Perceived Factors That Contributed to Task Termination during Fatiguing Tasks Anchored to Perceptual Intensities
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Time Course of Procedures
2.3. OMNI-RES Scale Standardized Anchoring Procedures
2.4. Orientation Session
2.5. Test Visits
2.6. Post-Test Questionnaire
2.7. Electromyographic and Torque Acquisition
2.8. Statistical Analysis
3. Results
3.1. Reliability
3.2. Time to Task Failure and Performance Fatigability
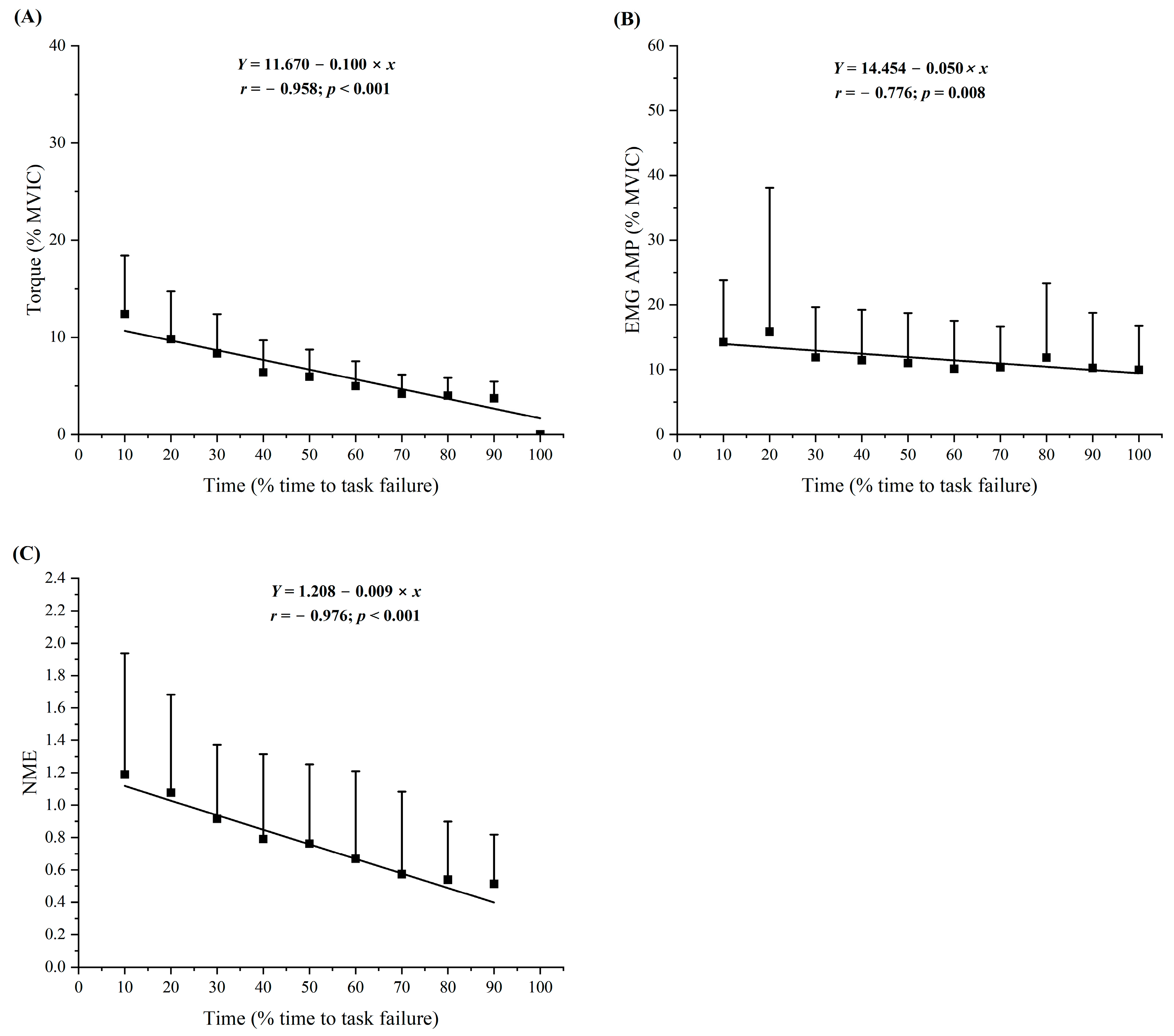
3.3. Torque Responses
3.4. Electromyographic Amplitude Responses
3.5. Neuromuscular Efficiency Responses
3.6. Relationships among Perceived Sensations, Psychological Factors, Time to Task Failure, and Performance Fatigability
3.7. Mean Differences between RPE2FT and RPE8FT for Perceived Sensations and Psychological Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enoka, R.M.; Stuart, D.G. Neurobiology of muscle fatigue. J. Appl. Physiol. 1992, 72, 1631–1648. [Google Scholar] [CrossRef] [PubMed]
- Zwarts, M.J.; Bleijenberg, G.; van Engelen, B.G.M. Clinical Neurophysiology of Fatigue. Clin. Neurophysiol. 2008, 119, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ronquillo, L.; Moien-Afshari, F.; Knox, K.; Britz, J.; Tellez-Zenteno, J.F. How to measure fatigue in epilepsy? The validation of three scales for clinical use. Epilepsy Res. 2011, 95, 119–129. [Google Scholar] [CrossRef]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Translating fatigue to human performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef]
- Arnett, J.E.; Smith, R.W.; Neltner, T.J.; Anders, J.P.V.; Ortega, D.G.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. Effects of joint angle on inter-and intra-individual variability for women during isometric fatiguing tasks anchored to a perceptual intensity. Am. J. Sports Sci. Med. 2023, 11, 7–21. [Google Scholar] [CrossRef]
- Marzouk, M.; McKeown, D.J.; Borg, D.N.; Headrick, J.; Kavanagh, J.J. Perceptions of fatigue and neuromuscular measures of performance fatigability during prolonged low-intensity elbow flexions. Exp. Physiol. 2023, 108, 465–479. [Google Scholar] [CrossRef]
- Ortega, D.G.; Housh, T.J.; Smith, R.W.; Arnett, J.E.; Neltner, T.J.; Schmidt, R.J.; Johnson, G.O. The effects of anchoring a fatiguing forearm flexion task to a high vs. low rating of perceived exertion on torque and neuromuscular responses. J. Strength. Cond. Res. 2024, 38, e219–e225. [Google Scholar] [CrossRef]
- Ortega, D.G.; Smith, R.W.; Arnett, J.E.; Neltner, T.J.; Roberts, T.D.; Schmidt, R.J.; Johnson, G.O.; Housh, T.J. Composite, inter-, and intra-individual torque and neuromuscular responses during fatiguing forearm flexion tasks are dependent on anchor scheme in men. Am. J. Sports Sci. Med. 2024, 12, 7–19. [Google Scholar] [CrossRef]
- Smith, R.W.; Housh, T.J.; Arnett, J.E.; Anders, J.P.V.; Neltner, T.J.; Ortega, D.G.; Schmidt, R.J.; Johnson, G.O. The effects of anchor schemes on performance fatigability, neuromuscular responses and the perceived sensations that contributed to task termination. J. Funct. Morphol. Kinesiol. 2023, 8, 49. [Google Scholar] [CrossRef]
- Smith, R.W.; Ortega, D.G.; Arnett, J.E.; Neltner, T.J.; Schmidt, R.J.; Johnson, G.O.; Weir, J.P.; Housh, T.J. The effects of sustained, low- and high-intensity isometric tasks on performance fatigability and the perceived responses that contributed to task termination. Eur. J. Appl. Physiol. 2024, 124, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R. The anticipatory regulation of performance: The physiological basis for pacing strategies and the development of a perception-based model for exercise performance. Br. J. Sports Med. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Keller, J.L.; Housh, T.J.; Hill, E.C.; Smith, C.M.; Schmidt, R.J.; Johnson, G.O. Neuromuscular responses of recreationally active women during a sustained, submaximal isometric leg extension muscle action at a constant perception of effort. Eur. J. Appl. Physiol. 2018, 118, 2499–2508. [Google Scholar] [CrossRef]
- Smith, R.W.; Anders, J.P.V.; Neltner, T.J.; Arnett, J.E.; Keller, J.L.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. Perceptual fatigability and neuromuscular responses during a sustained, isometric forearm flexion muscle action anchored to a constant level of perceived exertion. NeuroSports 2021, 1, 2. [Google Scholar]
- Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.S.; Vieira, T.M. Interpreting signal amplitudes in surface electromyography studies in sport and rehabilitation sciences. Front. Physiol. 2018, 8, 985. [Google Scholar] [CrossRef]
- Deschenes, M.R.; Brewer, R.E.; Bush, J.A.; McCoy, R.W.; Volek, J.S.; Kraemer, W.J. Neuromuscular disturbance outlasts other symptoms of exercise-induced muscle damage. J. Neurol. Sci. 2000, 174, 92–99. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Hureau, T.J.; Broxterman, R.M.; Weavil, J.C.; Lewis, M.T.; Layec, G.; Amann, M. On the role of skeletal muscle acidosis and inorganic phosphates as determinants of central and peripheral fatigue: A 31P-MRS study. J. Physiol. 2022, 600, 3069–3081. [Google Scholar] [CrossRef]
- Smith, C.M.; Housh, T.J.; Hill, E.C.; Cochrane, K.C.; Jenkins, N.D.; Schmidt, R.J.; Johnson, G.O. Differences between the time course of changes in neuromuscular responses and pretest versus posttest measurements for the examination of fatigue. J. Nat. Sci. 2017, 3, e456. [Google Scholar]
- McKay, A.K.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Walker, E.H.E.; Perreault, E.J. Arm dominance affects feedforward strategy more than feedback sensitivity during a postural task. Exp. Brain Res. 2015, 233, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.G.; Smith, R.W.; Arnett, J.E.; Neltner, T.J.; Schmidt, R.J.; Johnson, G.O.; Housh, T.J. Are performance and perceived fatigability dependent on the anchor scheme of fatiguing isometric tasks in men? J. Sports Med. Phys. Fit. 2024, 64, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Kulig, K.; Andrews, J.G.; Hay, J.G. Human strength curves. Exerc. Sport Sci. Rev. 1984, 12, 417–466. [Google Scholar] [CrossRef]
- Gearhart, R.F.; Goss, F.L.; Lagally, K.M.; Jakicic, J.M.; Gallagher, J.; Robertson, R.J. Standardized scaling procedures for rating perceived exertion during resistance exercise. J. Strength. Cond. Res. 2001, 15, 320–325. [Google Scholar] [PubMed]
- Boone, H.; Boone, D. Analyzing likert data. J. Ext. 2012, 50, 48. [Google Scholar] [CrossRef]
- Harpe, S.E. How to analyze likert and other rating scale data. Curr. Pharm. Teach. Learn. 2015, 7, 836–850. [Google Scholar] [CrossRef]
- Likert, R. A technique for the measurement of attitudes. Arch. Psychol. 1932, 22, 1–55. [Google Scholar]
- Bigland-Ritchie, B.; Woods, J.J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 1984, 7, 691–699. [Google Scholar] [CrossRef]
- Cook, D.B.; O’Connor, P.J.; Eubanks, S.A.; Smith, J.C.; Lee, M. Naturally occurring muscle pain during exercise: Assessment and experimental evidence. Med. Sci. Sports Exerc. 1997, 29, 999–1012. [Google Scholar] [CrossRef]
- Brehm, J.W.; Self, E.A. The intensity of motivation. Annu. Rev. Psychol. 1989, 40, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Jones, A.A.; Power, G.A.; Herzog, W. History dependence of the electromyogram: Implications for isometric steady-state EMG parameters following a lengthening or shortening contraction. J. Electromyogr. Kinesiol. 2016, 27, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength. Cond. Res. 2005, 19, 231–240. [Google Scholar] [PubMed]
- Weir, J.P.; Vincent, W.J. Analysis of nonparametric data. In Statistics in Kinesiology, 5th ed.; Human Kinetics Publishers: Champaign, IL, USA, 2020; pp. 381–492. [Google Scholar]
- Scheff, S.W. Nonparametric statistics. In Fundamental Statistical Principles for the Neurobiologist: A Survival Guide; Academic Press: Cambridge, MA, USA, 2016; p. 162. [Google Scholar]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Smith, R.W.; Housh, T.J.; Arnett, J.E.; Anders, J.P.V.; Neltner, T.J.; Ortega, D.G.; Schmidt, R.J.; Johnson, G.O. The effects of anchor scheme and sex on performance fatigability and neuromuscular responses following sustained, isometric forearm flexion tasks to failure. J. Exerc. Physiol. Online 2023, 26, 69–92. [Google Scholar]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Halaki, M.; Ginn, K. Normalization of EMG signals: To normalize or not to normalize and what to normalize to. Comput. Intell. Electromyogr. Anal-Perspect. Curr. Appl. Future Chall. 2012, 10, 49957. [Google Scholar]
- Keller, M.L.; Pruse, J.; Yoon, T.; Schlinder-Delap, B.; Harkins, A.; Hunter, S.K. Supraspinal fatigue is similar in men and women for a low-force fatiguing contraction. Med. Sci. Sports Exerc. 2011, 43, 1873–1883. [Google Scholar] [CrossRef]
- Petrofsky, J.S.; Phillips, C.A. The effect of elbow angle on the isometric strength and endurance of the elbow flexors in men and women. J. Hum. Ergol. 1980, 9, 125–131. [Google Scholar]
- Rudroff, T.; Justice, J.N.; Holmes, M.R.; Matthews, S.D.; Enoka, R.M. Muscle activity and time to task failure differ with load compliance and target force for elbow flexor muscles. J. Appl. Physiol. 2011, 110, 125–136. [Google Scholar] [CrossRef]
- Thomas, K.; Goodall, S.; Howatson, G. Performance fatigability is not regulated to a peripheral critical threshold. Exerc. Sport Sci. Rev. 2018, 46, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Hureau, T.J.; Romer, L.M.; Amann, M. The ‘sensory tolerance limit’: A hypothetical construct determining exercise performance? Eur. J. Sport Sci. 2018, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.L.; Housh, T.J.; Hill, E.C.; Smith, C.M.; Schmidt, R.J.; Johnson, G.O. Self-regulated force and neuromuscular responses during fatiguing isometric leg extensions anchored to a rating of perceived exertion. Appl. Psychophysiol. Biofeedback 2019, 44, 343–350. [Google Scholar] [CrossRef]
- Strojnik, V.; Komi, P.V. Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J. Appl. Physiol. 1998, 84, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Gandevia, S.C. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J. Appl. Physiol. 2008, 104, 542–550. [Google Scholar] [CrossRef]
- Bigland-Ritchie, B.; Furbush, F.; Woods, J.J. Fatigue of intermittent submaximal voluntary contractions: Central and peripheral factors. J. Appl. Physiol. 1986, 61, 421–429. [Google Scholar] [CrossRef]
- Merton, P.A. Voluntary strength and fatigue. J. Physiol. 1954, 123, 553. [Google Scholar] [CrossRef]
- Smirmaul, B. de P.C. Sense of effort and other unpleasant sensations during exercise: Clarifying concepts and mechanisms. Br. J. Sports Med. 2012, 46, 308–311. [Google Scholar] [CrossRef]
- Staiano, W.; Bosio, A.; de Morree, H.M.; Rampinini, E.; Marcora, S. The cardinal exercise stopper: Muscle fatigue, muscle pain or perception of effort? Prog. Brain Res. 2018, 240, 175–200. [Google Scholar]
- Greenhouse-Tucknott, A.; Wrightson, J.G.; Raynsford, M.; Harrison, N.A.; Dekerle, J. Interactions between perceptions of fatigue, effort, and afect decrease knee extensor endurance performance following upper body motor activity, independent of changes in neuromuscular function. Psychophysiology 2020, 57, e13602. [Google Scholar] [CrossRef] [PubMed]


| Orientation Session | Test Visits 1 and 2 |
|---|---|
|
|
| Variables (mean ± SD) | Visit 1 | Visit 2 | P | ICC | ICC95% |
|---|---|---|---|---|---|
| MVIC (Nm) | 78.1 ± 19.6 | 80.4 ± 19.4 | 0.379 | 0.902 | 0.707–0.970 |
| EMG AMP (μVrms) | 1123.2 ± 626.0 | 1325.6 ± 712.5 | 0.020 * | 0.892 | 0.523–0.971 |
| NME | 0.09 ± 0.05 | 0.08 ± 0.05 | 0.088 | 0.903 | 0.681–0.972 |
| Subjects | Torque | EMG AMP | NME | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Corr. | p-Value | Model | Corr. | p-Value | Model | Corr. | p-Value | |
| 1 | Linear | −0.770 | 0.009 | - | - | NS | Linear | −0.708 | 0.033 |
| 2 | Linear | −0.949 | <0.001 | Linear | −0.792 | 0.006 | - | - | NS |
| 3 | Linear | −0.953 | <0.001 | - | - | NS | Linear | −0.923 | <0.001 |
| 4 | Linear | −0.861 | 0.001 | Linear | −0.807 | 0.005 | Linear | −0.940 | <0.001 |
| 5 | Linear | −0.956 | <0.001 | Linear | −0.930 | <0.001 | Linear | −0.704 | 0.034 |
| 6 | Linear | −0.978 | <0.001 | Linear | −0.885 | <0.001 | Linear | −0.955 | <0.001 |
| 7 | Linear | −0.967 | <0.001 | Linear | −0.756 | 0.011 | Linear | −0.817 | 0.007 |
| 8 | Linear | −0.799 | 0.006 | - | - | NS | - | - | NS |
| 9 | Linear | −0.897 | <0.001 | - | - | NS | Linear | −0.764 | 0.016 |
| 10 | Linear | −0.956 | <0.001 | Linear | −0.848 | 0.002 | Linear | −0.953 | <0.001 |
| 11 | Linear | −0.836 | 0.003 | - | - | NS | Linear | −0.916 | <0.001 |
| 12 | Linear | −0.855 | 0.002 | Linear | −0.723 | 0.018 | Linear | −0.921 | <0.001 |
| Composite | Linear | −0.958 | <0.001 | Linear | −0.776 | 0.008 | Linear | −0.976 | <0.001 |
| Subjects | Torque | EMG AMP | NME | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Corr. | p-Value | Model | Corr. | p-Value | Model | Corr. | p-Value | |
| 1 | Linear | −0.872 | <0.001 | Linear | −0.635 | 0.049 | - | - | NS |
| 2 | Linear | −0.908 | <0.001 | Linear | −0.947 | <0.001 | Linear | −0.865 | 0.003 |
| 3 | Quadratic | −0.969 | 0.021 | Linear | −0.635 | 0.048 | Linear | −0.699 | 0.036 |
| 4 | Quadratic | −0.959 | 0.024 | Quadratic | −0.88 | 0.012 | - | - | NS |
| 5 | Linear | −0.933 | <0.001 | Linear | −0.714 | 0.02 | Quadratic | −0.408 | 0.005 |
| 6 | Linear | −0.761 | 0.011 | Linear | −0.731 | 0.016 | - | - | NS |
| 7 | Linear | −0.947 | <0.001 | - | - | NS | Linear | −0.799 | 0.010 |
| 8 | Linear | −0.884 | <0.001 | - | - | NS | - | - | NS |
| 9 | Linear | −0.905 | <0.001 | - | - | NS | Linear | −0.716 | 0.030 |
| 10 | Linear | −0.933 | <0.001 | Quadratic | −0.436 | 0.031 | Linear | −0.940 | <0.001 |
| 11 | Linear | −0.944 | <0.001 | - | - | NS | Linear | −0.831 | 0.006 |
| 12 | Quadratic | −0.993 | 0.003 | Quadratic | −0.476 | 0.007 | Linear | −0.946 | <0.001 |
| Composite | Linear | −0.969 | <0.001 | Linear | −0.857 | 0.002 | Linear | −0.969 | <0.001 |
| BB Fatigue | BB Pain | FM Fatigue | FM Pain | HM Fatigue | HM Pain | LOF | Motivation 1 | Motivation 2 | Motivation 3 | TTF | PF | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BB Fatigue | 1.000 | |||||||||||
| BB Pain | 0.663 * | 1.000 | ||||||||||
| FM Fatigue | 0.694 * | 0.539 | 1.000 | |||||||||
| FM Pain | 0.497 | 0.675 * | 0.788 ** | 1.000 | ||||||||
| HM Fatigue | 0.654 * | 0.720 ** | 0.763 ** | 0.689 * | 1.000 | |||||||
| HM Pain | 0.520 | 0.648 * | 0.617 * | 0.585 * | 0.942 ** | 1.000 | ||||||
| LOF | 0.294 | 0.287 | 0.241 | 0.449 | −0.017 | −0.120 | 1.000 | |||||
| Motivation 1 | 0.210 | −0.027 | 0.172 | 0.000 | 0.309 | 0.242 | −0.442 | 1.000 | ||||
| Motivation 2 | −0.186 | 0.033 | 0.085 | 0.233 | −0.092 | −0.204 | 0.703 * | −0.308 | 1.000 | |||
| Motivation 3 | −0.173 | −0.216 | 0.106 | 0.155 | −0.360 | −0.443 | 0.691 * | −0.311 | 0.822 * | 1.000 | ||
| TTF | −0.074 | 0.249 | 0.167 | 0.458 | 0.253 | 0.306 | −0.167 | 0.159 | −0.015 | 0.017 | 1.000 | |
| PF | 0.315 | −0.256 | 0.278 | 0.137 | −0.121 | −0.263 | 0.289 | 0.397 | −0.015 | 0.309 | −0.210 | 1.000 |
| BB Fatigue | BB Pain | FM Fatigue | FM Pain | HM Fatigue | HM Pain | LOF | Motivation 1 | Motivation 2 | Motivation 3 | TTF | PF | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BB Fatigue | 1.000 | |||||||||||
| BB Pain | 0.518 | 1.000 | ||||||||||
| FM Fatigue | 0.051 | 0.302 | 1.000 | |||||||||
| FM Pain | −0.008 | 0.157 | 0.904 ** | 1.000 | ||||||||
| HM Fatigue | 0.142 | 0.482 | 0.726 ** | 0.630 * | 1.000 | |||||||
| HM Pain | 0.222 | 0.442 | 0.679 * | 0.632 * | 0.856 ** | 1.000 | ||||||
| LOF | 0.170 | −0.125 | 0.238 | 0.496 | 0.175 | 0.377 | 1.000 | |||||
| Motivation 1 | 0.064 | 0.204 | 0.408 | 0.184 | 0.310 | 0.008 | −0.219 | 1.000 | ||||
| Motivation 2 | 0.343 | 0.129 | 0.437 | 0.565 | 0.077 | 0.329 | 0.690 * | −0.026 | 1.000 | |||
| Motivation 3 | −0.419 | −0.274 | 0.272 | 0.611 * | 0.000 | 0.134 | 0.548 | −0.309 | 0.378 | 1.000 | ||
| TTF | −0.158 | −0.137 | −0.092 | −0.165 | −0.223 | −0.166 | −0.315 | −0.148 | −0.220 | 0.000 | 1.000 | |
| PF | 0.415 | 0.547 | 0.392 | 0.319 | 0.037 | 0.090 | −0.158 | 0.070 | 0.318 | 0.065 | 0.042 | 1.000 |
| Subjects | RPE2FT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BB Fatigue | BB Pain | FM Fatigue | FM Pain | HM Fatigue | HM Pain | LOF | Motivation 1 | Motivation 2 | Motivation 3 | |
| 1 | 2 | 2 | 4 | 4 | 5 | 5 | 1 | 5 | 1 | 1 |
| 2 | 5 | 2 | 2 | 2 | 4 | 3 | 2 | 5 | 1 | 1 |
| 3 | 1 | 2 | 1 | 2 | 4 | 4 | 2 | 3 | 2 | 1 |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 4 | 3 | 3 |
| 5 | 2 | 1 | 2 | 1 | 4 | 4 | 1 | 4 | 1 | 1 |
| 6 | 4 | 3 | 3 | 2 | 5 | 4 | 2 | 4 | 2 | 1 |
| 7 | 2 | 1 | 2 | 2 | 2 | 2 | 4 | 3 | 2 | 2 |
| 8 | 2 | 2 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 |
| 9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 5 | 1 | 1 |
| 10 | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 2 | 2 | 2 |
| 11 | 4 | 4 | 2 | 2 | 4 | 4 | 2 | 3 | 1 | 1 |
| 12 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mean ± SD | 2.3 ± 1.4 | 1.8 ± 0.9 | 2.1 ± 1.1 | 2.0 ± 1.0 | 3.1 ± 1.6 | 2.8 ± 1.4 | 2.1 ± 1.1 | 3.5 ± 1.2 | 1.7 ± 0.8 | 1.5 ± 0.8 |
| Subjects | RPE8FT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BB Fatigue | BB Pain | FM Fatigue | FM Pain | HM Fatigue | HM Pain | LOF | Motivation 1 | Motivation 2 | Motivation 3 | |
| 1 | 4 | 4 | 4 | 3 | 4 | 3 | 1 | 5 | 1 | 1 |
| 2 | 5 | 4 | 2 | 2 | 1 | 1 | 1 | 4 | 2 | 1 |
| 3 | 4 | 5 | 4 | 3 | 4 | 4 | 1 | 5 | 2 | 1 |
| 4 | 5 | 4 | 2 | 2 | 3 | 3 | 3 | 4 | 2 | 1 |
| 5 | 4 | 5 | 2 | 2 | 4 | 2 | 1 | 5 | 1 | 1 |
| 6 | 5 | 4 | 4 | 4 | 5 | 4 | 2 | 5 | 2 | 1 |
| 7 | 4 | 4 | 3 | 4 | 2 | 2 | 2 | 4 | 2 | 2 |
| 8 | 2 | 3 | 3 | 4 | 4 | 4 | 3 | 3 | 2 | 2 |
| 9 | 4 | 2 | 1 | 1 | 1 | 1 | 1 | 5 | 1 | 1 |
| 10 | 5 | 5 | 2 | 2 | 3 | 3 | 2 | 4 | 2 | 1 |
| 11 | 5 | 5 | 2 | 2 | 4 | 4 | 1 | 1 | 1 | 1 |
| 12 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mean ± SD | 4.0 ± 1.3 | 3.8 ± 1.3 | 2.5 ± 1.1 | 2.5 ± 1.1 | 3.0 ± 1.4 | 2.7 ± 1.2 | 1.6 ± 0.8 | 3.8 ± 1.5 | 1.6 ± 0.5 | 1.2 ± 0.4 |
| Sustained Task | Likert-Type Scale Ratings | Percentage (% of Sample) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BB Fatigue | BB Pain | FM Fatigue | FM Pain | HM Fatigue | HM Pain | LOF | Motivation 1 | Motivation 2 | Motivation 3 | ||
| RPE2FT | 1—Strongly disagree | 33 | 42 | 33 | 33 | 25 | 25 | 33 | 8 | 50 | 67 |
| 2—Disagree | 42 | 42 | 42 | 50 | 17 | 17 | 42 | 8 | 33 | 17 | |
| 3—Neither agree or disagree | 0 | 8 | 8 | 0 | 0 | 17 | 8 | 33 | 17 | 17 | |
| 4—Agree | 17 | 8 | 17 | 17 | 42 | 33 | 17 | 25 | 0 | 0 | |
| 5—Strongly agree | 8 | 0 | 0 | 0 | 17 | 8 | 0 | 25 | 0 | 0 | |
| RPE8FT | 1—Strongly disagree | 8 | 8 | 17 | 17 | 25 | 25 | 58 | 17 | 42 | 83 |
| 2—Disagree | 8 | 8 | 42 | 42 | 8 | 17 | 25 | 0 | 58 | 17 | |
| 3—Neither agree or disagree | 0 | 8 | 17 | 17 | 17 | 25 | 17 | 8 | 0 | 0 | |
| 4—Agree | 42 | 42 | 25 | 25 | 42 | 33 | 0 | 33 | 0 | 0 | |
| 5—Strongly agree | 42 | 33 | 0 | 0 | 8 | 0 | 0 | 42 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, D.G.; Smith, R.W.; Arnett, J.E.; Neltner, T.J.; Roberts, T.D.; Schmidt, R.J.; Housh, T.J. Perceived Factors That Contributed to Task Termination during Fatiguing Tasks Anchored to Perceptual Intensities. J. Funct. Morphol. Kinesiol. 2024, 9, 152. https://doi.org/10.3390/jfmk9030152
Ortega DG, Smith RW, Arnett JE, Neltner TJ, Roberts TD, Schmidt RJ, Housh TJ. Perceived Factors That Contributed to Task Termination during Fatiguing Tasks Anchored to Perceptual Intensities. Journal of Functional Morphology and Kinesiology. 2024; 9(3):152. https://doi.org/10.3390/jfmk9030152
Chicago/Turabian StyleOrtega, Dolores G., Robert W. Smith, Jocelyn E. Arnett, Tyler J. Neltner, Trevor D. Roberts, Richard J. Schmidt, and Terry J. Housh. 2024. "Perceived Factors That Contributed to Task Termination during Fatiguing Tasks Anchored to Perceptual Intensities" Journal of Functional Morphology and Kinesiology 9, no. 3: 152. https://doi.org/10.3390/jfmk9030152
APA StyleOrtega, D. G., Smith, R. W., Arnett, J. E., Neltner, T. J., Roberts, T. D., Schmidt, R. J., & Housh, T. J. (2024). Perceived Factors That Contributed to Task Termination during Fatiguing Tasks Anchored to Perceptual Intensities. Journal of Functional Morphology and Kinesiology, 9(3), 152. https://doi.org/10.3390/jfmk9030152






