Studies on Application of Ion Beam Breeding to Industrial Microorganisms at TIARA
Abstract
:1. Introduction
2. Mutagenic Effects of Radiations with Different LET in Microorganisms
3. Ion-Beam Breeding of Microorganisms
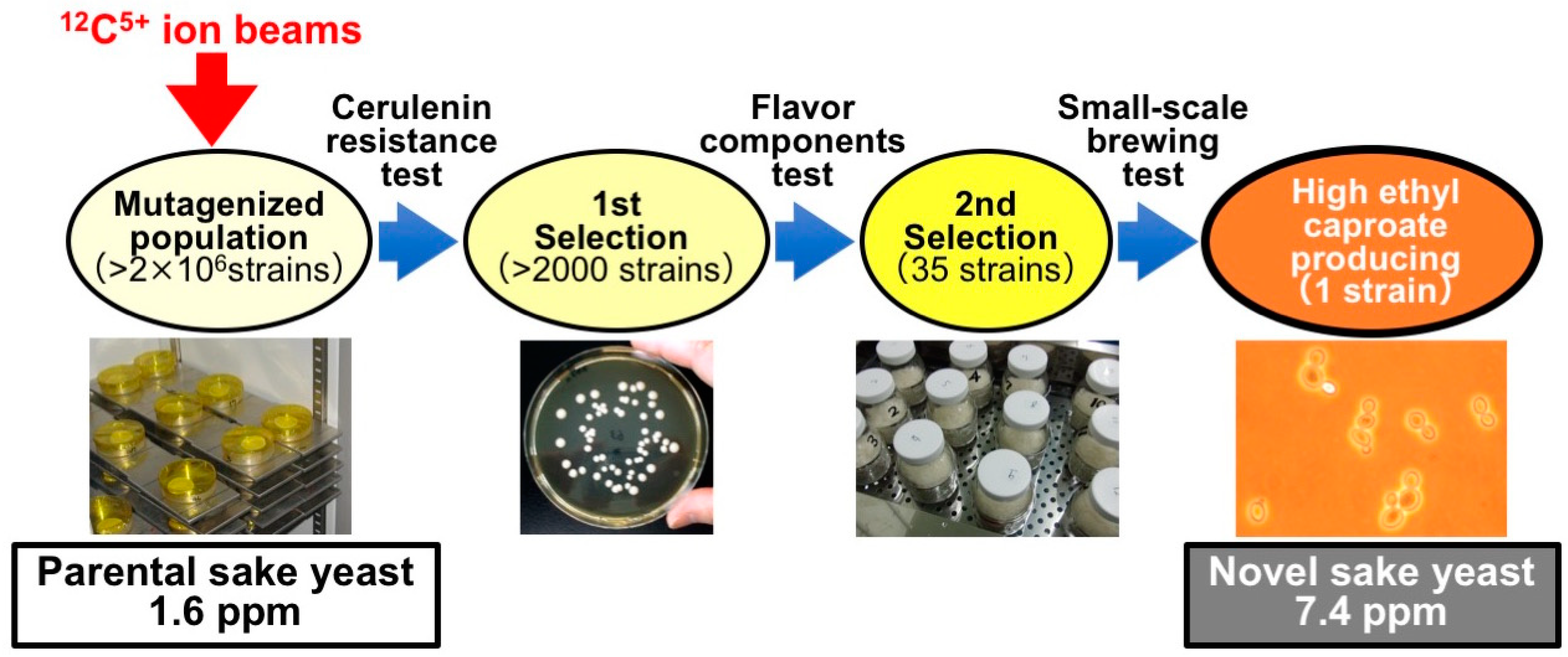
3.1. Sake Brewing
3.2. Industrial Enzyme Productions
3.3. Biopesticides
3.4. Biofertilizers
3.5. Bioremediation
3.6. Biofuels
4. Ion-Beam Breeding of Microorganisms in Other Facilities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Joint FAO/IAEA Mutant Variety Database. Available online: https://mvd.iaea.org/ (accessed on 6 March 2019).
- Kurashima, S.; Satoh, T.; Saitoh, Y.; Yokota, W. Irradiation facilities of the Takasaki advanced radiation research institute. Quantum Beam Sci. 2017, 1, 2. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakamoto, A.; Ishigaki, Y.; Nikaido, O.; Sun, G.; Hase, Y.; Shikazono, N.; Tano, S.; Watanabe, H. An ultraviolet-B-resistant mutant with enhanced DNA repair in Arabidopsis. Plant Physiol. 2002, 129, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Lan, V.T.; Hase, Y.; Shikazono, N.; Matsunaga, T.; Tanaka, A. Disruption of the AtREV3 gene causes hypersensitivity to ultraviolet B light and γ-rays in Arabidopsis: Implication of the presence of a translesion synthesis mechanism in plants. Plant Cell 2003, 15, 2042–2057. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Shikazono, N.; Tanaka, A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004, 37, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Nakasone, A.; Chhun, T.; Ooura, C.; Biswas, K.K.; Uchimiya, H.; Tsurumi, S.; Baskin, T.I.; Tanaka, A.; Oono, Y. A small acidic protein 1 (SMAP1) mediates responses of the Arabidopsis root to the synthetic auxin 2,4-dichlorophenoxyacetic acid. Plant J. 2006, 47, 788–801. [Google Scholar] [CrossRef] [Green Version]
- Nikjoo, H.; Uehara, S.; Wilson, W.E.; Hoshi, M.; Goodhead, D.T. Track structure in radiation biology: Theory and applications. Int. J. Radiat. Biol. 1998, 73, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Yatagai, F. Mutations induced by heavy charged particles. Biol. Sci. Space 2004, 18, 224–234. [Google Scholar] [CrossRef]
- Shikazono, N.; Suzuki, C.; Kitamura, S.; Watanabe, H.; Tano, S.; Tanaka, A. Analysis of mutations induced by carbon ions in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 587–596. [Google Scholar] [CrossRef]
- Shikazono, N.; Yokota, Y.; Kitamura, S.; Suzuki, C.; Watanabe, H.; Tano, S.; Tanaka, A. Mutation rate and novel tt mutants of Arabidopsis thaliana induced by carbon ions. Genetics 2003, 163, 1449–1455. [Google Scholar]
- Okamura, M.; Yasuno, N.; Ohtsuka, M.; Tanaka, A.; Shikazono, N.; Hase, Y. Wide variety of flower-color and -shape mutants regenerated from leaf cultures irradiated with ion beams. Nucl. Instrum. Methods Phys. Res. B 2003, 206, 574–578. [Google Scholar] [CrossRef]
- Nagatomi, S. Development of flower mutation breeding through ion beam irradiation. Res. J. Food Agric. 2003, 26, 33–38. (In Japanese) [Google Scholar]
- Okamura, M.; Tanaka, A.; Momose, M.; Umemoto, N.; da Silva, J.A.T.; Toguri, T. Advances of mutagenesis in flowers and their industrialization. In Floriculture, Ornamental and Plant Biotechnology; da Silva, J.A.T., Ed.; Global Science Books: Isleworth, UK, 2006; Volume I, pp. 619–628. [Google Scholar]
- Iizuka, M.; Kimura, Y.; Hase, Y.; Tanaka, A. Mutation induction from osteospermum leaf cultures with ion beam Irradiation. JAEA Takasaki Ann. Rep. 2006, 2005, 81. [Google Scholar]
- Taneishi, M.; Katai, H.; Yamada, H.; Otsuka, H.; Hase, Y.; Shikazono, N.; Tanaka, A. Effect of ion beam irradiation on the growth of netted melon (Cucumis melo L.). TIARA Ann. Rep. 2002, 2001, 60–61. [Google Scholar]
- Nakai, H.; Watanabe, H.; Kitayama, S.; Tanaka, A.; Kobayashi, Y.; Takahashi, T.; Asai, T.; Imada, T. Studies on induced mutations by ion beam in plant. JAERI TIARA Ann. Rep. 1995, 1994, 34–36. [Google Scholar]
- Kitamura, H.; Mori, M.; Sato, D.; Nakagawa, J.; Yoshida, T.; Yoshizawa, K.; Kawai, T.; Hase, Y.; Tanaka, A. Carbon ion beam breeding of rice suitable for low nitrogen input. TIARA Ann. Rep. 2006, 2004, 100–101. [Google Scholar]
- Ishikawa, S.; Ishimaru, Y.; Igura, M.; Kuramata, M.; Abe, T.; Senoura, T.; Hase, Y.; Arao, T.; Nishizawa, N.K.; Nakanishi, H. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc. Natl. Acad. Sci. USA 2012, 109, 19166–19171. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, A.; Shikazono, N.; Hase, Y. Studies on biological effects of ion beams on lethality, molecular nature of mutation, mutation rate, and spectrum of mutation phenotype for mutation breeding in higher plants. J. Radiat. Res. 2010, 51, 223–233. [Google Scholar] [CrossRef]
- Hamada, N.; Funayama, T.; Wada, S.; Sakashita, T.; Kakizaki, T.; Ni, M.; Kobayashi, Y. LET-dependent survival of irradiated normal human fibroblasts and their descendents. Radiat. Res. 2006, 166, 24–30. [Google Scholar] [CrossRef]
- Hase, Y.; Yamaguchi, M.; Inoue, M.; Tanaka, A. Reduction of survival and induction of chromosome aberrations in tobacco irradiated by carbon ions with different linear energy transfers. Int. J. Radiat. Biol. 2002, 78, 799–806. [Google Scholar] [CrossRef]
- Tanaka, S.; Fukuda, K.; Nishimura, K.; Watanabe, H.; Yamano, N. IRAC M: A Code System to Calculate Induced Radioactivity Produced by Ions and Neutrons; JAERI-Data/Code 97-019; Japan Atomic Energy Research Institute: Tokyo, Japan, 1997.
- Machida, M.; Yamada, O.; Gomi, K. Genomics of Aspergillus oryzae: Learning from the history of koji mold and exploration of its future. DNA Res. 2008, 15, 173–183. [Google Scholar] [CrossRef]
- Toyoshima, Y.; Takahashi, A.; Tanaka, H.; Watanabe, J.; Mogi, Y.; Yamazaki, T.; Hamada, R.; Iwashita, K.; Satoh, K.; Narumi, I. Lethal and mutagenic effects of ion beams and γ-rays in Aspergillus oryzae. Mutat. Res. 2012, 740, 43–49. [Google Scholar] [CrossRef]
- Imamura, M.; Murata, T.; Akagi, K.; Tanaka, Y.; Imamura, M.; Inoue, K.; Mizuma, N.; Kobayashi, Y.; Watanabe, H.; Hachiya, M.; et al. Relationship between LET and RBE values for Escherichia coli determined using carbon ion beams from the TIARA cyclotron and HIMAC synchrotron. J. Gen. Appl. Microbiol. 1997, 43, 175–177. [Google Scholar] [CrossRef]
- Hase, Y.; Satoh, K.; Chiba, A.; Hirano, Y.; Tomita, S.; Saito, Y.; Narumi, K. Experimental Study on the Biological Effect of Cluster Ion Beams in Bacillus subtilis Spores. Quantum Beam Sci. 2019, 3, 8. [Google Scholar] [CrossRef]
- Satoh, K.; Tejima, K.; Narumi, I. Lethal effects of different LET radiations in Deinococcus radiodurans. JAEA Takasaki Ann. Rep. 2010, 2009, 80. [Google Scholar]
- Ito, K.; Takeichi, J.; Hanya, Y.; Satoh, K.; Hase, Y.; Sakashita, T.; Kobayashi, Y.; Narumi, I. Mutation breeding of koji mold induced by ion beams. JAEA Takasaki Ann. Rep. 2006, 2005, 98. [Google Scholar]
- Matuo, Y.; Nishijima, S.; Hase, Y.; Sakamoto, A.; Tanaka, A.; Shimizu, K. Specificity of mutations induced by carbon ions in budding yeast Saccharomyces cerevisiae. Mutat. Res. 2006, 602, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Ozawa, S.; Hayashi, H.; Oono, Y. Low cesium-accumulating mutants of Rhodococcus erythropolis CS98 generated by ion beam breeding. QST Takasaki Ann. Rep. 2017, 2016, 105. [Google Scholar]
- Sakai, K.; Kobayashi, H.; Ohshima, A.; Kato, S.; Satoh, K.; Hase, Y.; Narumi, I.; Sakashita, T.; Kobayashi, Y. Deletion of minor enzyme activities of Rhizomucor miehei by irradiation of heavy-ion beam. JAEA Takasaki Ann. Rep. 2006, 2005, 97. [Google Scholar]
- Sakai, K.; Kobayashi, H.; Ohshima, A.; Kato, S.; Satoh, K.; Narumi, I. Deletion of Minor Enzyme Activities of Rhizomucor miehei by Heavy Ion Beam Irradiation. JAEA Takasaki Ann. Rep. 2007, 2006, 95. [Google Scholar]
- Satoh, K.; Tejima, K.; Onodera, T.; Narumi, I. Analysis of mutant frequencies for different LET radiations in Deinococcus radiodurans. JAEA Takasaki Ann. Rep. 2012, 2011, 103. [Google Scholar]
- Satoh, K.; Oono, Y. Lethal Effects of gamma rays and carbon ion beam radiations in Bacillus subtilis. QST Takasaki Ann. Rep. 2016, 2015, 135. [Google Scholar]
- Amsal, A.; Takigami, M.; Ito, H. Increased digestibility of raw starches by mutant strains of Aspergillus awamori. Food Sci. Technol. Res. 1999, 5, 153–155. [Google Scholar] [CrossRef]
- Hanya, Y.; Koyama, T.; Ito, K.; Takeichi, J.; Otsuka, T.; Narumi, I.; Satoh, K.; Kobayashi, Y.; Sakashita, T.; Hase, Y. Koji Mold, Method for Breeding the Same, and Method for Producing Soy Sauce. Japanese Unexamined Patent Application Publication No. JP2009095279A, 5 May 2009. (In Japanese). [Google Scholar]
- Ngamnit, S.; Takigami, M.; Suchada, P.; Orawan, S.; Saovapong, C.; Ito, H. Decolorization of dark brown pigments in molasses wastewater by mutant strains of Aspergillus usamii and Coriolus versicolor. Biocontrol Sci. 1999, 4, 109–113. [Google Scholar]
- Fitriana, Y.; Shinohara, S.; Satoh, K.; Narumi, I.; Saito, T. Benomyl-resistant Beauveria bassiana (Hypocreales: Clavicipitaceae) mutants induced by ion beams. Appl. Entomol. Zool. 2015, 50, 123–129. [Google Scholar] [CrossRef]
- Shinohara, S.; Fitriana, Y.; Satoh, K.; Narumi, I.; Saito, T. Enhanced fungicide resistance in Isaria fumosorosea following ionizing radiation-induced mutagenesis. FEMS Microbiol. Lett. 2013, 349, 54–60. [Google Scholar] [PubMed]
- Fitriana, Y.; Satoh, K.; Narumi, I.; Saito, T. Ion-beam and gamma-ray irradiation induces thermotolerant mutants in the entomopathogenic fungus Metarhizium anisopliae s.l. Biocontrol Sci. Technol. 2014, 24, 1052–1061. [Google Scholar] [CrossRef]
- Kawashima, Y.; Hase, Y.; Yokota, Y. Development of new commercial strains in functional mushroom by ion beam irradiation. JAEA Takasaki Ann. Rep. 2008, 2007, 76. (In Japanese) [Google Scholar]
- Ogino, C.; Yamada, R.; Satoh, K.; Oono, Y. Screening of yeast strain for ethanol fermentation after carbon ion beam irradiation. JAEA Takasaki Ann. Rep. 2015, 2013, 122. [Google Scholar]
- Masubuchi, T.; Satoh, K.; Narumi, I.; Kamiyama, O. Ion beam breeding of “Sake Yeast”. Bioindustry 2013, 30, 65–71. (In Japanese) [Google Scholar]
- Ito, K.; Hanya, Y.; Satoh, S.; Hase, Y.; Narumi, I. Mutation breeding of Zygosaccharomyces rouxii induced by ion beams. JAEA Takasaki Ann. Rep. 2009, 2008, 83. [Google Scholar]
- Kato, Y.; Ho, S.H.; Vavricka, C.J.; Chang, J.S.; Hasunuma, T.; Kondo, A. Evolutionary engineering of salt-resistant Chlamydomonas sp. strains reveals salinity stress-activated starch-to-lipid biosynthesis switching. Bioresour. Technol. 2017, 245, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Araie, H.; Hase, Y.; Oono, Y.; Suzuki, I.; Shiraiwa, Y. Breeding of the oil-producing algae by heavy ion beam irradiation. QST Takasaki Ann. Rep. 2018, 2016, 95. [Google Scholar]
- Takeda, K.; Satoh, K.; Narumi, I.; Ohtsu, N.; Yokoyama, T. Identification of DNA mutation sites in a high temperature tolerant mutant of Bradyrhizobium japonicum USDA110 generated by ion-beam irradiation. JAEA Takasaki Ann. Rep. 2014, 2012, 114. [Google Scholar]
- Satoh, K.; Ueda, R.; Hase, Y.; Narumi, I.; Oono, Y. Development of cesium-accumulating bacteria by ion beam breeding technology. JAEA Takasaki Ann. Rep. 2015, 2013, 117. [Google Scholar]
- Aino, M.; Matsuura, K.; Satoh, K.; Narumi, I. Characteristics of mutant endophytic bacteria strains improved using ion beams. JAEA Takasaki Ann. Rep. 2011, 2010, 109. (In Japanese) [Google Scholar]
- Yanagisawa, M.; Asamizu, S.; Sugai, Y.; Satoh, K.; Oono, Y.; Onaka, H. Screening of mutants generated by heavy ion beam for identification of genes involved in bacterial interaction. QST Takasaki Ann. Rep. 2017, 2016, 97. [Google Scholar]
- Masubuchi, T.; Takashima, C.; Kamiyama, O.; Ikenaga, H.; Satoh, K.; Narumi, I. Identification of mutation sites in high ethyl caproate producing sake yeasts generated by ion beam breeding. JAEA Takasaki Ann. Rep. 2012, 2011, 110. (In Japanese) [Google Scholar]
- Akada, R.; Matsuo, K.; Aritomi, K.; Nishizawa, Y. Construction of recombinant sake yeast containing a dominant FAS2 mutation without extraneous sequences by a two-step gene replacement protocol. J. Biosci. Bioeng. 1999, 87, 43–48. [Google Scholar] [CrossRef]
- Takeda, K.; Satoh, K.; Narumi, I.; Oono, Y.; Ohkama-Ohtsu, N.; Yokoyama, T. Genome analysis of the high temperature tolerant mutant of Bradyrhizobium japonicum USDA110 generated by ion-beam irradiation. JAEA Takasaki Ann. Rep. 2015, 2013, 120. [Google Scholar]
- Tomioka, N.; Uchiyama, H.; Yagi, O. Isolation and characterization of cesium-accumulating bacteria. Appl. Environ. Microbiol. 1992, 58, 1019–1023. [Google Scholar] [Green Version]
- Satoh, K.; Ueda, R.; Hase, Y.; Narumi, I.; Oono, Y. Screening of cesium-accumulating mutant of radioresistant bacterium Deinococcus radiodurans by ion beam breeding technology. JAEA Takasaki Ann. Rep. 2016, 2014, 100. [Google Scholar]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F.; Ogino, C.; Yamada, R.; Satoh, K.; et al. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar]
- Ogino, C.C.; Kahar, P.; Lee, J.M.; Satoh, K.; Oono, Y.; Kondo, A. Breeding of high ethanol producing yeast by screening from mutant library. JAEA Takasaki Ann. Rep. 2016, 2014, 105. [Google Scholar]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Fact. 2018, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- RIKEN Nishina Center for Accelerator-Based Science. Available online: https://www.nishina.riken.jp/index_e.html (accessed on 6 March 2019).
- The Wakasa Wan Energy Research Center. Available online: http://www.werc.or.jp/enenews/pdf/pamphlet_english.pdf (accessed on 6 March 2019).
- National Institute of Radiological Sciences. Available online: https://www.nirs.qst.go.jp/ENG/index.html (accessed on 6 March 2019).
- Hu, W.; Li, W.; Che, J. Recent advances of microbial breeding via heavy-ion mutagenesis at IMP. Lett. Appl. Microbiol. 2017, 65, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Ota, S.; Matsuda, T.; Takeshita, T.; Yamazaki, T.; Kazama, Y.; Abe, T.; Kawano, S. Phenotypic spectrum of Parachlorella kessleri (Chlorophyta) mutants produced by heavy-ion irradiation. Bioresour Technol. 2013, 49, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Suzuki, H.; Takeuchi, T.; Kazama, Y.; Mitra, S.; Abe, T.; Goda, K.; Suzuki, K.; Iwata, O. Efficient selective breeding of live oil-rich Euglena gracilis with fluorescence-activated cell sorting. Sci. Rep. 2016, 6, 26327. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Effects of additives on cordycepin production using a Cordyceps militaris mutant induced by ion beam irradiation. Afr. J. Biotechnol. 2009, 8, 3041–3047. [Google Scholar]
- Moeller, R.; Raguse, M.; Reitz, G.; Okayasu, R.; Li, Z.; Klein, S.; Setlow, P.; Nicholsone, W.L. Resistance of Bacillus subtilis spore DNA to lethal ionizing radiation damage relies primarily on spore core components and DNA repair, with minor effects of oxygen radical detoxification. Appl. Environ. Microbiol. 2014, 80, 104–109. [Google Scholar] [CrossRef]
- Yokota, Y.; Yamada, S.; Hase, Y.; Shikazono, N.; Narumi, I.; Tanaka, A.; Inoue, M. Initial yields of DNA double-strand breaks and DNA fragmentation patterns depend on linear energy transfer in tobacco BY-2 protoplasts irradiated with helium, carbon and neon ions. Radiat. Res. 2007, 167, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Hase, Y.; Yoshihara, R.; Nozawa, S.; Narumi, I. Mutagenic effects of carbon ions near the range end in plants. Radiat. Res. 2012, 731, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hase, Y.; Tanaka, A.; Shikazono, N.; Degi, K.; Shimizu, A.; Morishita, T. Mutagenic effects of ion beam irradiation on rice. Breed. Sci. 2009, 59, 169–177. [Google Scholar] [CrossRef] [Green Version]


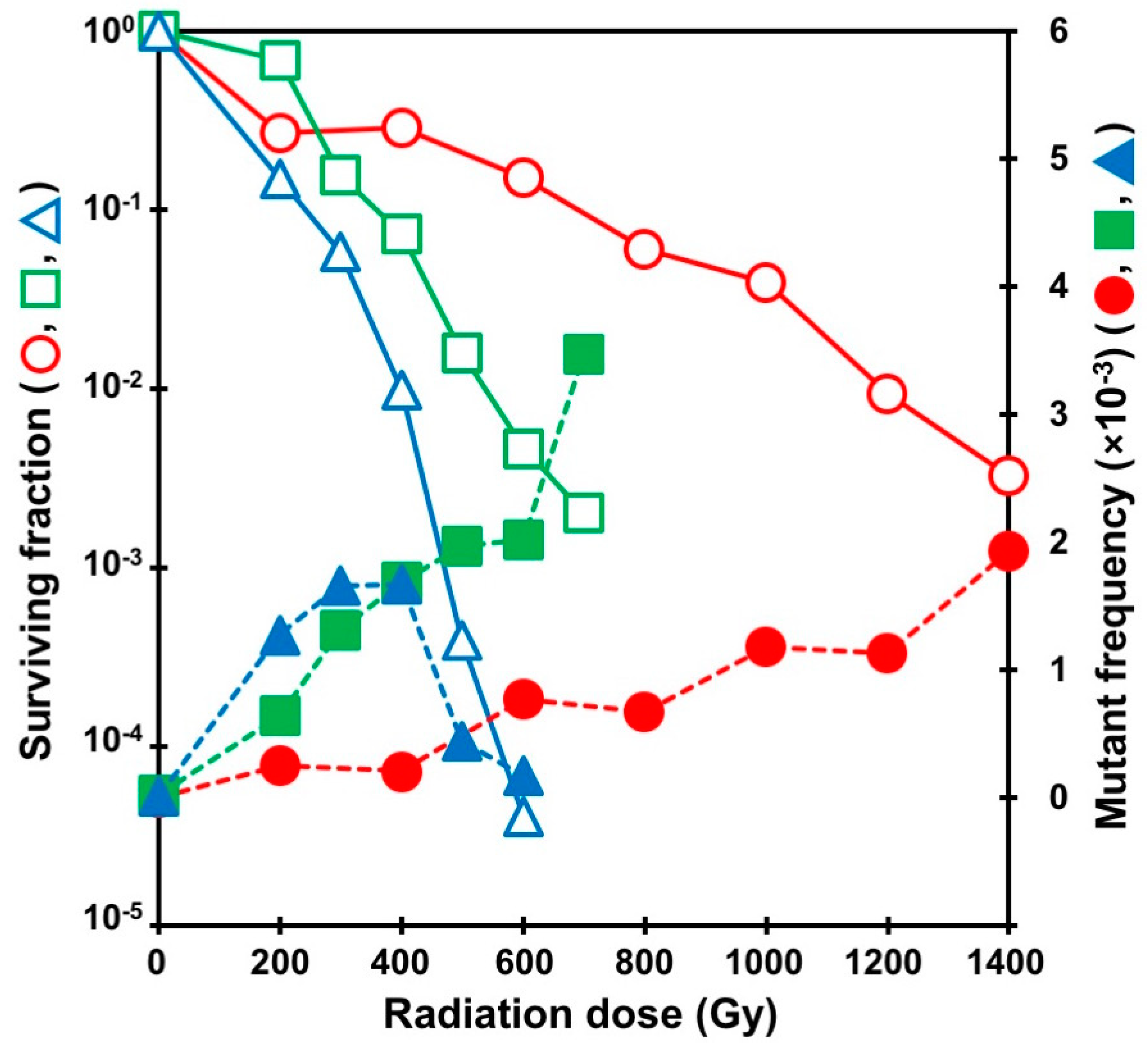
| Ion | Total Accelerated Energy (MeV) | Total Energy at Target Surface (MeV) | LET at Target Surface (keV/µm) | Penetration Range in Water (µm) |
|---|---|---|---|---|
| 4He2+ | 50 | 48 | 16 | 1670 |
| 12C6+ | 320 | 311 | 76 | 2270 |
| 12C5+ | 220 | 208 | 107 | 1110 |
| 20Ne8+ | 350 | 316 | 317 | 600 |
| 40Ar13+ | 460 | 310 | 1550 | 150 |
| Microorganism | Radiation | Dose (Gy) | Mutant Frequency | Target Gene |
|---|---|---|---|---|
| Fungi and Yeast | ||||
| Aspergillus oryzae | 60Co gamma rays | 1400 | 1.93 × 10−3 | sB and sC |
| 12C5+ | 400 | 1.67 × 10−3 | ||
| 12C6+ | 700 | 3.47 × 10−3 | ||
| Aspergillus sojae | UV | No data | 0.26 × 10−5 | thiA |
| 4He2+ | 300 | 3.56 × 10−5 | ||
| 20Ne8+ | 200 | 1.88 × 10−5 | ||
| 12C5+ | 200 | 5.60 × 10−5 | ||
| Saccharomyces cerevisiae | 60Co gamma rays | 66 | 0.16 × 10−5 | ura3 |
| 12C5+ | 100 | 1.85 × 10−5 | ||
| Bacteria | ||||
| Deinococcus radiodurans | 60Co gamma rays | 8000 | 1.93 × 10−6 | rpoB |
| 4He2+ | 6000 | 0.92 × 10−6 | ||
| 12C6+ | 6000 | 0.74 × 10−6 | ||
| 12C5+ | 6000 | 1.38 × 10−6 | ||
| 20Ne8+ | 8000 | 1.71 × 10−6 | ||
| 40Ar13+ | 15,000 | 1.08 × 10−6 | ||
| Rhodococcus erythropolis | 4He2+ | 600 | 8.67 × 10−7 | rpoB |
| 12C5+ | 800 | 9.45 × 10−7 | ||
| Radiation | 60Co Gamma Rays | 12C5+ Ion Beams |
|---|---|---|
| Lethal effect*1 | 1.0 | 4.7 |
| Mutagenic Effect*2 | 1.93 × 10−3 | 1.67 × 10−3 |
| A multitude of mutations*3 | 5.26, 2.06 | 1.30, 1.09 |
| Large-scale mutations*4 | 71.25% | 48.89% |
| Chromosomal rearrangement | Observed | Observed |
| Microorganism | Ion | Dose Range (Gy) | Breeding Objective | References |
|---|---|---|---|---|
| Fungi and Yeast | ||||
| Aspergillus awamori | 12C5+ | 100 to 1200 | High amylase activity | [35] |
| Aspergillus sojae | 12C5+ | 100 to 500 | High producing of protease | [36] |
| Aspergillus usamii | 12C5+ | 10 to 1000 | High decompose ability of dark brown pigments | [37] |
| Coriolus versicolor | 12C5+ | 300 | High decompose ability of dark brown pigments | [37] |
| Rhizomucor miehei | 4He2+ 12C5+ 20Ne8+ 40Ar13 | 100, 200 | Low lipase activity Low coagulation activity | [31,32] |
| Beauveria bassiana | 12C5+ | 50 to 400 | High fungicide resistance | [38] |
| Isaria fumosorosea | 12C5+ | 50 to 600 | High fungicide resistance | [39] |
| Metarhizium anisopliae | 12C5+ | 100 to 500 | High thermotolerance | [40] |
| Pleurotus osutreatus | 12C6+ | 50 to 1000 | New variety | [41] |
| Lyophyllum decastes | 12C6+ | 50 to 1000 | New variety | [41] |
| Pleurotus eryngii | 12C6+ | 50 to 1000 | New variety | [41] |
| Ganoderma lucidum | 12C6+ | 50 to 1000 | New variety | [41] |
| Grifora flondosa | 12C6+ | 50 to 1000 | New variety | [41] |
| Alcohol fermentative yeast | 12C5+ | 10 to 300 | High producing of ethanol | [42] |
| Saccharomyces cerevisiae | 12C5+ | 50 to 300 | High producing of ethyl caproate | [43] |
| Zygosaccharomyces rouxii | 12C5+ | 50 to 300 | Auxotrophy | [44] |
| Microalgae | ||||
| Chlamydomonas sp. | 12C5+ | 50 to 100 | High salinity tolerance | [45] |
| Tisochrysis lutea | 12C6+ | 5 to 320 | High oil productivity | [46] |
| Bacteria | ||||
| Bradyrhizobium japonicum | 12C5 | 50 to 800 | High thermotolerance | [47] |
| Deinococcus radiodurans | 4He2+ 12C6+ 12C5+ 20Ne8+ 40Ar13 | 2000 to 20000 | Cs-accumulating ability | [48] |
| Pseudomonas fluorescens | 12C5+ | 10 to 300 | High suppression effect of tomato bacterial wilt | [49] |
| Rhodococcus erythropolis | 12C5+ | 200 to 2000 | Cs-accumulating ability | [30] |
| Streptomyces coelicolor | 12C5+ | 10 to 1000 | Pigment producing ability | [50] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satoh, K.; Oono, Y. Studies on Application of Ion Beam Breeding to Industrial Microorganisms at TIARA. Quantum Beam Sci. 2019, 3, 11. https://doi.org/10.3390/qubs3020011
Satoh K, Oono Y. Studies on Application of Ion Beam Breeding to Industrial Microorganisms at TIARA. Quantum Beam Science. 2019; 3(2):11. https://doi.org/10.3390/qubs3020011
Chicago/Turabian StyleSatoh, Katsuya, and Yutaka Oono. 2019. "Studies on Application of Ion Beam Breeding to Industrial Microorganisms at TIARA" Quantum Beam Science 3, no. 2: 11. https://doi.org/10.3390/qubs3020011
APA StyleSatoh, K., & Oono, Y. (2019). Studies on Application of Ion Beam Breeding to Industrial Microorganisms at TIARA. Quantum Beam Science, 3(2), 11. https://doi.org/10.3390/qubs3020011





