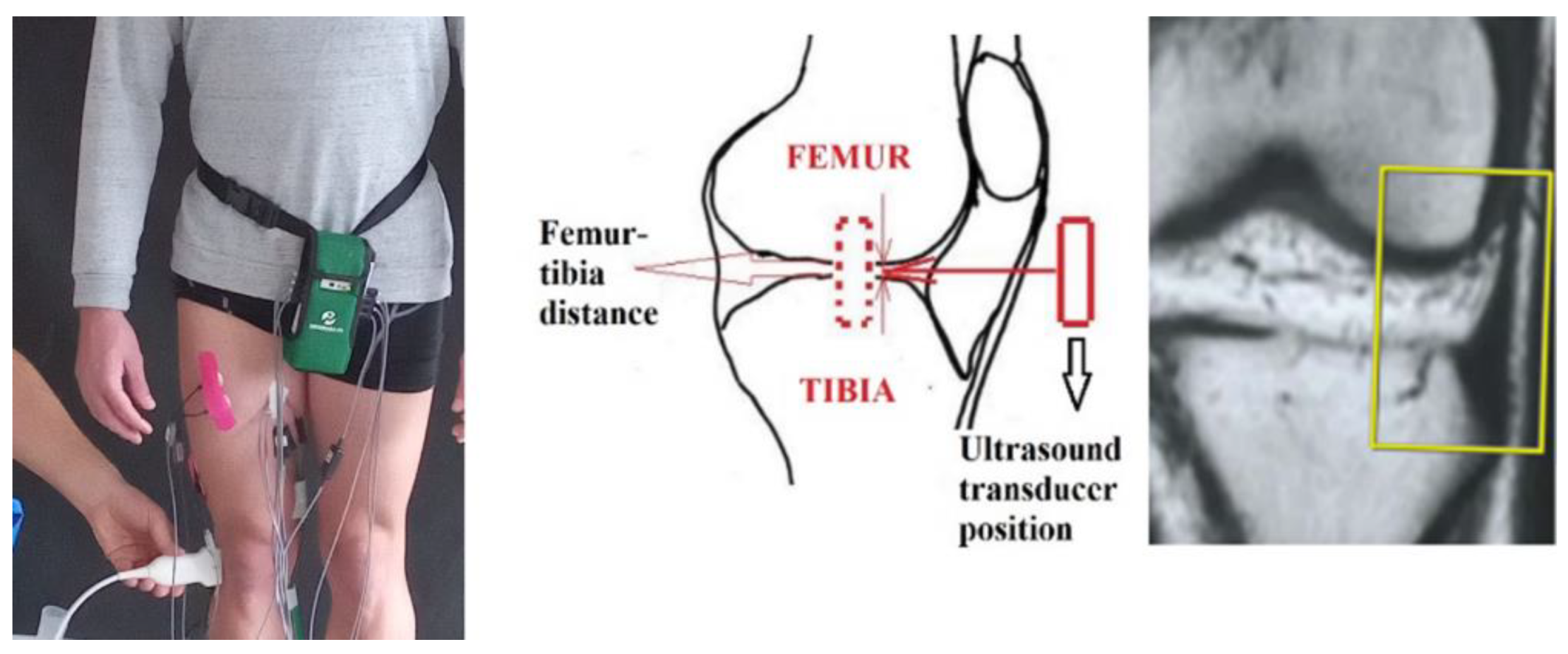

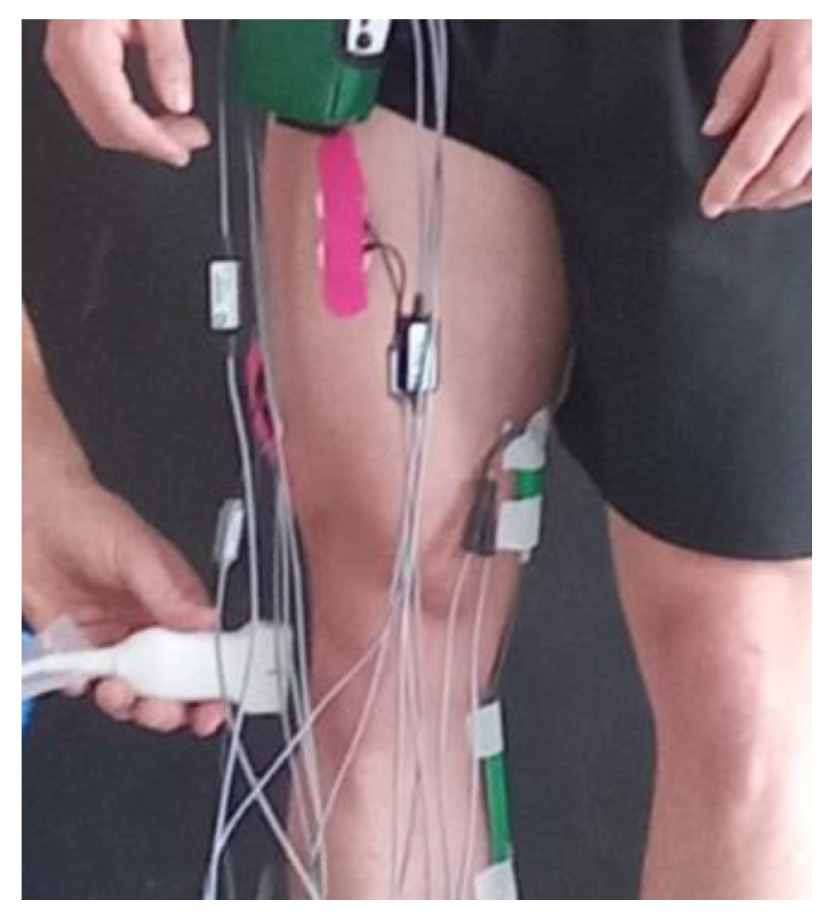

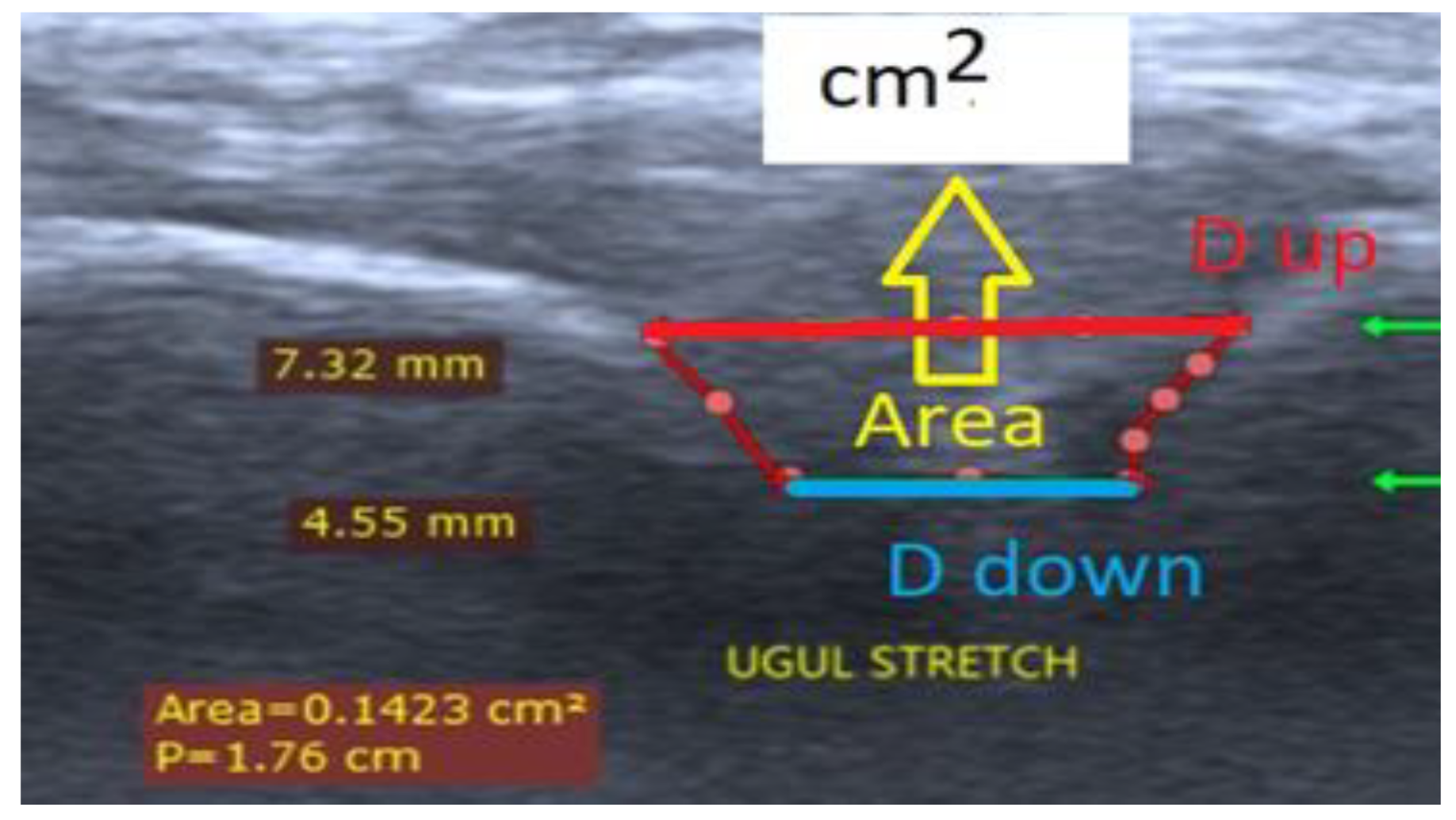



| Participants | D, mm | 0 kg | 2 kg | 5 kg | 10 kg | 15 kg | 17 kg | 20 kg | Angle Coefficient | %, Decrease | Stage | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Age | Height, cm | Weight, kg | BMI (kg/m2) | BSA (m2) | |||||||||||
| N11 | 20 | 175 | 70 | 22.86 | 1.85 | 1 | 1.5 | 1.58 | 1.3 | 1.39 | 1.26 | 1.23 | 1.36 | −0.0117 | 15.74 | II |
| N12 | 22 | 171 | 70 | 23.94 | 1.82 | 1 | 1.65 | 1.57 | 1.6 | 1.55 | 1.46 | 1.7 | 1.37 | −0.0072 | 8.81 | |
| N13 | 21 | 176 | 77 | 24.86 | 1.93 | 1 | 1.28 | 1.24 | 1.14 | 1.13 | 1.09 | 1.06 | 1.02 | −0.0116 | 18.55 | |
| N14 | 22 | 188 | 103 | 29.14 | 2.29 | 1.5 | 2.21 | 1.9 | 2.05 | 2.06 | 1.53 | 1.46 | 1.6 | −0.0327 | 30.35 | |
| N15 | 20 | 183 | 76 | 22.69 | 1.98 | 1 | 1.45 | 1.02 | 1.09 | 1.16 | 0.996 | 1.03 | 1.05 | −0.0119 | 19.18 | |
| N16 | 20 | 181 | 86 | 26.25 | 2.07 | 2.05 | 2 | 1.85 | 1.8 | 1.78 | 1.71 | −0.0147 | 14.61 | |||
| N17 | 20 | 183 | 90 | 26.87 | 2.12 | 1 | 1.78 | 1.54 | 1.52 | 1.47 | 1.62 | 1.71 | 1.49 | −0.0032 | 3.94 | |
| N18 | 19 | 1 | 0.895 | 0.651 | 0.673 | 0.456 | 0.439 | −0.022 | 53.92 | |||||||
| N19 | 19 | 170 | 78 | 26.99 | 1.89 | 1 | 1.41 | 1.36 | 1.4 | 1.32 | 1.25 | 1.19 | 1.17 | −0.0121 | 17.08 | |
| N20 | 20 | 177 | 96 | 30.64 | 2.13 | 1.25 | 1.66 | 1.58 | 1.51 | 1.47 | 1.57 | 1.38 | 1.37 | −0.0112 | 13.89 | |
| N21 | 22 | 175 | 64 | 20.90 | 1.78 | 0.75 | 1.62 | 1.44 | 1.51 | 1.52 | 1.44 | −0.0094 | 11.76 | |||
| N22 | 20 | 190 | 80 | 22.16 | 2.08 | 0.9 | 1.80 | 1.71 | 1.57 | 1.55 | 1.56 | 1.54 | −0.0132 | 14.9 | ||
| N23 | 20 | 185 | 90 | 26.30 | 2.14 | 1 | 2.13 | 1.87 | 2.05 | 1.75 | 1.65 | 1.71 | 1.52 | −0.0256 | 24.78 | |
| N24 | 191 | 85 | 23.30 | 2.14 | 1 | 1.84 | 1.39 | −0.0225 | 24.46 | |||||||
| N25 | 21 | 167 | 70 | 25.10 | 1.79 | 1.25 | 1.93 | 1.82 | 1.76 | 1.54 | 1.41 | −0.0233 | 24.27 | |||
| N26 | 22 | 184 | 73 | 21.56 | 1.95 | 0.875 | 1.09 | 1.08 | 1.07 | 1.03 | 0.979 | 0.99 | −0.0059 | 10.737 | III | |
| N27 | 20 | 184 | 69 | 20.38 | 1.90 | 1.5 | 0.595 | 0.489 | −0.0053 | 17.81 | ||||||
| N28 | 21 | 183 | 86 | 1 | 1.25 | 1.22 | 1.2 | 1.25 | 1.21 | 1.18 | −0.0022 | 3.54 | ||||
| N29 | 24 | 185 | 79 | 23.08 | 2.03 | 1 | 1.86 | 1.8 | 1.77 | 1.75 | 1.77 | −0.0052 | 5.7 | |||
| N30 | 20 | 193 | 79 | 21.21 | 2.09 | 1.125 | 1.84 | 1.8 | 1.89 | 1.66 | 1.61 | 1.61 | −0.0139 | 15 | ||
| N31 | 24 | 178 | 80 | 25.25 | 1.98 | 1 | 1.19 | 1.18 | 1.14 | 1.05 | 1.07 | 1.08 | −0.0064 | 10.92 | ||
| N32 | 19 | 187 | 74 | 21.16 | 1.99 | 1.5 | 0.648 | 0.656 | 0.629 | 0.619 | −0.0684 | 6.87 | ||||
| Stage/Component | 1 | 2 | 3 | Total |
|---|---|---|---|---|
| Stage 1 | ≤5% | ≤20% | ≤5% | ≤30% |
| Stage 2 | ≤5% | ≤7% | ≤5% | ≤17% |
| Stage 3 | ≤5% | ≤2% | ≤2% | ≤9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, I.; Ranchev, S.; Stoychev, S. Experimental Ultrasound Approach for Studying Knee Intra-Articular Femur–Tibia Movements under Different Loads. J. Funct. Morphol. Kinesiol. 2024, 9, 8. https://doi.org/10.3390/jfmk9010008
Ivanov I, Ranchev S, Stoychev S. Experimental Ultrasound Approach for Studying Knee Intra-Articular Femur–Tibia Movements under Different Loads. Journal of Functional Morphology and Kinesiology. 2024; 9(1):8. https://doi.org/10.3390/jfmk9010008
Chicago/Turabian StyleIvanov, Ivan, Sergey Ranchev, and Stoyan Stoychev. 2024. "Experimental Ultrasound Approach for Studying Knee Intra-Articular Femur–Tibia Movements under Different Loads" Journal of Functional Morphology and Kinesiology 9, no. 1: 8. https://doi.org/10.3390/jfmk9010008
APA StyleIvanov, I., Ranchev, S., & Stoychev, S. (2024). Experimental Ultrasound Approach for Studying Knee Intra-Articular Femur–Tibia Movements under Different Loads. Journal of Functional Morphology and Kinesiology, 9(1), 8. https://doi.org/10.3390/jfmk9010008






