Testing of a Low-Cost Dry Cell Prototype for Oxyhydrogen Production
Abstract
:1. Introduction
- in dry cells, the electrolyte is stored in an electrolytic container, which also acts as a bubbler, and the electrolyte enters the cell by gravity;
- dry cells typically require lower electrical current, compared to wet cells, for the production of the same amount of gas, since the gap between the electrodes can be smaller in dry cells than in wet cells;
- unlike wet cells, dry cells do not require rare metals such as platinum, which reduces their production costs;
- the maintenance costs of dry cells are also lower [9].
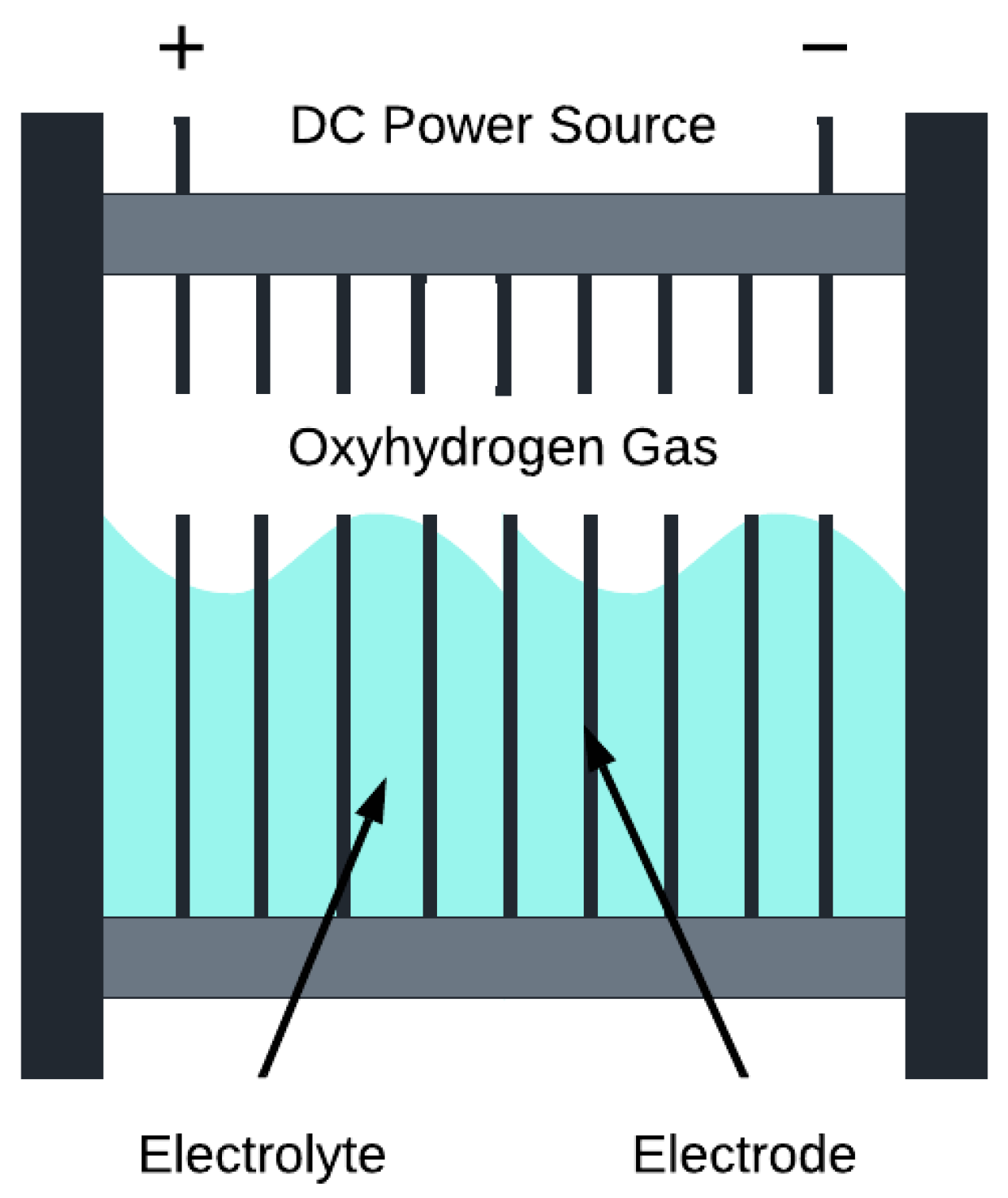
2. Experimental Prototype: Components, Assembly and Operation Principles
2.1. Components and Assembly
2.2. Operation Principles
3. Results
3.1. Experimental Preparation and Procedure
3.2. Molecular Composition of the Gas
3.3. Efficiency of the Dry Cell
4. Discussion
- For an easier filling of the dry cell, reservoir and bubbler, a hole could be inserted at the top of the bubbler and reservoir, so that the air inside can escape during filling. This is because during the filling of these reservoirs, a funnel placed in the upper holes in the bases was used, and sometimes distilled water overflowed. This additional hole must be closed during the operation of the equipment, with, for example, a rubber stopper. Instead of a hole, a T-bond could be used, where one side would serve to fix the tube and the other to let the air escape during filling, and then closed with a lid;
- To remove the electrolyte from the inside of the dry cell without the need to disassemble it, which may, over time, lead to damage to the seals and, mainly, the threaded rods; therefore, a hole could be inserted in the lower corner of the dry cell bases and all stainless steel plates, the same being closed, during filling and operation, with a rubber stopper that would pass through the different cells so that they remain separate;
- To ensure that there is no potential difference between the stainless-steel plates at the ends, with the poles for the power source, and the adjacent dry cell bases, it would be best to use a rectangular, blunt EPDM seal on the inside, as suggested by the manufacturer’s manual. This would also avoid unnecessary electrolyte consumption;
- To control the temperature inside the dry cell, which should not exceed 50 °C, as it may damage the EPDM seals, a thermocouple with its temperature indicator could be put inside. Alternatively, a closed circuit with the water from the reservoir and the bubbler circulating around the dry cell for cooling it could be built. Or, use heat-dissipating materials (with good thermal conductivity) that are thermally resistant;
- During the tests, some hydrogen leaks were found in the connections and along the tubes, in the order of the parts per million (ppm). Although relatively small, they reduce the efficiency of the equipment, in addition to decrease its safety. As a solution, pipes less permeable to this gas should be used.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- Ghosh, T.K.; Mark, A. Prelas, Energy Resources and Systems, Volume 2: Renewable Resources; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Chang, R.; Overby, J. Chemistry; McGraw-Hill Education: New York, NY, USA, 2019; ISBN 1-260-08531-7. [Google Scholar]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Santos, D.M.; Sequeira, C.A.; Figueiredo, J.L. Hydrogen Production by Alkaline Water Electrolysis. Química Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- Ezzahra, C.F.; Kaddami, M.; Mikou, M. Effect of Operating Parameters on Hydrogen Production by Electrolysis of Water. Int. J. Hydrogen Energy 2017, 42, 25550–25557. [Google Scholar]
- Godula-Jopek, A. Hydrogen Production: By Electrolysis; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 3-527-67652-X. [Google Scholar]
- Leludak, F.T.; Haus, T.L. Célula de Hidrogênio: Estudo e Construção de Uma Célula Eletrolítica Para Produção de Hidrogênio Gasoso. Cad. PAIC 2013, 14, 383–397, (In Brazilian Portuguese). [Google Scholar]
- Choodum, N. A Study of the Optimized Conditions for a Closed-Loop HHO Production System Using A/C Power Supply. Ph.D. Thesis, Prince of Songkla University, Hat Yai, Thailand, 2017. [Google Scholar]
- Manu, P.V.; Sunil, A.; Jayaraj, S. Experimental Investigation Using an On-Board Dry Cell Electrolyzer in a CI Engine Working on Dual Fuel Mode. Energy Procedia 2016, 90, 209–216. [Google Scholar] [CrossRef]
- Hydrogen Fuel Cell Engines and Related Technologies, Module 3: Hydrogen Use In Internal Combustion Engines, College of the Desert. 2001. Available online: http://www.tuks.nl/pdf/Reference_Material/ICE_and_Water_Injection/College%20of%20the%20Destert%20-%20Hydrogen%20Fuel%20Cell%20Engines%20and%20Related%20Technologies-%202001.pdf (accessed on 16 July 2022).
- Hydrogen Fuel Cell Engines and Related Technologies, Module 1: Hydrogen Properties, College of the Desert. 2001. Available online: http://www.tuks.nl/pdf/Reference_Material/ICE_and_Water_Injection/College%20of%20the%20Destert%20-%20Hydrogen%20Fuel%20Cell%20Engines%20and%20Related%20Technologies-%202001.pdf (accessed on 16 July 2022).
- Fuels and Chemicals Autoignition Temperatures, The Engineering Toolbox. Available online: https://www.engineeringtoolbox.com/fuels-ignition-temperatures-d_171.html (accessed on 16 July 2022).
- Minimum Ignition Energy (MIE). Available online: https://www.euratex.co.uk/110411020.pdf (accessed on 8 July 2021).
- Adiabatic Flame Temperatures, The Engineering Toolbox. Available online: https://www.engineeringtoolbox.com/adiabatic-flame-temperature-d_996.html (accessed on 16 July 2022).
- Turns, S.R. An Introduction to Combustion: Concepts and Applications; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- HHO Workshop. Wasser Statt Sprit. Available online: https://www.wasserstattsprit.info/ (accessed on 16 July 2022). (In German).
- Standard Thermodynamic Values at 25 °C, Chemistry Handbook. Available online: http://chemistry-reference.com/Standard%20Thermodynamic%20Values.pdf (accessed on 16 July 2022).
- Sterner, M.; Stadler, I. (Eds.) Handbook of Energy Storage; Springer: Berlin, Germany, 2019; Available online: https://link.springer.com/book/10.1007/978-3-662-55504-0 (accessed on 16 July 2022).
- Koponen, J. Review of Water Electrolysis Technologies and Design of Renewable Hydrogen Production Systems. Master’s Thesis, Lappeenranta University of Technology, Lappeenranta, Finland, 2015. [Google Scholar]
- Gilliam, R.J.; Graydon, J.W.; Kirk, D.W.; Thorpe, S.J. A Review of Specific Conductivities of Potassium Hydroxide Solutions for Various Concentrations and Temperatures. Int. J. Hydrogen Energy 2007, 32, 359–364. [Google Scholar] [CrossRef]
- “Electrical Resistivity and Conductivity,” Wikipedia. Available online: https://en.wikipedia.org/wiki/Electrical_resistivity_and_conductivity (accessed on 16 July 2022).
- Micro GC Fusion Gas Analyser, INFICON. Available online: https://www.inficon.com/v1/attachment/4b9f2fc3-5b8e-4245-ae33-749f6fcab95c (accessed on 16 July 2022).
- Matthews, J.C.; Wright, M.D.; Martin, D.; Bacak, A.; Priestley, M.; Bannan, T.J.; Silva, H.G.; Flynn, M.; Percival, C.J.; Shallcross, D.E. Urban tracer dispersion and infiltration into buildings over a 2-km scale. Bound. Layer Meteorol. 2020, 175, 113–134. [Google Scholar] [CrossRef] [Green Version]







| Oxyhydrogen | Hydrogen-H2 | Gasoline | Diesel | Methane-CH4 | Propane-C3H8 | |
|---|---|---|---|---|---|---|
| Autoignition temperature at 1 atm | 570 °C [8] | 585 °C [11] | 230–480 °C [11] | 210 °C [12] | 540 °C [11] | 490 °C [11] |
| Minimum ignition energy | 20 μJ (STP *) [8] | 20 μJ (25 °C and 1 atm) [11] | 800 μJ [13] | - | 280–300 μJ [13] | 250–260 μJ [13] |
| HHV ** at 25 °C and 1 atm | - | 141.86 kJ/g [11] | 44.5 kJ/g [11] | 42.5 kJ/g [11] | 55.53 kJ/g [11] | 50.36 kJ/g [11] |
| LHV *** at 25 °C and 1 atm | 120.90 kJ/g (H2) [8] | 119.93 kJ/g [11] | 47.5 kJ/g [11] | 44.8 kJ/g [11] | 50.02 kJ/g [11] | 45.6 kJ/g [11] |
| Flame temperature | 2800 °C [8] | 2210 °C [14] **** | 2002 °C (C8H18) [15] **** | 2004 °C (C12H26) [15] **** | 1953 °C [15] **** | 1994 °C [15] **** |
| H2 [%] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tests | V [V] | I [A] | P [W] | T [°C] | Injections | Mean | σ | |||||
| 1 | 11.0 | 1.0 | 11.0 | 28.5 | 71.3109 | 71.7860 | 73.5770 | 72.5380 | 74.0864 | 75.0888 | 73.0645 | 1.4416 |
| 2 | 11.5 | 2.0 | 23.0 | 29.0 | 68.6623 | 68.5332 | 69.1098 | 69.954 | 70.5681 | - | 69.3655 | 0.8725 |
| 3 | 12.0 | 4.0 | 48.0 | 30.1 | 67.8310 | 68.8524 | 68.3066 | 68.2974 | 70.3263 | - | 68.7227 | 0.9666 |
| 4 | 12.0 | 6.0 | 72.0 | 31.2 | 67.1471 | 67.0147 | 66.9715 | 67.2175 | 67.2253 | - | 67.1152 | 0.1166 |
| 5 | 12.0 | 8.0 | 96.0 | 32.2 | 67.3574 | 67.4225 | 67.1704 | 67.8528 | 67.9407 | - | 67.5488 | 0.3323 |
| 6 | 10.5 | 1.0 | 10.5 | 38.2 | 66.6453 | 67.9731 | 66.7826 | 66.4719 | 66.5821 | - | 66.8910 | 0.6152 |
| O2 [%] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tests | V [V] | I [A] | P [W] | T [°C] | Injections | Mean | σ | |||||
| 1 | 11.0 | 1.0 | 11.0 | 28.5 | 31.2340 | 31.5441 | 32.9292 | 32.1266 | 33.229 | 34.0856 | 32.5248 | 1.0846 |
| 2 | 11.5 | 2.0 | 23.0 | 29.0 | 29.2484 | 29.2053 | 29.5592 | 30.1957 | 30.6151 | - | 29.7647 | 0.6186 |
| 3 | 12.0 | 4.0 | 48.0 | 30.1 | 28.6650 | 29.3586 | 29.0067 | 28.9888 | 30.4064 | - | 29.2851 | 0.6732 |
| 4 | 12.0 | 6.0 | 72.0 | 31.2 | 28.1822 | 28.0950 | 28.0376 | 28.2243 | 28.2087 | - | 28.1496 | 0.0801 |
| 5 | 12.0 | 8.0 | 96.0 | 32.2 | 28.2920 | 28.3377 | 28.1369 | 28.6258 | 28.6713 | - | 28.4127 | 0.2283 |
| 6 | 10.5 | 1.0 | 10.5 | 38.2 | 27.9692 | 28.8481 | 28.0476 | 27.6933 | 27.6198 | - | 28.0356 | 0.4886 |
| Tests | V [V] | I [A] | P [W] | T [°C] | H2 + O2 [%] | σ | H2O [%] |
|---|---|---|---|---|---|---|---|
| 1 | 11.0 | 1.0 | 11.0 | 28.5 | 105.5893 * | 2.5263 | - |
| 2 | 11.5 | 2.0 | 23.0 | 29.0 | 99.1302 | 1.4911 | 0.8698 |
| 3 | 12.0 | 4.0 | 48.0 | 30.1 | 98.0078 | 1.6398 | 1.9922 |
| 4 | 12.0 | 6.0 | 72.0 | 31.2 | 95.2648 | 0.1967 | 4.7352 |
| 5 | 12.0 | 8.0 | 96.0 | 32.2 | 95.9615 | 0.5607 | 4.0385 |
| 6 | 10.5 | 1.0 | 10.5 | 38.2 | 94.9266 | 1.1038 | 5.0734 |
| H2 [%] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tests | V [V] | I [A] | P [W] | T [°C] | Injections | Mean | σ | ||||
| 1 | 10.5 | 2.0 | 21.0 | 25.5 | 69.7686 | 71.1656 | 69.7499 | 72.6915 | 73.5868 | 71.3925 | 1.7240 |
| 2 | 11.5 | 4.0 | 46.0 | 27.5 | 67.3373 | 67.2757 | 67.8946 | 68.5800 | 68.9232 | 68.0022 | 0.7355 |
| 3 | 12.0 | 6.0 | 72.0 | 30.7 | 67.9280 | 67.8105 | 68.4318 | 67.7515 | 68.2048 | 68.0253 | 0.2864 |
| 4 | 12.0 | 8.0 | 96.0 | 33.2 | 67.3552 | 67.5519 | 67.4448 | 67.8942 | 67.3227 | 67.5138 | 0.2305 |
| 5 | 11.0 | 2.0 | 22.0 | 36.7 | 67.0117 | 66.7328 | 67.3999 | 67.0985 | 67.2338 | 67.0953 | 0.2502 |
| O2 [%] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tests | V [V] | I [A] | P [W] | T [°C] | Injections | Mean | σ | ||||
| 1 | 10.5 | 2.0 | 21.0 | 25.5 | 30.1326 | 30.8042 | 29.9082 | 32.4402 | 32.9199 | 31.2410 | 1.3650 |
| 2 | 11.5 | 4.0 | 46.0 | 27.5 | 28.1489 | 28.3364 | 28.7623 | 29.0552 | 29.3325 | 28.7271 | 0.4905 |
| 3 | 12.0 | 6.0 | 72.0 | 30.7 | 28.6800 | 28.6056 | 29.0372 | 28.5520 | 28.8635 | 28.7477 | 0.2002 |
| 4 | 12.0 | 8.0 | 96.0 | 33.2 | 28.3091 | 28.4352 | 28.3762 | 28.6529 | 28.2425 | 28.4032 | 0.1571 |
| 5 | 11.0 | 2.0 | 22.0 | 36.7 | 28.0844 | 28.0439 | 28.2716 | 28.1194 | 28.1377 | 28.1314 | 0.0862 |
| Tests | V [V] | I [A] | P [W] | T [°C] | H2 + O2 [%] | σ | H2O [%] |
|---|---|---|---|---|---|---|---|
| 1 | 10.5 | 2.0 | 21.0 | 25.5 | 102.6335 * | 3.0890 | - |
| 2 | 11.5 | 4.0 | 46.0 | 27.5 | 96.7292 | 1.2260 | 3.2708 |
| 3 | 12.0 | 6.0 | 72.0 | 30.7 | 96.7730 | 0.4866 | 3.2270 |
| 4 | 12.0 | 8.0 | 96.0 | 33.2 | 95.9169 | 0.3877 | 4.0831 |
| 5 | 11.0 | 2.0 | 22.0 | 36.7 | 95.2267 | 0.3364 | 4.7733 |
| Test | V [V] | V/Cell [V] | I [A] | Current Density [A/cm2] | P [W] | δP [W] | T [°C] | ηV [%] | δηV [%] |
|---|---|---|---|---|---|---|---|---|---|
| Start-up | 11.5 | 3.0 | 34.5 | - | 25.3 | ||||
| 1 | 11.0 | 1.8333 | 1.0 | 0.0052 | 11.0 | 3.3 | 28.4 | 66.9194 | 0.5070 |
| 2 | 11.5 | 1.9167 | 2.0 | 0.0104 | 23.0 | 3.9 | 28.4 | 64.0098 | 0.4638 |
| 3 | 11.5 | 1.9167 | 3.0 | 0.0156 | 34.5 | 4.4 | 28.6 | 64.0098 | 0.4638 |
| 4 | 12.0 | 2.0000 | 4.0 | 0.0208 | 48.0 | 5.0 | 28.5 | 61.3428 | 0.4260 |
| 5 | 12.5 | 2.0833 | 5.0 | 0.0261 | 62.5 | 5.6 | 28.6 | 58.8890 | 0.3926 |
| 6 | 12.5 | 2.0833 | 6.0 | 0.0313 | 75.0 | 6.1 | 28.6 | 58.8890 | 0.3926 |
| 7 | 12.5 | 2.0833 | 7.0 | 0.0365 | 87.5 | 6.6 | 28.9 | 58.8890 | 0.3926 |
| 8 | 13.0 | 2.1667 | 8.0 | 0.0417 | 104.0 | 7.3 | 28.9 | 56.6241 | 0.3630 |
| 9 | 13.0 | 2.1667 | 9.0 | 0.0469 | 117.0 | 7.8 | 28.9 | 56.6241 | 0.3630 |
| 10 | 13.0 | 2.1667 | 10.0 | 0.0521 | 130.0 | 8.3 | 28.7 | 56.6241 | 0.3630 |
| 11 | 13.5 | 2.2500 | 11.0 | 0.0573 | 148.5 | 8.9 | 28.9 | 54.5269 | 0.3366 |
| 12 | 13.5 | 2.2500 | 12.0 | 0.0625 | 162.0 | 9.4 | 29.0 | 54.5269 | 0.3366 |
| Average temperature [°C] | 28.7 | ||||||||
| Test | V [V] | V/Cell [V] | I [A] | Current Density [A/cm2] | P [W] | δP [W] | T [°C] | ηV [%] | δηV [%] |
|---|---|---|---|---|---|---|---|---|---|
| Start-up | 10.5 | 2.0 | 21.0 | - | 25.5 | ||||
| 1 | 10.5 | 1.7500 | 2.0 | 0.0104 | 21.0 | 3.6 | 35.4 | 69.7806 | 0.5564 |
| 2 | 11.0 | 1.8333 | 3.0 | 0.0156 | 33.0 | 4.3 | 35.5 | 66.6087 | 0.5070 |
| 3 | 11.5 | 1.9167 | 4.0 | 0.0208 | 46.0 | 4.9 | 35.5 | 63.7127 | 0.4638 |
| 4 | 11.5 | 1.9167 | 5.0 | 0.0261 | 57.5 | 5.4 | 35.4 | 63.7127 | 0.4638 |
| 5 | 12.0 | 2.0000 | 6.0 | 0.0313 | 72.0 | 6.0 | 35.5 | 61.0580 | 0.4260 |
| 6 | 12.0 | 2.0000 | 7.0 | 0.0365 | 84.0 | 6.5 | 35.4 | 61.0580 | 0.4260 |
| 7 | 12.5 | 2.0833 | 8.0 | 0.0417 | 100.0 | 7.1 | 35.4 | 58.6157 | 0.3926 |
| 8 | 12.5 | 2.0833 | 9.0 | 0.0469 | 112.5 | 7.6 | 35.3 | 58.6157 | 0.3926 |
| 9 | 12.5 | 2.0833 | 10.0 | 0.0521 | 125.0 | 8.1 | 35.2 | 58.6157 | 0.3926 |
| 10 | 12.5 | 2.0833 | 11.0 | 0.0573 | 137.5 | 8.6 | 35.1 | 58.6157 | 0.3926 |
| 11 | 13.0 | 2.1667 | 12.0 | 0.0625 | 156.0 | 9.3 | 35.2 | 56.3612 | 0.3630 |
| Average temperature [°C] | 35.4 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunge, L.; Silva, H.G.; Cruz, P.L.; Iten, M. Testing of a Low-Cost Dry Cell Prototype for Oxyhydrogen Production. Designs 2022, 6, 79. https://doi.org/10.3390/designs6050079
Bunge L, Silva HG, Cruz PL, Iten M. Testing of a Low-Cost Dry Cell Prototype for Oxyhydrogen Production. Designs. 2022; 6(5):79. https://doi.org/10.3390/designs6050079
Chicago/Turabian StyleBunge, Lisa, Hugo G. Silva, Pedro L. Cruz, and Muriel Iten. 2022. "Testing of a Low-Cost Dry Cell Prototype for Oxyhydrogen Production" Designs 6, no. 5: 79. https://doi.org/10.3390/designs6050079
APA StyleBunge, L., Silva, H. G., Cruz, P. L., & Iten, M. (2022). Testing of a Low-Cost Dry Cell Prototype for Oxyhydrogen Production. Designs, 6(5), 79. https://doi.org/10.3390/designs6050079









