Formation of Fe Nanoparticles by Ion Implantation Technique for Catalytic Graphitization of a Phenolic Resin
Abstract
:1. Introduction
- (1)
- The formation of a graphitic nanostructure such as shell-like structure consisted of warped graphene layers, and/or a turbostratic graphite structure consisting of the hexagonal carbon layers piled up randomly or uniformly in a part;
- (2)
- Incorporation of nitrogen atoms into the graphitic structure as pyridinic nitrogen and/or quaternary nitrogen.
- (1)
- Crosslinking of the carbon precursor polymer due to high energy deposition;
- (2)
- Reduction reaction of metal ions (M+) with secondary electrons (e−) generated in the carbon precursor polymer (M+ + e− → M);
- (3)
- Formation of metal NPs during the heat treatment after the implantation;
- (4)
- Catalytic graphitization of the carbon precursor polymer by the formed metal NPs;
2. Materials and Methods
2.1. Materials and Ion Implantation
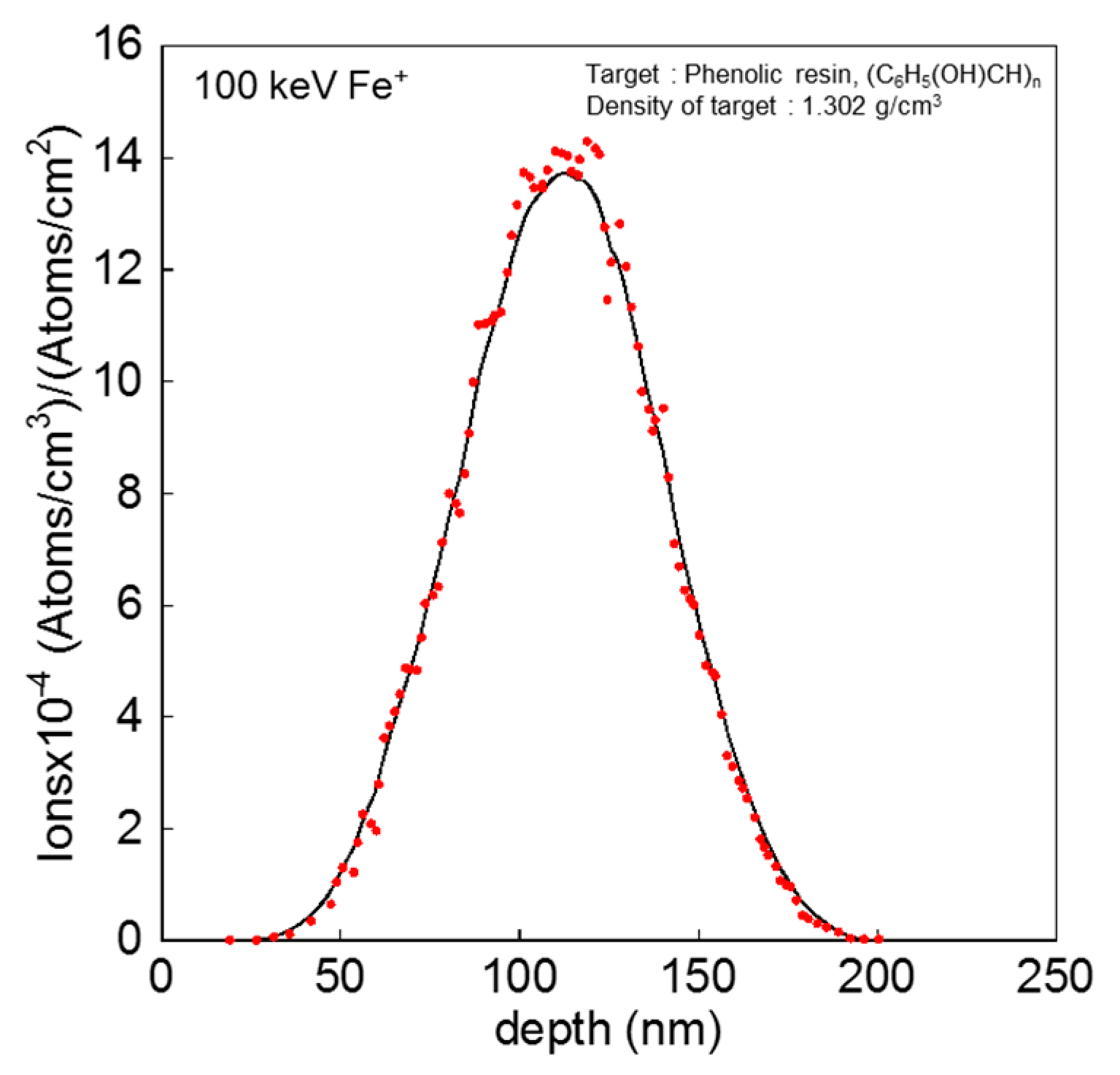
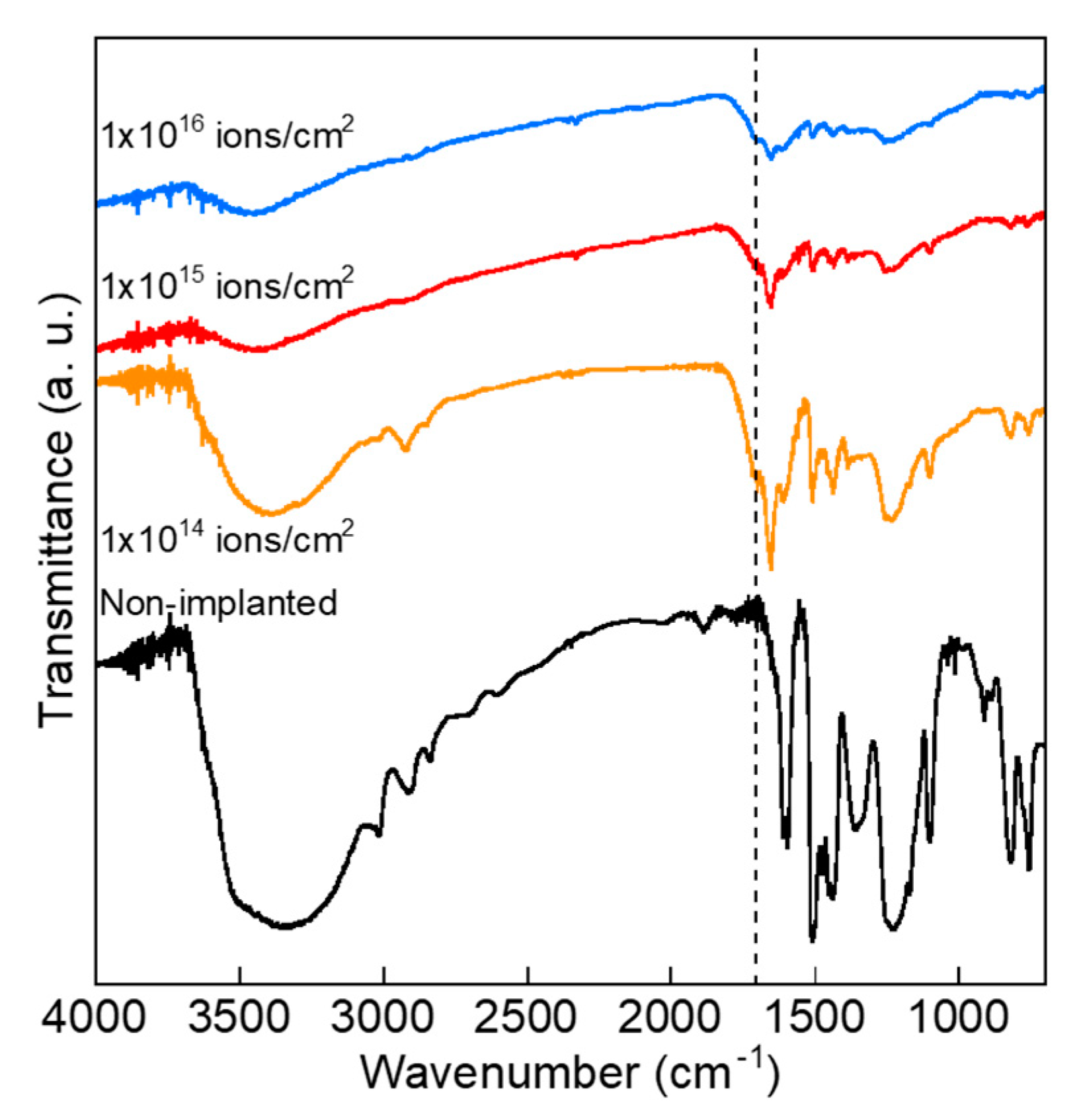
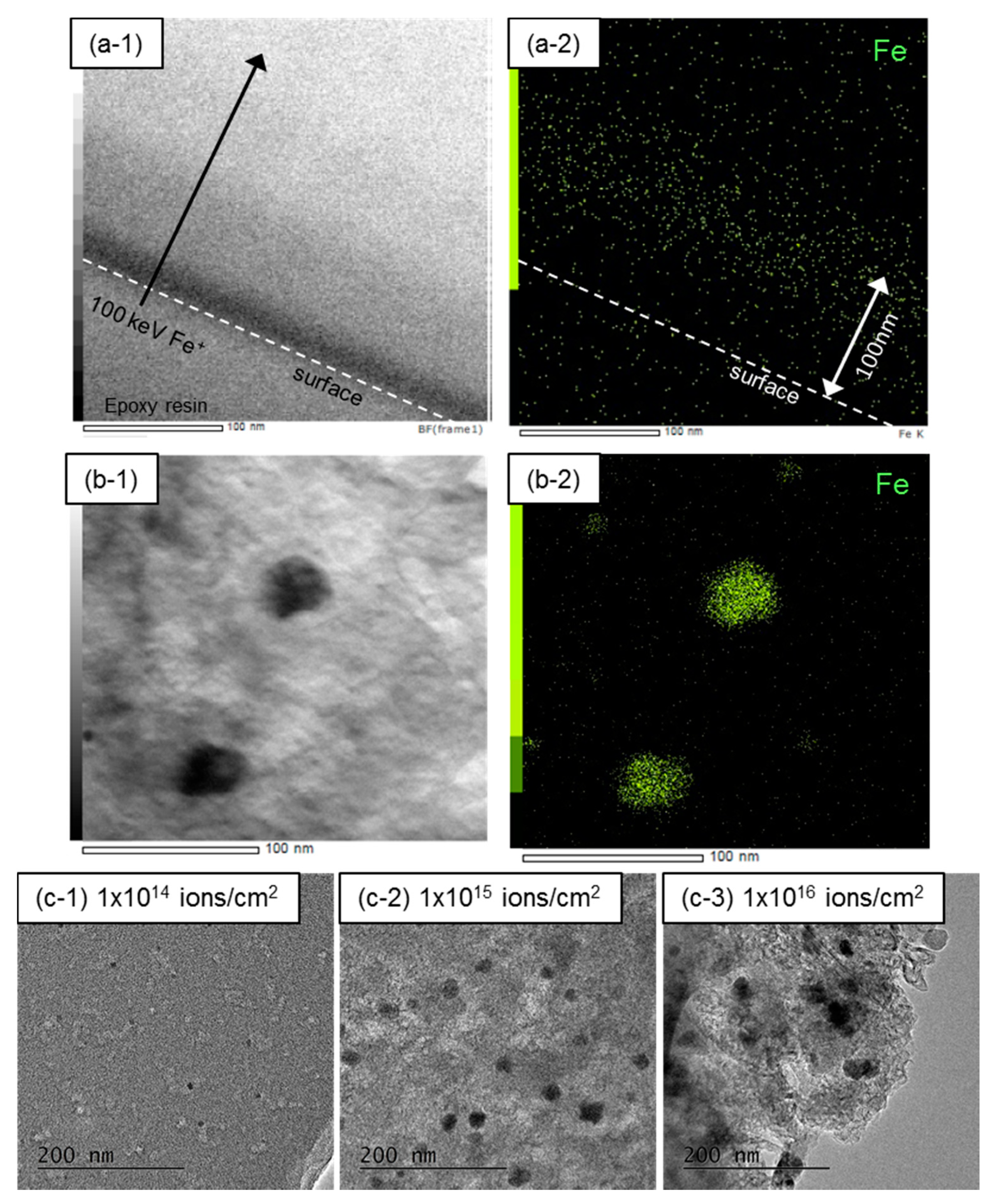
2.2. Measurements and Analyses
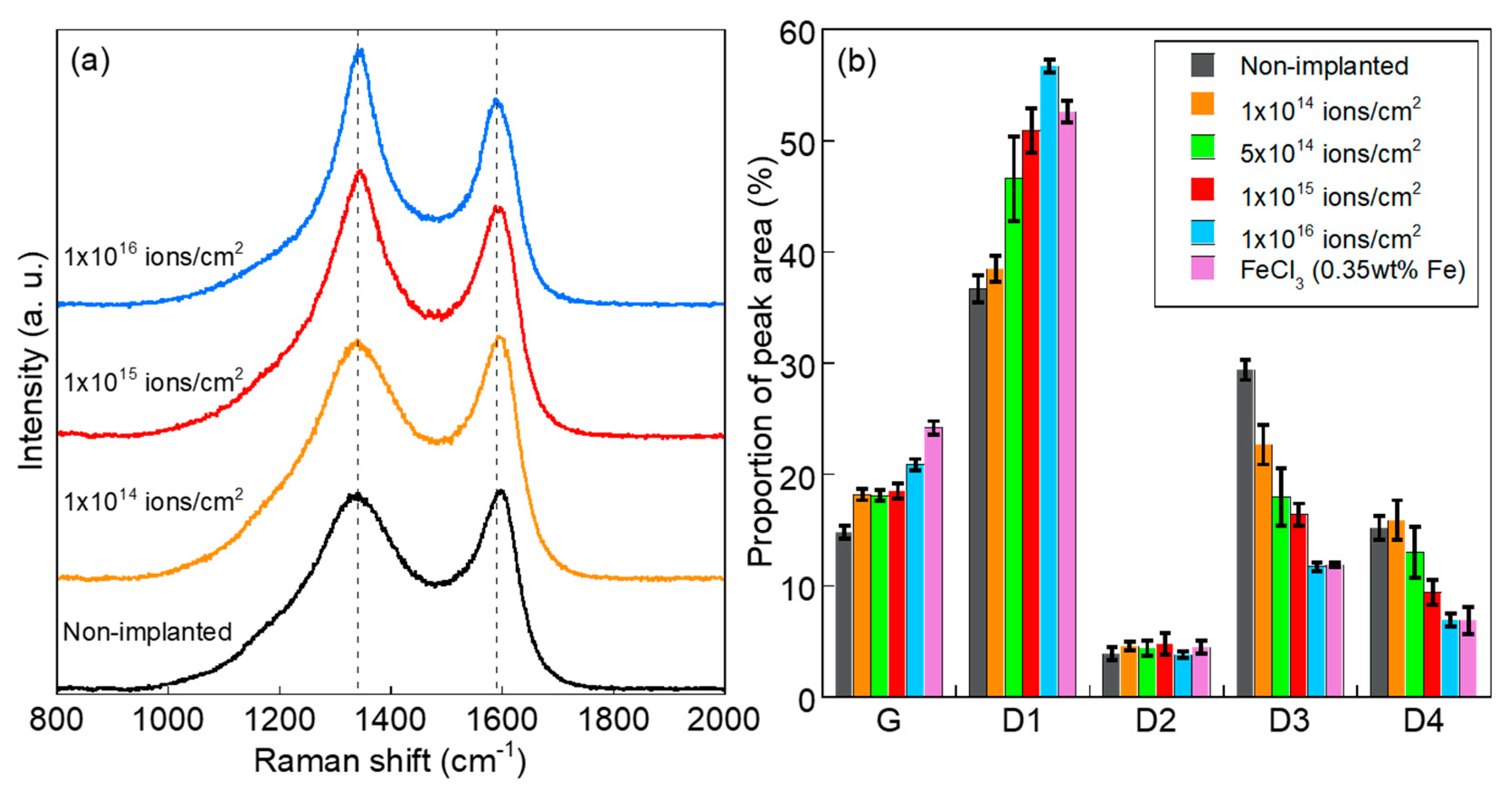
3. Results and Discussion
4. Conclusions
- (1)
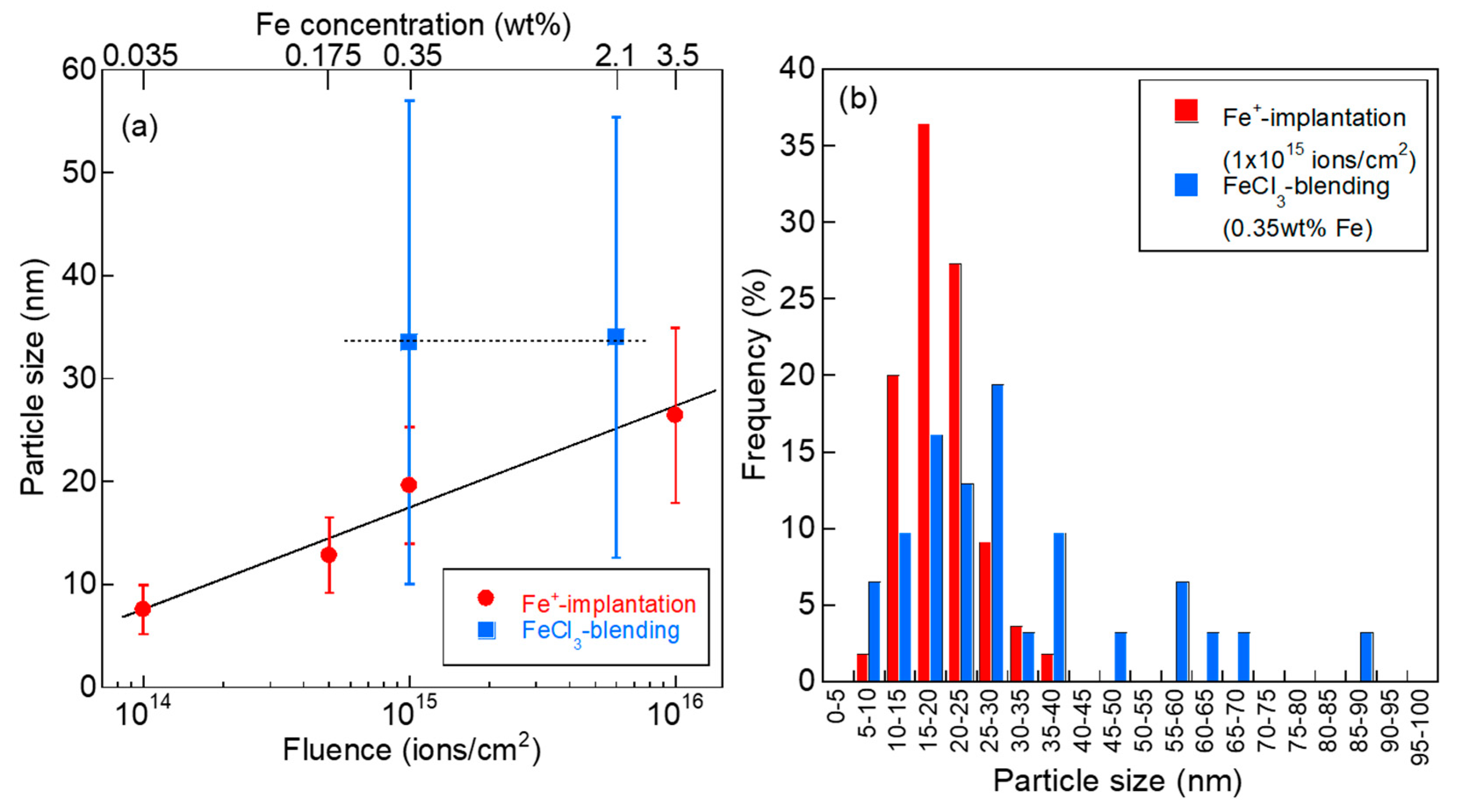
- The mean particle size of Fe NPs could be controlled in the range of 5–30 nm by the ion fluence of 1 × 1014–1 × 1016 ions/cm2. Because the mean particle size of Fe NPs could not be controlled in the case of the FeCl3-blended PhR, it can be concluded that the ion implantation technique is one of the advantageous ways to introduce fine metal NPs.
- (2)
- The turbostratic graphite structure with shell-like carbon layers and intricately distorted carbon layers was formed by the heat treatment of Fe+-implanted PhR at 800 °C. The peak analyses of Raman spectra revealed that carbonization at 800 °C was promoted even in the case of ion implantation with the ion fluence of 1 × 1014 ions/cm2 (0.035 wt% as Fe). Compared with the conventional FeCl3-blending method (0.35 wt% as Fe), the fine Fe NPs introduced by the ion implantation (1 × 1015 ions/cm2, 0.35 wt% as Fe) suppress the formation of graphite structure and induce the formation of turbostratic graphite structure.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozaki, J.; Tanifuji, S.; Furuichi, A.; Oya, A. Enhancement of oxygen reduction activity by carbonization of furan resin in the presence of phthalocyanines. Carbon 2006, 44, 1324–1326. [Google Scholar] [CrossRef]
- Ozaki, J.; Tanifuji, S.; Furuichi, A.; Yabutsuka, K. Enhancement of oxygen reduction activity of nanoshell carbons by introducing nitrogen atoms from metal phthalocyanines. Electrochim. Acta 2010, 55, 1864–1871. [Google Scholar] [CrossRef]
- Kannari, N.; Ozaki, J. Formation of uniformly and finely dispersed nanoshells by carbonization of cobalt-coordinated oxine-formaldehyde resin and their electrochemical oxygen reduction activity. Carbon 2012, 50, 2941–2952. [Google Scholar] [CrossRef]
- Ozaki, J.; Imashiro, Y. Carbon Alloy catalysts for polymer electrolyte fuel cells: Exploration of materials and understanding of mechanisms. Electrochemistry 2015, 83, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Ning, X.; Li, Y.; Ming, J.; Wang, Q.; Wang, H.; Cao, Y.; Peng, F.; Yang, Y.; Yu, H. Electronic synergism of pyridinic- and graphitic-nitrogen on N-doped carbons for the oxygen reduction reaction. Chem. Sci. 2019, 10, 1589–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takigami, M.; Kobayashi, R.; Ishii, T.; Imashiro, Y.; Ozaki, J. Warped graphitic layers generated by oxidation of fullerene extraction residue and its oxygen reduction catalytic activity. Beilstein J. Nanotechnol. 2019, 10, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Park, S.H.; Woo, S.I. N-doped carbon prepared by pyrolysis of dicyandiamide with various MeCl2·xH2O (Me = Co, Fe, and Ni) composites: Effect of type and amount of metal seed on oxygen reduction reactions. Appl. Catal. B Environ. 2012, 119–120, 123–131. [Google Scholar] [CrossRef]
- Muthukrishnan, A.; Nabae, Y.; Hayakawa, T.; Okajima, T.; Ohsaka, T. Fe-containing polyimide-based high-performance ORR catalysts in acidic medium: A kinetic approach to study the durability of catalysts. Catal. Sci. Technol. 2015, 5, 475–483. [Google Scholar] [CrossRef]
- Oya, A.; Marsh, H. Phenomena of catalytic graphitization. J. Mater. Sci. 1982, 17, 309–322. [Google Scholar] [CrossRef]
- Maksimova, N.I.; Krivoruchko, O.P.; Mestl, G.; Zaikovskii, V.I.; Chuvillin, A.L.; Salanov, A.N.; Burgina, E.B. Catalytic synthesis of carbon nanosturctures from polymer precursor. J. Mol. Catal. A Chem. 2000, 158, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Song, H. Catalytic graphitization of phenolic resin. J. Mater. Sci. 2011, 27, 266–270. [Google Scholar] [CrossRef]
- Kodama, Y.; Sato, K.; Suzuki, K.; Saito, Y.; Suzuki, T.; Konno, T.J. Electron microscope study of the formation of graphitic nanostructures in nickel-loaded wood char. Carbon 2012, 50, 3486–3496. [Google Scholar] [CrossRef]
- Wang, J.; Deng, X.; Zhang, H.; Zhang, Y.; Duan, H.; Lu, L.; Song, J.; Tian, L.; Song, S.; Zhang, S. Synthesis of carbon nanotubes via Fe-catalyzed pyrolysis of phenolic resin. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 86, 24–35. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, H.; Zhang, H.; Li, J.; Ding, X. Growth of carbon nanofibers in phenolic resin for carbon-contained refractory using different catalysts. J. Nanomater. 2017, 2017, 4826785. [Google Scholar] [CrossRef]
- Rastegar, H.; Bavand-vandchali, M.; Nemati, A.; Fard, F.G. Catalytic graphitization behavior of phenolic resins by addition of in situ formed nano-Fe particles. Phys. E Low-Demens. Sys. Nanostruct. 2018, 101, 50–61. [Google Scholar] [CrossRef]
- Stepanov, A.L. Optical properties of metal nanoparticles synthesized in a polymer by ion implantation: A review. Tech. Phys. 2004, 49, 143–153. [Google Scholar] [CrossRef]
- Popok, V.N. Ion implantation of polymers: Formation of nanoparticulate materials. Rev. Adv. Mater. Sci. 2012, 30, 1–26. [Google Scholar]
- Boldyryeva, H.; Umeda, N.; Plaksin, O.A.; Kishimoto, N. High-fluence implantation of negative metal ions into polymers for surface modification and nanoparticle formation. Surf. Coat. Technol. 2005, 196, 373–377. [Google Scholar] [CrossRef]
- Mailinsky, P.; Mackova, A.; Hnatowicz, V.; Khaibullin, R.I.; Valeev, V.F.; Slepicka, P.; Svorcik, V.; Slouf, M.; Perina, V. Properties of polyimide, polyetheretherketone and polyethyleneterephthalate implanted by Ni ions to high fluences. Res. Sect. B Beam Interact. Mater. At. 2012, 272, 396–399. [Google Scholar] [CrossRef]
- Salvadori, M.C.; Teixeira, F.S.; Sgubin, L.G.; Cattani, M.; Brown, I.G. Surface modification by metal ion implantation forming metallic nanoparticles in an insulating matrix. Appl. Surf. Sci. 2014, 310, 158–163. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy, A Textbook for Material Science; Plenum Press: New York, NY, USA, 1996. [Google Scholar]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Ziegler, J.F. The Stopping and Ranges of Ions in Matter, SRIM 2013 Software. Available online: www.srim.org (accessed on 4 February 2020).
- Poljanšek, I.; Krajnc, M. Characterization of Phenol-Formaldehyde Prepolymer Resins by In Line FT-IR Spectroscopy. Acta Chim. Slov. 2005, 52, 238–244. [Google Scholar]
- Renda, C.G.; Bertholdo, R. Study of phenolic resin and their tendency for carbon graphitization. J. Polym. Res. 2018, 25, 241. [Google Scholar] [CrossRef]
- Idesaki, A.; Sugimoto, M.; Yamamoto, S.; Yamaki, T. Effect of ion implantation on a precursor polymer for synthesis of carbon material with catalytic performance. Ceram. Trans. 2018, 264, 33–40. [Google Scholar]
- Yamashita, Y.; Ouchi, K. A study on carbonization of phenol-formaldehyde resin labelled with deuterium and 13C. Carbon 1981, 19, 89–94. [Google Scholar] [CrossRef]
- Moisala, A.; Nasibulin, A.G.; Kauppinen, E.I. The role of metal nanoparticles in the catalytic production of single-walled carbon nanotubes—A review. J. Phys. Condens. Matter 2003, 15, S3011–S3035. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray diffraction study of carbon black. J. Chem. Phys. 1934, 2, 551–555. [Google Scholar] [CrossRef]
- Biscoe, J.; Warren, B.E. An X-ray study of carbon black. J. Appl. Phys. 1942, 13, 364–371. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idesaki, A.; Yamamoto, S.; Sugimoto, M.; Yamaki, T.; Maekawa, Y. Formation of Fe Nanoparticles by Ion Implantation Technique for Catalytic Graphitization of a Phenolic Resin. Quantum Beam Sci. 2020, 4, 11. https://doi.org/10.3390/qubs4010011
Idesaki A, Yamamoto S, Sugimoto M, Yamaki T, Maekawa Y. Formation of Fe Nanoparticles by Ion Implantation Technique for Catalytic Graphitization of a Phenolic Resin. Quantum Beam Science. 2020; 4(1):11. https://doi.org/10.3390/qubs4010011
Chicago/Turabian StyleIdesaki, Akira, Shunya Yamamoto, Masaki Sugimoto, Tetsuya Yamaki, and Yasunari Maekawa. 2020. "Formation of Fe Nanoparticles by Ion Implantation Technique for Catalytic Graphitization of a Phenolic Resin" Quantum Beam Science 4, no. 1: 11. https://doi.org/10.3390/qubs4010011
APA StyleIdesaki, A., Yamamoto, S., Sugimoto, M., Yamaki, T., & Maekawa, Y. (2020). Formation of Fe Nanoparticles by Ion Implantation Technique for Catalytic Graphitization of a Phenolic Resin. Quantum Beam Science, 4(1), 11. https://doi.org/10.3390/qubs4010011





