High Versus Low-Energy Extracorporeal Shockwave Therapy for Chronic Lateral Epicondylitis: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
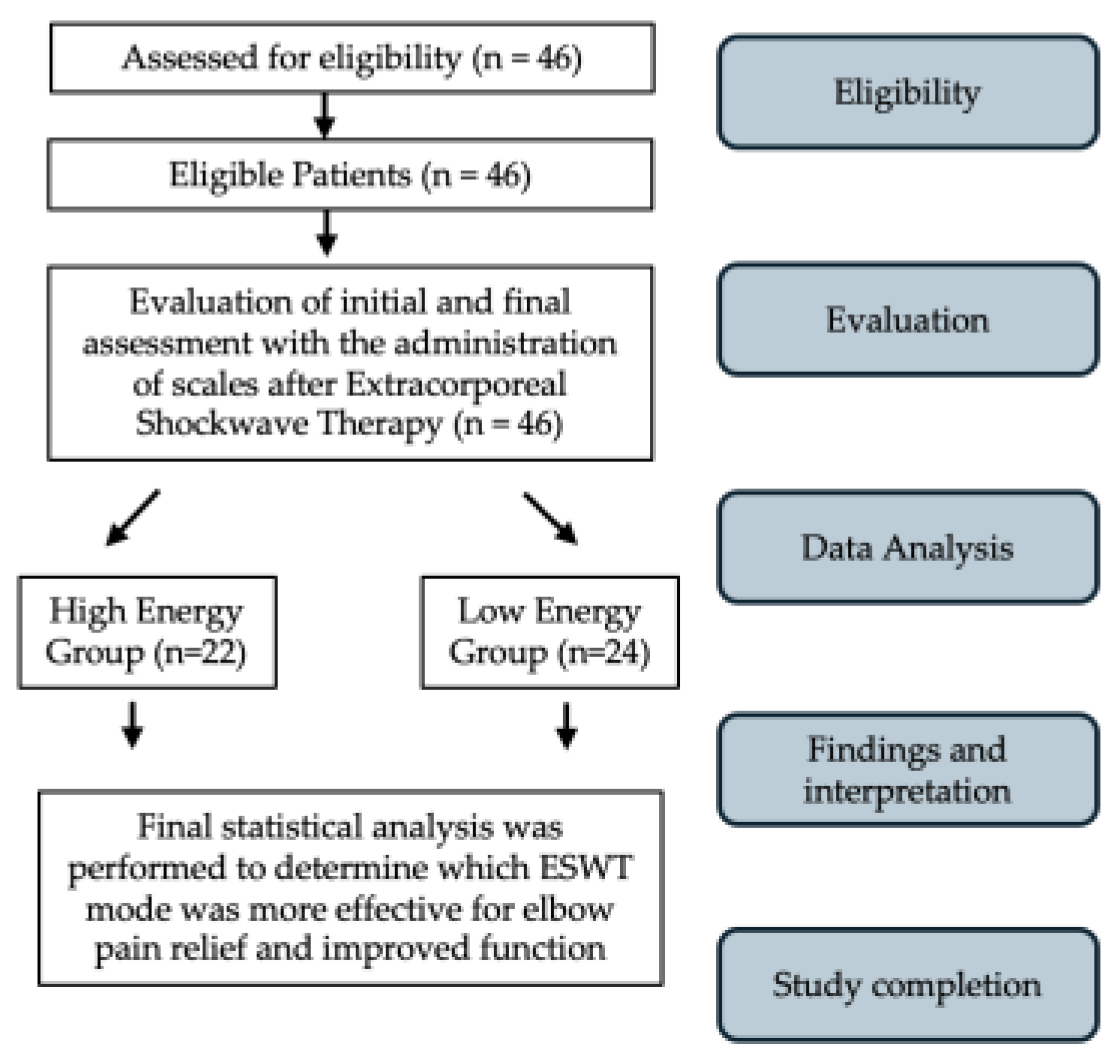
2.1. Study Design and Population
2.2. Intervention
Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Demographic and Clinical Characteristics
3.2. Safety
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CET | Common extensor tendon |
| ECRB | Extensor carpi radialis brevis |
| EDC | Extensor digitorum communis |
| EFD | Energy flux density |
| ESWT | Extracorporeal shockwave therapy |
| LE | Lateral epicondylitis |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PRTEE | Patient-Rated Tennis Elbow Evaluation Questionnaire |
| US | Ultrasonography |
| VAS | Visual analog scale |
References
- Ahmed, A.F.; Rayyan, R.; Zikria, B.A.; Salameh, M. Lateral epicondylitis of the elbow: An up-to-date review of management. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Vaquero-Picado, A.; Barco, R.; Antuña, S.A. Lateral epicondylitis of the elbow. EFORT Open Rev. 2017, 1, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Stegink-Jansen, C.W.; Bynum, J.G.; Lambropoulos, A.L.; Patterson, R.M.; Cowan, A.C. Lateral epicondylosis: A literature review to link pathology and tendon function to tissue-level treatment and ergonomic interventions. J. Hand Ther. 2021, 34, 263–297. [Google Scholar] [CrossRef]
- Greenbaum, B.; Itamura, J.; Vangsness, C.T.; Tibone, J.; Atkinson, R. Extensor carpi radialis brevis. J. Bone Jt. Surg. 1999, 81-B, 926–929. [Google Scholar] [CrossRef][Green Version]
- Nimura, A.; Fujishiro, H.; Wakabayashi, Y.; Imatani, J.; Sugaya, H.; Akita, K. Joint capsule attachment to the extensor carpi radialis brevis origin: An anatomical study with possible implications regarding the etiology of lateral epicondylitis. J. Hand Surg. 2014, 39, 219–225. [Google Scholar] [CrossRef]
- Omoumi, P.; Teixeira, P.A.G.; Ward, S.R.; Trudell, D.; Resnick, D. Practical ultrasonographic technique to precisely identify and differentiate tendons and ligaments of the elbow at the level of the humeral epicondyles: Anatomical study. Skelet. Radiol. 2021, 50, 1369–1377. [Google Scholar] [CrossRef]
- Waseem, M.; Nuhmani, S.; Ram, C.; Sachin, Y. Lateral epicondylitis: A review of the literature. J. Back Musculoskelet. Rehabil. 2012, 25, 131–142. [Google Scholar] [CrossRef]
- Descatha, A.; Dale, A.M.; Jaegers, L.; Herquelot, E.; Evanoff, B. Self-reported physical exposure association with medial and lateral epicondylitis incidence in a large longitudinal study: Table 1. Occup. Environ. Med. 2013, 70, 670–673. [Google Scholar] [CrossRef]
- Lee, S.H.; Gong, H.S.; Kim, S.; Kim, J.; Baek, G.H. Is There a Relation Between Lateral Epicondylitis and Total Cholesterol Levels? Arthroscopy 2019, 35, 1379–1384. [Google Scholar] [CrossRef]
- Factor, S.; Snopik, P.G.; Albagli, A.; Rath, E.; Amar, E.; Atlan, F.; Morag, G. The “Selfie Test”: A Novel Test for the Diagnosis of Lateral Epicondylitis. Medicina 2023, 59, 1159. [Google Scholar] [CrossRef]
- Karanasios, S.; Korakakis, V.; Moutzouri, M.; Drakonaki, E.; Koci, K.; Pantazopoulou, V.; Tsepis, E.; Gioftsos, G. Diagnostic accuracy of examination tests for lateral elbow tendinopathy (LET)—A systematic review. J. Hand Ther. 2021, 35, 541–551. [Google Scholar] [CrossRef]
- Lee, M.H.; Cha, J.G.; Jin, W.; Kim, B.S.; Park, J.S.; Lee, H.K.; Hong, H.S. Utility of sonographic measurement of the common tensor tendon in patients with lateral epicondylitis. Am. J. Roentgenol. 2011, 196, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.; Nazarian, L.N.; Miller, T.T.; O’Kane, P.L.; Feld, R.I.; Parker, L.; McShane, J.M. Lateral epicondylitis of the elbow: US findings. Radiology 2005, 237, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.J.; Mackie, K.; Fallon, M.; Butler, R.; Breidahl, W.; Zheng, M.H.; Wang, A. The reliability and validity of magnetic resonance imaging in the assessment of chronic lateral epicondylitis. J. Hand Surg. Am. 2011, 36, 475–479. [Google Scholar] [CrossRef]
- Kim, G.M.; Yoo, S.J.; Choi, S.; Park, Y.-G. Current trends for treating lateral epicondylitis. Clin. Shoulder Elb. 2019, 22, 227–234. [Google Scholar] [CrossRef]
- Staples, M.P.; Forbes, A.; Ptasznik, R.; Gordon, J.; Buchbinder, R. A randomized controlled trial of extracorporeal shock wave therapy for lateral epicondylitis (tennis elbow). J. Rheumatol. 2008, 35, 2038–2046. [Google Scholar]
- Wang, C.; Wang, F.; Yang, K.D.; Weng, L.; Hsu, C.; Huang, C.; Yang, L. Shock wave therapy induces neovascularization at the tendon–bone junction. A study in rabbits. J. Orthop. Res. 2003, 21, 984–989. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Yang, K. Biological effects of extracorporeal shockwave in bone healing: A study in rabbits. Arch. Orthop. Trauma Surg. 2008, 128, 879–884. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, C.J.; Yang, K.D.; Kuo, Y.R.; Huang, H.C.; Huang, Y.T.; Sun, Y.C.; Wang, F.S. Extracorporeal shock waves promote healing of collagenase-induced Achilles tendinitis and increase TGF-beta1 and IGF-I expression. J. Orthop. Res. 2004, 22, 854–861. [Google Scholar] [CrossRef]
- Zhang, D.; Kearney, C.J.; Cheriyan, T.; Schmid, T.M.; Spector, M. Extracorporeal shockwave-induced expression of lubricin in tendons and septa. Cell Tissue Res. 2011, 346, 255–262. [Google Scholar] [CrossRef]
- Yao, G.; Chen, J.; Duan, Y.; Chen, X. Efficacy of extracorporeal shock wave therapy for lateral epicondylitis: A systematic review and meta-analysis. Biomed Res. Int. 2020, 2020, 2064781. [Google Scholar] [CrossRef]
- Xiong, Y.; Xue, H.; Zhou, W.; Sun, Y.; Liu, Y.; Wu, Q.; Liu, J.; Hu, L.; Panayi, A.C.; Chen, L.; et al. Shock-wave therapy versus corticosteroid injection on lateral epicondylitis: A meta-analysis of randomized controlled trials. Physician Sportsmed. 2019, 47, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Ioppolo, F.; Tattoli, M.; Di Sante, L.; Attanasi, C.; Venditto, T.; Servidio, M.; Cacchio, A.; Santilli, V. Extracorporeal shock-wave therapy for supraspinatus calcifying tendinitis: A randomized clinical trial comparing two different energy levels. Phys. Ther. 2012, 92, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-C.; Ye, J.; Yao, M.; Cui, X.-J.; Xia, Y.; Shen, Q.-X.; Tong, Z.-Y.; Wu, X.-Q.; Ma, J.-M.; Mo, W. Is extracorporeal shock wave therapy clinical efficacy for relief of chronic, recalcitrant plantar fasciitis? A systematic review and meta-analysis of randomized placebo or active-treatment controlled trials. Arch. Phys. Med. Rehabil. 2014, 95, 1585–1593. [Google Scholar] [CrossRef]
- Chow, I.H.W.; Cheing, G.L. Comparison of different energy densities of extracorporeal shock wave therapy (ESWT) for the management of chronic heel pain. Clin. Rehabil. 2007, 21, 131–141. [Google Scholar] [CrossRef]
- Gezgİnaslan, Ö.; GÜmÜŞ Atalay, S.G. High-Energy Flux Density Extracorporeal Shock Wave Therapy Versus Traditional Physical Therapy Modalities in Myofascial Pain Syndrome: A Randomized-controlled, Single-Blind Trial. Turk. J. Rheumatol. 2019, 35, 78–89. [Google Scholar] [CrossRef]
- Park, K.D.; Lee, W.Y.; Park, M.-H.; Ahn, J.K.; Park, Y. High- versus low-energy extracorporeal shock-wave therapy for myofascial pain syndrome of upper trapezius: A prospective randomized single blinded pilot study. Medicine 2018, 97, e11432. [Google Scholar] [CrossRef]
- Tassinari, R.; Cavallini, C.; Olivi, E.; Facchin, F.; Taglioli, V.; Zannini, C.; Marcuzzi, M.; Ventura, C. Cell Responsiveness to Physical Energies: Paving the Way to Decipher a Morphogenetic Code. Int. J. Mol. Sci. 2022, 23, 3157. [Google Scholar] [CrossRef]
- Maxwell, L.J.; Zochling, J.; Boonen, A.; Singh, J.; Veras, M.M.; Ghogomu, E.T.; Jandu, M.B.; Tugwell, P.; Wells, G. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst. Rev. 2015, 4, CD005468. [Google Scholar] [CrossRef]
- Taheri, P.; Emadi, M.; Poorghasemian, J. Comparison the Effect of Extra Corporeal Shockwave Therapy with Low Dosage Versus High Dosage in Treatment of the Patients with Lateral Epicondylitis. Adv. Biomed. Res. 2017, 6, 61. [Google Scholar] [CrossRef]
- Reilly, J.M.; Bluman, E.; Tenforde, A.S. Effect of Shockwave Treatment for Management of Upper and Lower Extremity Musculoskeletal Conditions: A Narrative Review. PM R 2018, 10, 1385–1403. [Google Scholar] [CrossRef]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of Digital Visual Analog Scale Pain Scoring With a Traditional Paper-based Visual Analog Scale in Adults. JAAOS Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef] [PubMed]
- Rompe, J.D.; Overend, T.J.; MacDermid, J.C. Validation of the Patient-Rated Tennis Elbow Evaluation Questionnaire. J. Hand Ther. 2007, 20, 3–11. [Google Scholar] [CrossRef]
- Speed, C.A.; Nichols, D.; Richards, C.; Humphreys, H.; Wies, J.T.; Burnet, S.; Hazleman, B.L. Extracorporeal shock wave therapy for lateral epicondylitis—A double blind randomized controlled trial. J. Orthop. Res. 2002, 20, 895–898. [Google Scholar] [CrossRef]
- El-Bably, S.; Ganeb, S.S.; El-shambaky, A.; Hassan, W. Extracorporeal Shock Wave Therapy versus Local Corticosteroid Injection and Platelet-Rich Plasma in The Treatment of Supraspinatus Tendinopathy. Egypt. J. Hosp. Med. 2023, 92, 5900–5906. [Google Scholar] [CrossRef]
- Testa, G.; Vescio, A.; Perez, S.; Petrantoni, V.; Mazzarella, G.; Costarella, L.; Pavone, V. Functional Outcome at Short and Middle Term of the Extracorporeal Shockwave Therapy Treatment in Lateral Epicondylitis: A Case-Series Study. J. Clin. Med. 2020, 9, 633. [Google Scholar] [CrossRef]
- Cacchio, A.; Necozione, S.; MacDermid, J.C.; Rompe, J.D.; Maffulli, N.; di Orio, F.; Santilli, V.; Paoloni, M. Cross-cultural adaptation and measurement properties of the italian version of the Patient-Rated Tennis Elbow Evaluation (PRTEE) questionnaire. Phys. Ther. 2012, 92, 1036–1045. [Google Scholar] [CrossRef]
- Riaz, S.; Sattar, A.; Seemal, P.; Majeed, R.; Naveed, A.; Abid, N.; Bashir, S. Comparison of Extracorporeal Shockwave and High-Intensity Laser in Treating Chronic Plantar Fasciitis. Pak. J. Med. Health Sci. 2023, 17, 46–47. [Google Scholar] [CrossRef]
- Ratner, B. The correlation coefficient: Its values range between +1/−1, or do they? J. Target. Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Available online: https://shockwavetherapy.org/wp-content/uploads/2024/01/ISMST-Guidelines-for-ESWT-_-engl-20240103.pdf (accessed on 17 September 2024).
- Karanasios, S.; Tsamasiotis, G.K.; Michopoulos, K.; Sakellari, V.; Gioftsos, G. Clinical effectiveness of shockwave therapy in lateral elbow tendinopathy: Systematic review and meta-analysis. Clin. Rehabil. 2021, 35, 1383–1398. [Google Scholar] [CrossRef]
- Sen, S.B.; Kosehasanogullari, M.; Yilmaz, N.O.; Kocyigit, B.F. Comparative analysis of the therapeutic effects of extracorporeal shock wave therapy and high-intensity laser therapy in lateral epicondylitis: A randomised clinical trial. Rheumatol. Int. 2024, 44, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Ozturan, K.E.; Yucel, I.; Cakici, H.; Guven, M.; Sungur, I. Autologous blood and corticosteroid injection and extracoporeal shock wave therapy in the treatment of lateral epicondylitis. Orthopedics 2010, 33, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, L.; Yuce, D.; Erbilici, A.; Baltaci, G. Does Kinesiotaping improve pain and functionality in patients with newly diagnosed lateral epicondylitis? Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Huang, Y.-C.; Lau, Y.-C.; Wang, L.-Y. Efficacy of radial extracorporeal shock wave therapy on lateral epicondylosis, and changes in the common extensor tendon stiffness with pretherapy and posttherapy in real-time sonoelastography: A randomized controlled study. Am. J. Phys. Med. Rehabil. 2017, 96, 93–100. [Google Scholar] [CrossRef]
- Speed, C.A.; Kolk, A.; Yang, K.G.A.; Tamminga, R.; van der Hoeven, H.; Wanner, S.; Gstöttner, M.; Meirer, R.; Hausdorfer, J.; Fille, M.; et al. Extracorporeal shock-wave therapy in the management of chronic soft-tissue conditions. J. Bone Jt. Surg. Br. Vol. 2004, 86, 165–171. [Google Scholar] [CrossRef]
- Van der Worp, H.; Akker-Scheek, I.v.D.; van Schie, H.; Zwerver, J. ESWT for tendinopathy: Technology and clinical implications. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1451–1458. [Google Scholar] [CrossRef]
- Chang, K.-V.; Chen, S.-Y.; Chen, W.-S.; Tu, Y.-K.; Chien, K.-L. Comparative effectiveness of focused shock wave therapy of different intensity levels and radial shock wave therapy for treating plantar fasciitis: A systematic review and network meta-analysis. Arch. Phys. Med. Rehabil. 2012, 93, 1259–1268. [Google Scholar] [CrossRef]
- Fatima, A.; Ahmad, A.; Gilani, S.A.; Darain, H.; Kazmi, S.; Hanif, K. Effects of High-Energy Extracorporeal Shockwave Therapy on Pain, Functional Disability, Quality of Life, and Ultrasonographic Changes in Patients with Calcified Rotator Cuff Tendinopathy. BioMed Res. Int. 2022, 2022, 1230857. [Google Scholar] [CrossRef]
- Hsu, C.J.; Wang, D.Y.; Tseng, K.F.; Fong, Y.C.; Hsu, H.C.; Jim, Y.F. Extra- corporeal shock wave therapy for calcifying tendinitis of the shoulder. J. Shoulder Elbow Surg. 2008, 17, 55–59. [Google Scholar] [CrossRef]
- Mouzopoulos, G.; Stamatakos, M.; Mouzo-poulos, D.; Tzurbakis, M. Extracorporeal shock wave treatment for shoulder calcific tendonitis: A systematic review. Skelet. Radiol. 2007, 36, 803–811. [Google Scholar] [CrossRef]
- Peters, J.; Luboldt, W.; Schwarz, W.; Jacobi, V.; Herzog, C.; Vogl, T.J. Extracorporeal shock wave therapy in calcific tendinitis of the shoulder. Skelet. Radiol. 2004, 33, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Rompe, J.D.; Rumler, F.; Hopf, C.; Nafe, B.; Heine, J. Extra- corporal shock wave therapy for calcifying tendinitis of the shoulder. Clin. Orthop. Relat. Res. 1995, 321, 196–201. [Google Scholar]
- Gerdesmeyer, L.; Wagenpfeil, S.; Haake, M.; Maie, M.; Loe, M.; Wortle, K.; Lamp, R.; Seil, R.; Handle, G.; Gassel, S.; et al. Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: A randomized controlled trial. JAMA 2003, 290, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.D.; Meadeb, J.; Guggenbuhl, P.; Marin, F.; Benkalfate, T.; Thomazeau, H.; Chales, G. High-energy extracorporeal shock-wave therapy for calcifying tendinitis of the rotator cuff: A randomised trial. J. Bone Jt. Surg. Br. 2007, 89, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Huang, H.-Y.; Chen, H.-H.; Pai, C.-H.; Yang, K.D. Effect of shock wave therapy on acute fractures of the tibia: A study in a dog model. Clin. Orthop. Relat. Res. 2001, 387, 112–118. [Google Scholar] [CrossRef]
- Ciampa, A.R.; de Prati, A.C.; Amelio, E.; Cavalieri, E.; Persichini, T.; Colasanti, M.; Musci, G.; Marlinghaus, E.; Suzuki, H.; Mariotto, S. Nitric oxide mediates anti-inflammatory action of extracorporeal shock waves. FEBS Lett. 2005, 579, 6839–6845. [Google Scholar] [CrossRef]
- Keijsers, R.; Koenraadt, K.L.; Turkenburg, J.L.; Beumer, A.; The, B.; Eygendaal, D. Ultrasound Measurements of the ECRB Tendon Shows Remarkable Variations in Patients with Lateral Epicondylitis. Arch. Bone Jt. Surg. 2020, 8, 168–172. [Google Scholar] [CrossRef]
- McClure, S.; Dorfmuller, C. Extracorporeal shock wave therapy: Theory and equipment. Clin. Tech. Equine Pract. 2003, 2, 348–357. [Google Scholar] [CrossRef]
- Ruotolo, C.; Fow, J.; Nottage, W.M. The supraspinatus footprint: An anatomic study of the supraspinatus insertion. Arthroscopy 2004, 20, 246–249. [Google Scholar] [CrossRef]
- Madaras, E.I.; Perez, J.; Sobel, B.E.; Mottley, J.G.; Miller, J.G. Anisotropy of the ultrasonic backscatter of myocardial tissue: II. Measurements in vivo. J. Acoust. Soc. Am. 1988, 83, 762–769. [Google Scholar] [CrossRef]
- Holland, M.R.; Lewis, S.H.; Hall, C.S.; Finch-Johnston, A.E.; Handley, S.M.; Wallace, K.D.; D’Sa, A.P.; Prater, D.M.; Perez, J.E.; Miller, J.G. Effects of tissue anisotropy on the spectral characteristics of ultrasonic backscatter measured with a clinical imaging system. Ultrason. Imaging 1998, 20, 178–190. [Google Scholar] [CrossRef]
- Connolly, D.J.; Berman, L.; McNally, E.G. The use of beam angulation to overcome anisotropy when viewing human tendon with high frequency linear array ultrasound. Br. J. Radiol. 2001, 74, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Ogden, J.A.; Alvarez, R.G.; Levitt, R.L.; Johnson, J.E.; Marlow, M.E. Electrohydraulic high-energy shock-wave treatment for chronic plantar fasciitis. J. Bone Jt. Surg. Am. 2004, 86, 2216–2228. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Jung, Y.J.; Lee, J.Y.; Choi, J.S.; Mun, J.H.; Park, W.Y.; Seo, C.H.; Jang, K.U. The effect of extracorporeal shock wave therapy on myofascial pain syndrome. Ann. Rehabil. Med. 2012, 36, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Rompe, J.D.; Meurer, A.; Nafe, B.; Hofmann, A.; Gerdesmeyer, L. Repetitive low-energy shock wave application without local anesthesia is more efficient than repetitive low- energy shock wave application with local anesthesia in the treatment of chronic plantar fasciitis. J. Orthop. Res. 2005, 23, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Haupt, G.; Chvapil, M. Effect of shock waves on the healing of partial-thickness wounds in piglets. J. Surg. Res. 1990, 49, 45–48. [Google Scholar] [CrossRef]
- Holsapple, J.S.; Cooper, B.; Berry, S.H.; Staniszewska, A.; Dickson, B.M.; Taylor, J.A.; Bachoo, P.; Wilson, H.M. Low Intensity Shockwave Treatment Modulates Macrophage Functions Beneficial to Healing Chronic Wounds. Int. J. Mol. Sci. 2021, 22, 7844. [Google Scholar] [CrossRef]
- Orhan, Z.; Cam, K.; Alper, M.; Ozturan, K. The effects of extracorporeal shock waves on the rat Achilles tendon: Is there a critical dose for tissue injury? Arch. Orthop. Trauma Surg. 2003, 124, 631–635. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Lopes-Martins, R.; Joensen, J.; Couppe, C.; Ljunggren, A.E.; Stergioulas, A.; Johnson, M.I. A systematic review with procedural assessments and meta-analysis of low level laser therapy in lateral elbow tendinopathy (tennis elbow). BMC Musculoskelet. Disord. 2008, 9, 75. [Google Scholar] [CrossRef]
- Jang, H.; Lee, H. Meta-analysis of pain relief effects by laser irradiation on joint areas. Photomed. Laser Surg. 2012, 30, 405–417. [Google Scholar] [CrossRef]
- Tumilty, S.; Munn, J.; McDonough, S.; Hurley, D.A.; Basford, J.R.; Baxter, G.D. Low level laser treatment of tendinopathy: A systematic review with meta-analysis. Photomed. Laser Surg. 2010, 28, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Haslerud, S.; Magnussen, L.H.; Joensen, J.; Lopes-Martins, R.A.B.; Bjordal, J.M. The efficacy of low-level laser therapy for shoulder tendinopathy: A systematic review and meta-analysis of randomized controlled trials. Physiother. Res. Int. 2015, 20, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Bernetti, A.; Santilli, G.; Paoloni, M.; Santilli, V.; Mangone, M. Laser and thermal therapy in athletes’ tennis elbow: An observational study. Med. Sport 2022, 75, 238–247. [Google Scholar] [CrossRef]
- Bellosta-López, P.; Blasco-Abadía, J.; Andersen, L.L.; Vinstrup, J.; Skovlund, S.V.; Doménech-García, V. Multimodal sensorimotor assessment of hand and forearm asymmetries: A reliability and correlational study. PeerJ 2024, 12, e17403. [Google Scholar] [CrossRef]
- Doménech-García, V.; Pecos-Martín, D.; Blasco-Abadía, J.; Bellosta-López, P.; López-Royo, M.P. Placebo and nocebo effects of per-cutaneous needle electrolysis and dry-needling: An intra and inter-treatment sessions analysis of a three-arm randomized double-blinded controlled trial in patients with patellar tendinopathy. Front. Med. 2024, 11, 1381515. [Google Scholar] [CrossRef]
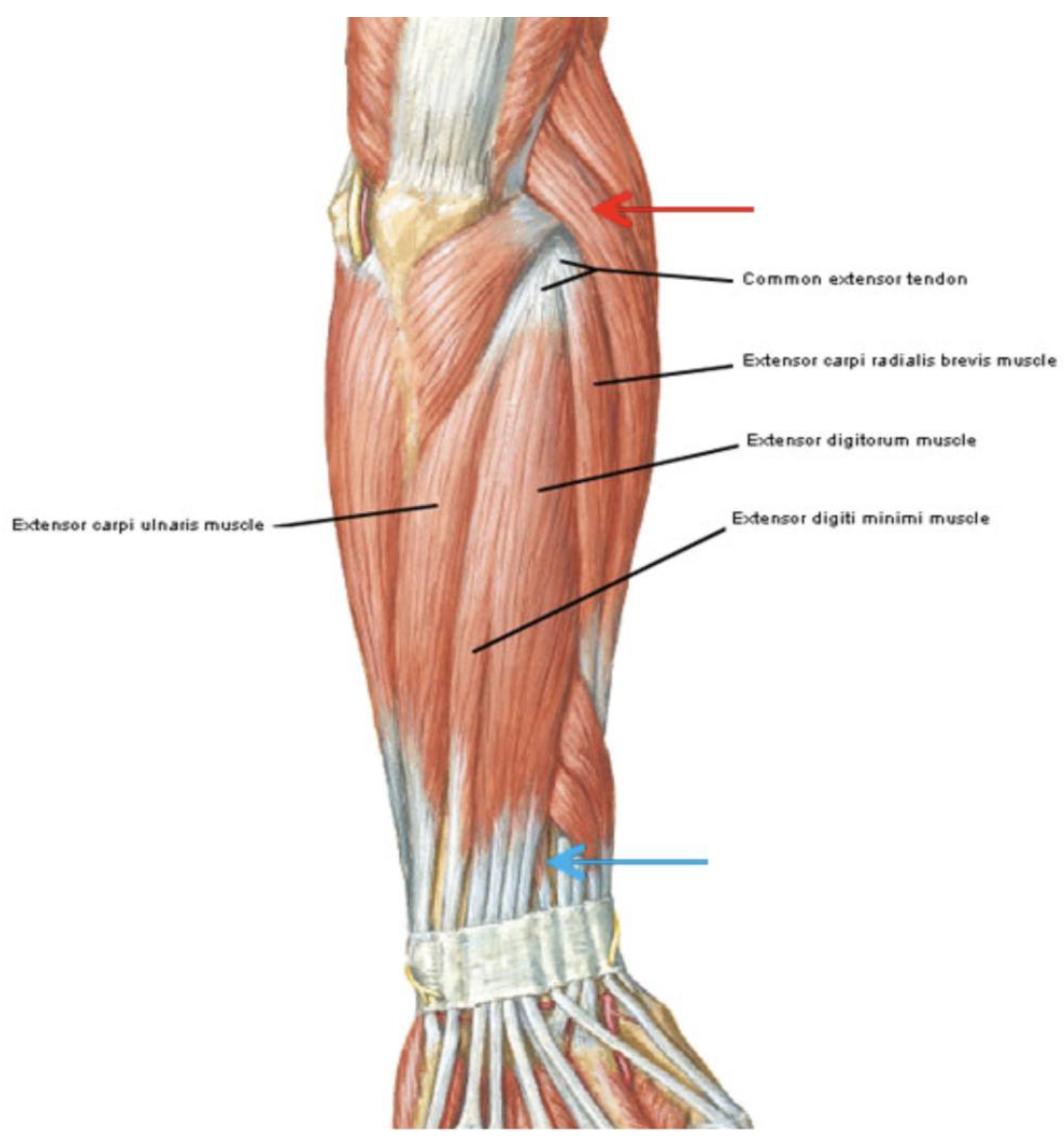
| Variables | High Energy Group (n = 22) | Low Energy Group (n = 24) | p Value |
|---|---|---|---|
| Age, year | 49.3 ± 9.8 | 50.2 ± 10.4 | 0.779 |
| Gender, M = 1 F = 2 | 1 = 13; 2 = 9 | 1 = 11; 2 = 13 | 0.369 |
| VAS T0, continuous | 6.8 ± 1.1 | 7.3 ± 1.1 | 0.112 |
| PRTEE T0 continuous | 49.6 ± 2.1 | 52.2 ± 3.2 | 0.515 |
| Variables | High Energy Group (n = 22) | Low Energy Group (n = 24) | p Value |
|---|---|---|---|
| VAS T1, continuous | 4.4± 0.1 | 3.6 ± 0.3 | 0.02 |
| PRTEE T1 continuous | 34 ± 6.5 | 27.1± 11.3 | 0.01 |
| VAS T2, continuous | 3.2± 0.2 | 2.1 ± 0.2 | 0.002 |
| PRTEE T2 continuous | 24.8 ± 7.4 | 17.5 ± 8.4 | 0.003 |
| Variable | Correlation with | Coefficient (r) | p-Value |
|---|---|---|---|
| VAS T0 | PRTEE T0 | 0.7 | <0.001 |
| VAS T1 | VAS T2 | 0.5 | <0.05 |
| VAS T1 | PRTEE T1 | 0.7 | <0.001 |
| VAS T1 | PRTEE T2 | 0.6 | <0.01 |
| VAS T2 | PRTEE T1 | 0.7 | <0.01 |
| VAS T2 | PRTEE T2 | 0.8 | <0.001 |
| VAS T1 | Low-power group | −0.332 | 0.024 |
| VAS T2 | Low-power group | −0.446 | <0.002 |
| PRTEE T1 | Low-power group | −0.339 | 0.032 |
| PRTEE T2 | Low-power group | −0.510 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santilli, G.; Ioppolo, F.; Mangone, M.; Agostini, F.; Bernetti, A.; Forleo, S.; Cazzolla, S.; Mannino, A.C.; Fricano, A.; Franchitto, A.; et al. High Versus Low-Energy Extracorporeal Shockwave Therapy for Chronic Lateral Epicondylitis: A Retrospective Study. J. Funct. Morphol. Kinesiol. 2024, 9, 173. https://doi.org/10.3390/jfmk9030173
Santilli G, Ioppolo F, Mangone M, Agostini F, Bernetti A, Forleo S, Cazzolla S, Mannino AC, Fricano A, Franchitto A, et al. High Versus Low-Energy Extracorporeal Shockwave Therapy for Chronic Lateral Epicondylitis: A Retrospective Study. Journal of Functional Morphology and Kinesiology. 2024; 9(3):173. https://doi.org/10.3390/jfmk9030173
Chicago/Turabian StyleSantilli, Gabriele, Francesco Ioppolo, Massimiliano Mangone, Francesco Agostini, Andrea Bernetti, Sara Forleo, Sara Cazzolla, Anna Camilla Mannino, Alessio Fricano, Antonio Franchitto, and et al. 2024. "High Versus Low-Energy Extracorporeal Shockwave Therapy for Chronic Lateral Epicondylitis: A Retrospective Study" Journal of Functional Morphology and Kinesiology 9, no. 3: 173. https://doi.org/10.3390/jfmk9030173
APA StyleSantilli, G., Ioppolo, F., Mangone, M., Agostini, F., Bernetti, A., Forleo, S., Cazzolla, S., Mannino, A. C., Fricano, A., Franchitto, A., Taurone, S., Ciccarelli, A., & Paoloni, M. (2024). High Versus Low-Energy Extracorporeal Shockwave Therapy for Chronic Lateral Epicondylitis: A Retrospective Study. Journal of Functional Morphology and Kinesiology, 9(3), 173. https://doi.org/10.3390/jfmk9030173









