Development of Advanced Biodevices Using Quantum Beam Microfabrication Technology
Abstract
:1. Introduction
2. Results and Discussion
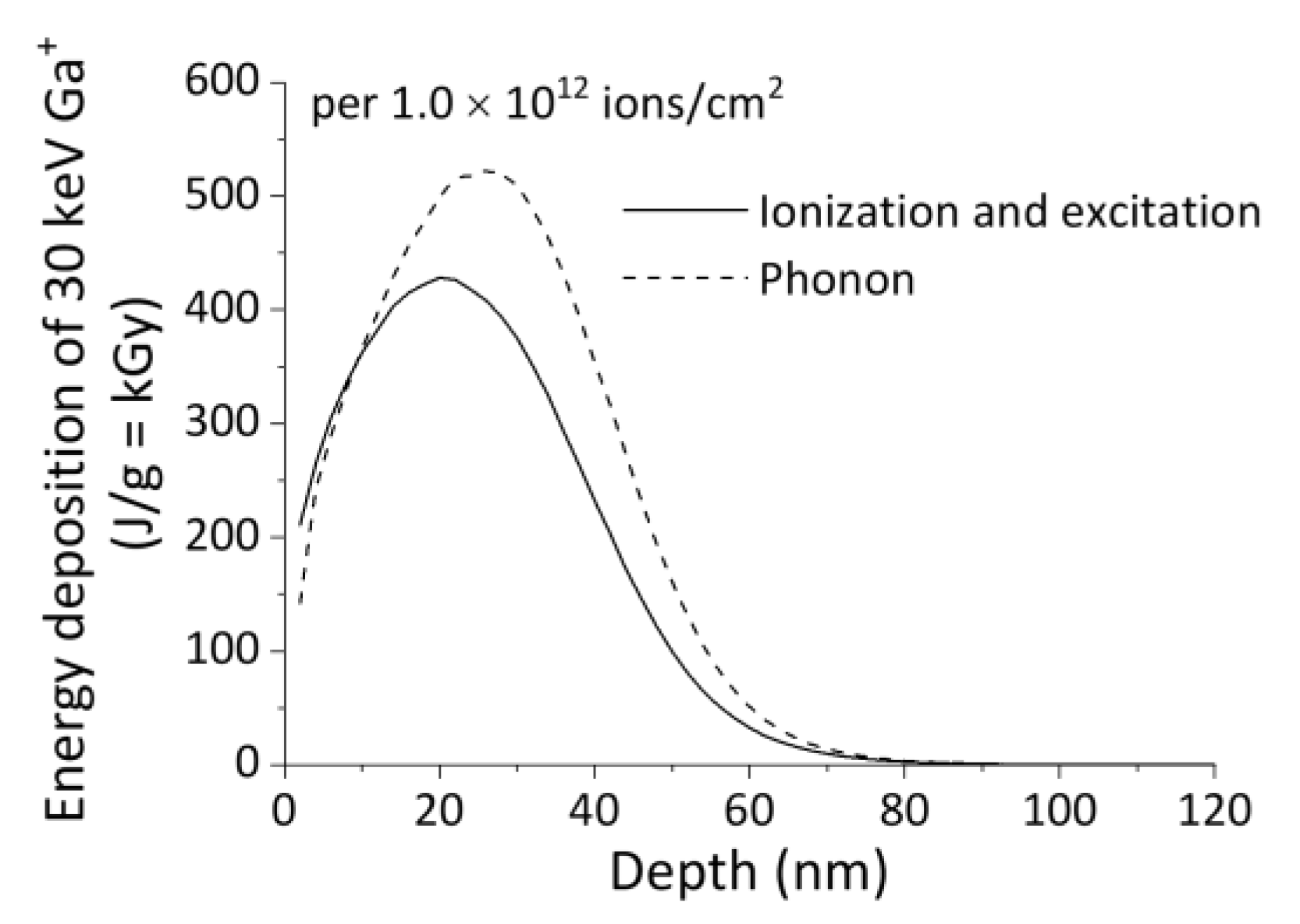
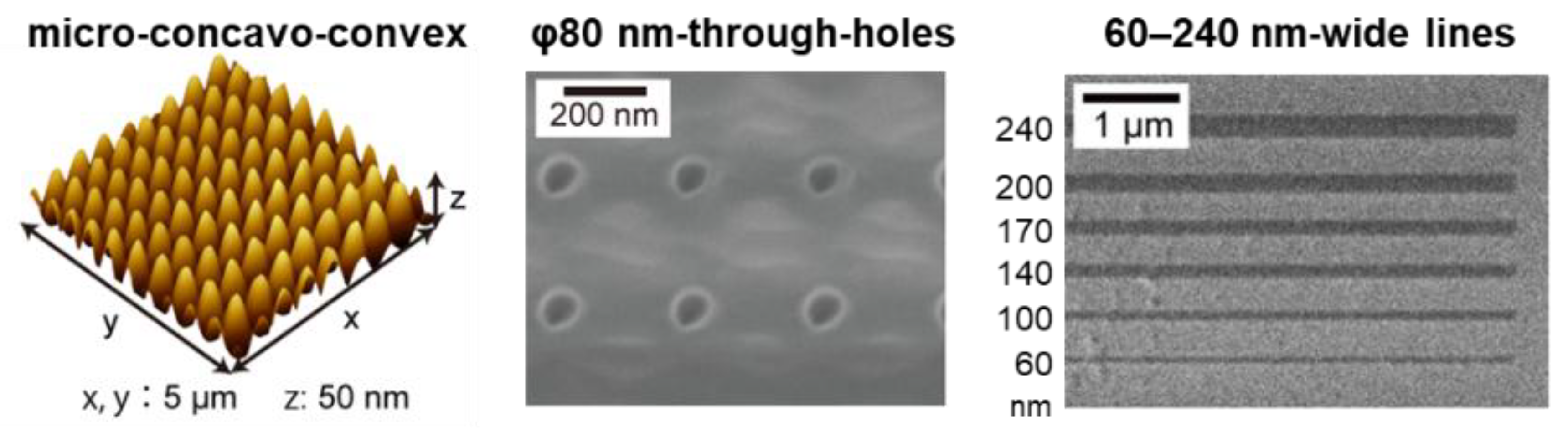
2.1. Micro/Nanopatterning of PLLA Using FIB Irradiation
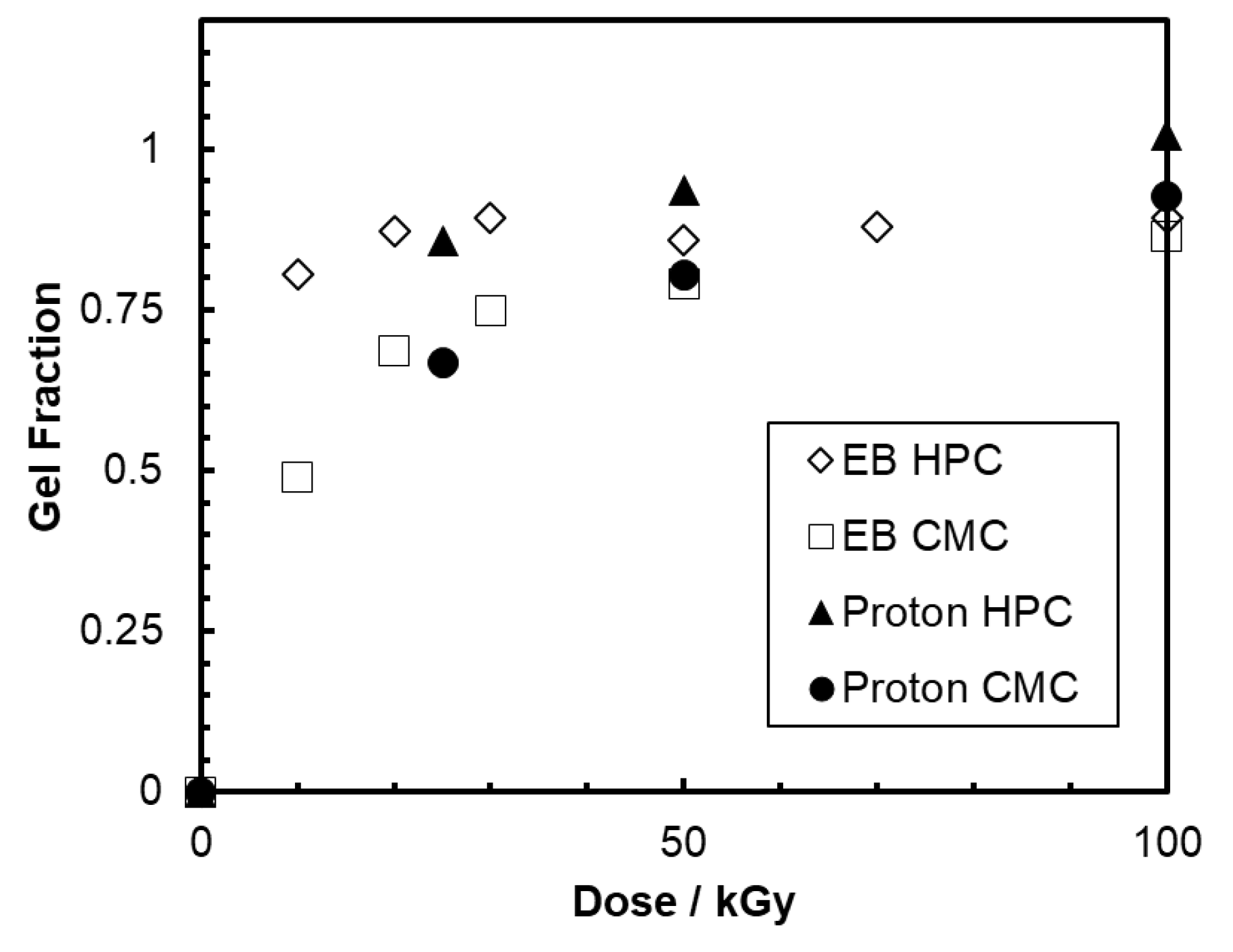
2.2. Microfabricated Polysaccharide Hydrogels by Proton Beam Writing
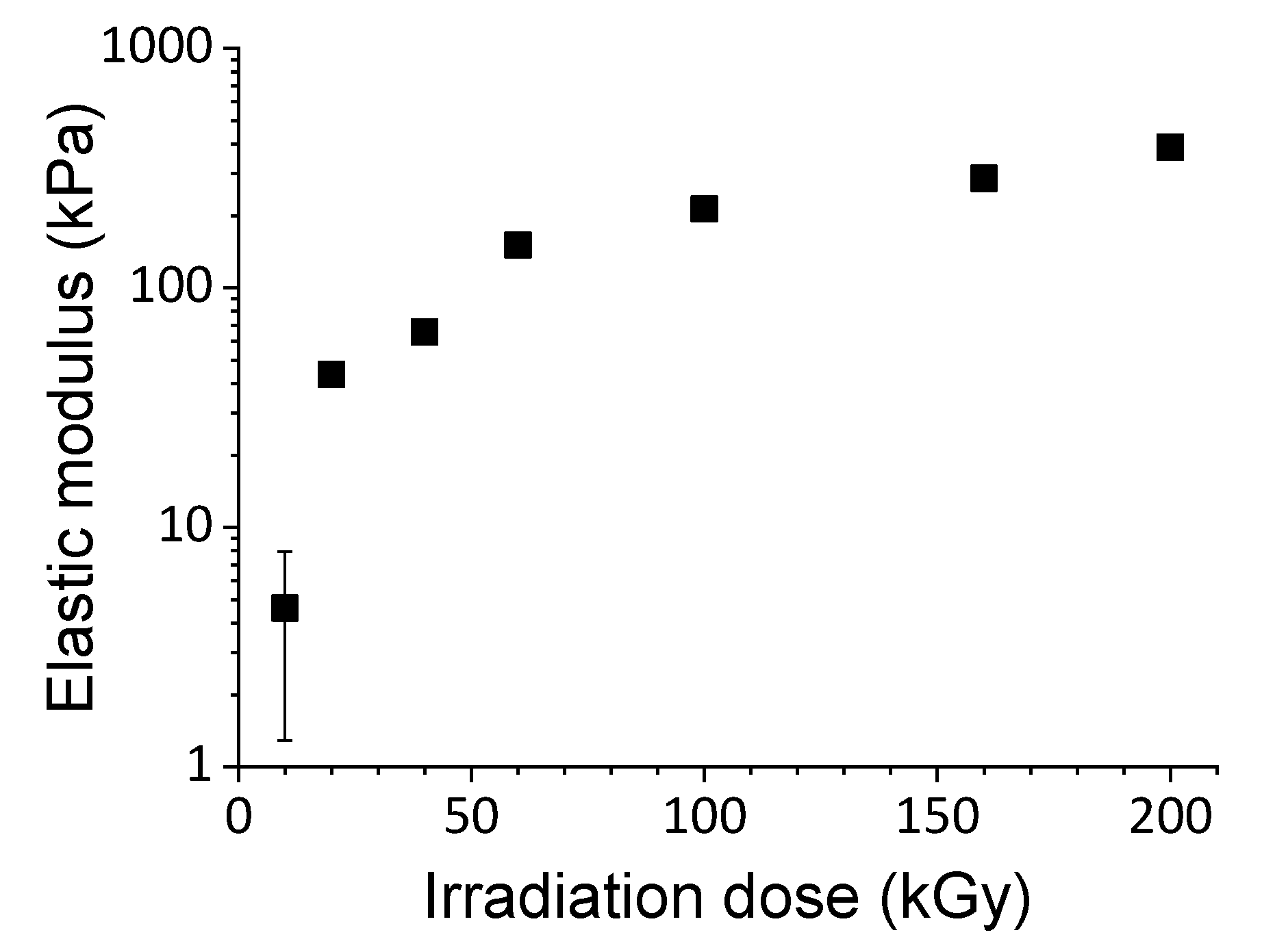
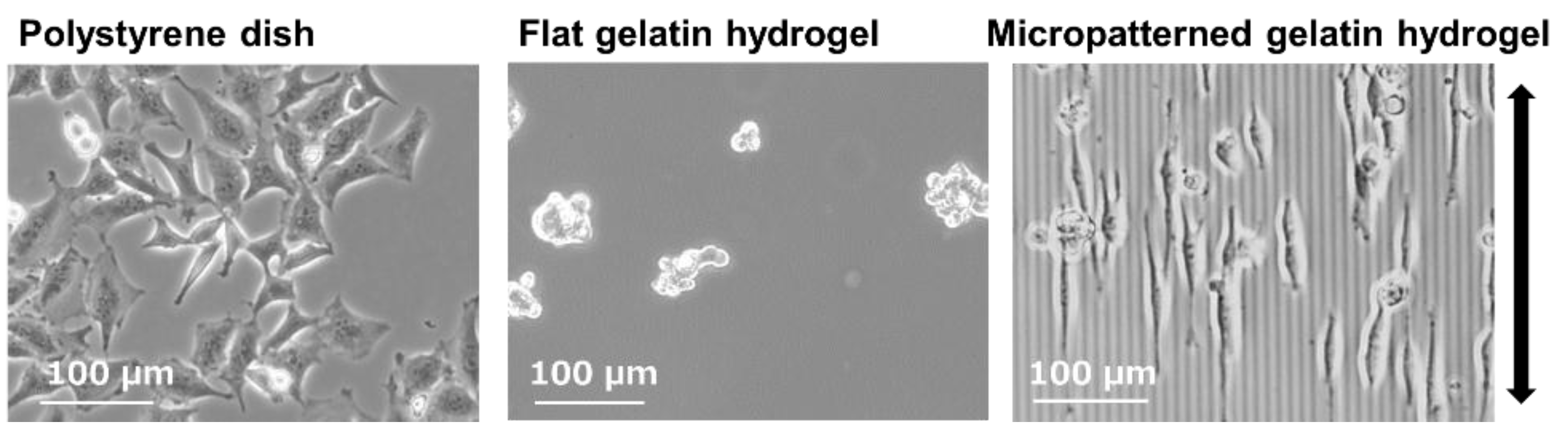
2.3. Gelatin Hydrogels for Mimicking In Vivo Extracellular Matrix
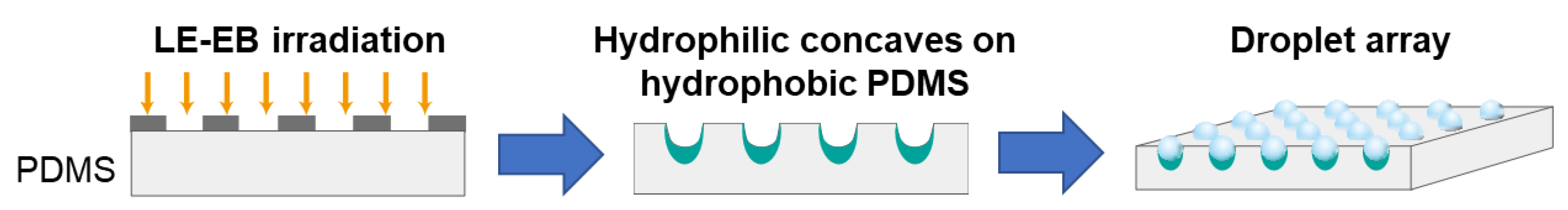
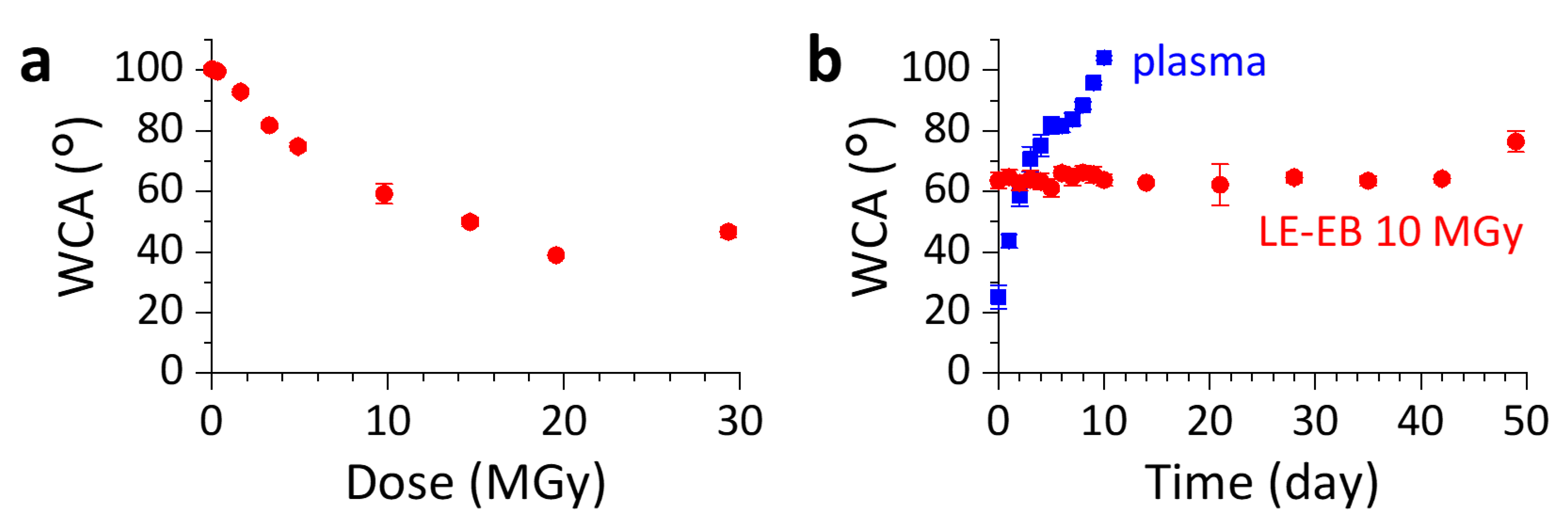
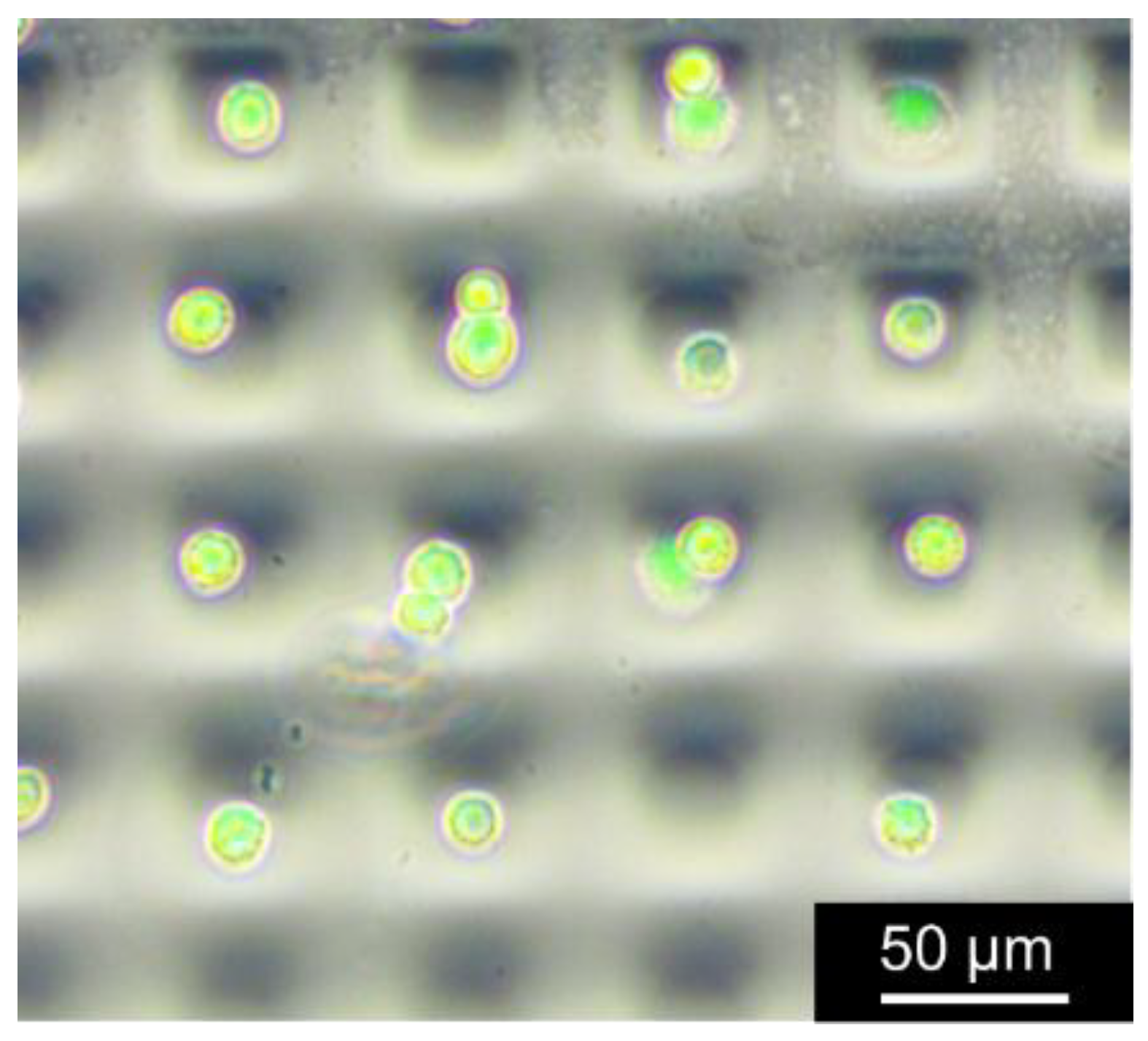
2.4. Long-Lasting Hydrophilic Polydimethylsiloxane (PDMS) Microwell Arrays
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Zhai, M.; Wang, M. (Eds.) Radiation Technology for Advanced Materials: From Basic to Modern Applications; Academic Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Kumar, V.; Chaudhary, B.; Sharma, V.; Verma, K. (Eds.) Radiation Effects in Polymeric Materials; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef] [Green Version]
- Makuuchi, K.; Cheng, S. Radiation Processing of Polymer Materials and Its Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Clough, R.L. High-energy radiation and polymers: A review of commercial processes and emerging applications. Nucl. Instrum. Methods Phys. Res. B 2001, 185, 8–33. [Google Scholar] [CrossRef]
- Drobny, J.G. (Ed.) Ionizing Radiation and Polymers, Principles, Technology and Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Kemmotsu, T.; Okada, M.; Ono, T. Improvement of the surface of polyethylene foamed sheet by irradiation. Radiat. Phys. Chem. 1993, 42, 97–100. [Google Scholar] [CrossRef]
- Cardoso, E.C.L.; Lugão, A.B.; Andrade, E.; Silva, L.G. Crosslinked polyethylene foams, via EB radiation. Radiat. Phys. Chem. 1998, 52, 197–200. [Google Scholar] [CrossRef]
- Chenguang, Y.; Zhe, X.; Mingxing, Z.; Quan, Z.; Mouhua, W.; Guozhong, W. Supercritical CO2 foaming of radiation crosslinked polypropylene/high-density polyethylene blend: Cell structure and tensile property. Radiat. Phys. Chem. 2017, 141, 276–283. [Google Scholar] [CrossRef]
- Ichikawa, H. Development of high performance SiC fibers derived from polycarbosilane using electron beam irradiation curing-A Review. J. Ceram. Soc. Jpn. 2006, 114, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Oshima, A.; Okubo, S.; Tsubokura, H.; Takahashi, T.; Oyama, T.G.; Tagawa, S.; Washio, M. Thermal and radiation process for nano-/micro-fabrication of crosslinked PTFE. Nucl. Instrum. Methods Phys. Res. B. 2013, 295, 76–80. [Google Scholar] [CrossRef]
- Oshima, A.; Tabata, Y.; Kudoh, H.; Seguchi, T. Radiation induced crosslinking of polytetrafluoroethylene. Radiat. Phys. Chem. 1995, 45, 269–273. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, J.; Peng, J.; Li, J.; Zhai, M. One-step radiation synthesis of gel polymer electrolytes with high ionic conductivity for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 12393–12399. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Bahry, T.; Dazzi, A.; Bui, T.-T.; Goubard, F.; Remita, S. Conducting polymers synthesized by γ-radiolysis in very acidic aqueous medium. Radiat. Phys. Chem. 2019, 159, 47–56. [Google Scholar] [CrossRef]
- Shinyama, K. Influence of electron beam irradiation on electrical insulating properties of PLA with soft resin added. Polymers 2018, 10, 898. [Google Scholar] [CrossRef] [Green Version]
- Rosiak, J.M.; Ulański, P.; Pajewski, L.A.; Yoshii, F.; Makuuchi, K. Radiation formation of hydrogels for biomedical purposes. Some remarks and comments. Radiat. Phys. Chem. 1995, 46, 161–168. [Google Scholar] [CrossRef]
- Yoshii, F.; Zhanshan, Y.; Isobe, K.; Shinozaki, K.; Makuuchi, K. Electron beam crosslinked PEO and PEO/PVA hydrogels for wound dressing. Radiat. Phys. Chem. 1999, 55, 133–138. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Dafader, N.C.; Hara, K.; Okabe, H.; Hidaka, Y.; Rahman, M.M.; Khan, M.M.R.; Rahman, N. Synthesis of potato starch-acrylic-acid hydrogels by gamma radiation and their application in dye adsorption. Int. J. Polym. Sci. 2016, 9867859. [Google Scholar] [CrossRef]
- Lugao, A.B.; Malmonge, S.M. Use of radiation in the production of hydrogels. Nucl. Instrum. Methods Phys. Res. B 2001, 185, 37–42. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly (lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Ju, J.; Peng, X.; Huang, K.; Li, L.; Liu, X.; Chitrakar, C.; Changf, L.; Gu, Z.; Kuangab, T. High-performance porous PLLA-based scaffolds for bone tissue engineering: Preparation, characterization, and in vitro and in vivo evaluation. Polymer 2019, 180, 121707. [Google Scholar] [CrossRef]
- Yao, J.; Chen, Y.; Li, W.; Chen, X.; Fan, X. Fabrication and characterization of electrospun PLLA/PANI/TSA fibers. RSC Adv. 2019, 9, 5610–5619. [Google Scholar] [CrossRef] [Green Version]
- Oyama, T.G.; Hinata, T.; Nagasawa, N.; Oshima, A.; Washio, M.; Tagawa, S.; Taguchi, M. Micro/nanofabrication of poly(L-lactic acid) using focused ion beam direct etching. Appl. Phys. Lett. 2013, 103, 163105. [Google Scholar] [CrossRef]
- Miyoshi, N.; Oshima, A.; Urakawa, T.; Fukutake, N.; Nagai, H.; Gowa, T.; Takasawa, Y.; Takahashi, T.; Numata, Y.; Katoh, T.; et al. Nano- and micro-fabrication of perfluorinated polymers using quantum beam technology. Radiat. Phys. Chem. 2011, 80, 230–235. [Google Scholar] [CrossRef]
- Fukutake, N.; Miyoshi, N.; Takasawa, Y.; Urakawa, T.; Gowa, T.; Okamoto, K.; Oshima, A.; Tagawa, S.; Washio, M. Micro- and nano-scale fabrication of fluorinated polymers by direct etching using focused ion beam. Jpn. J. Appl. Phys. 2010, 49, 065201. [Google Scholar] [CrossRef]
- Okubo, S.; Takahashi, T.; Takasawa, Y.; Gowa, T.; Sasaki, T.; Nagasawa, N.; Tamada, M.; Oshima, A.; Tagawa, S.; Washio, M. Micro-fabrication of biodegradable polymers using focused ion beam. J. Photopolym. Sci. Technol. 2010, 23, 393–397. [Google Scholar] [CrossRef] [Green Version]
- Oshima, A.; Okubo, S.; Oyama, T.G.; Washio, M.; Tagawa, S. Nano- and micro-fabrications of polystyrene having atactic and syndiotactic structures using focused ion beams lithography. Radiat. Phys. Chem. 2012, 81, 584–588. [Google Scholar] [CrossRef]
- Oyama, T.G.; Oshima, A.; Washio, M.; Tagawa, S. Fabrication of nanobeads from nanocups by controlling scission/crosslinking in organic polymer materials. Nanotechnology 2012, 23, 495307. [Google Scholar] [CrossRef]
- Ziegler, F.; Ziegler, M.D.; Biersack, J.P. SRIM–The stopping and range of ions in matter. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 1818–1823. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Suzuki, Y.; Tsuchida, K.; Yajima, H. Improvement of cell attachment capabilities of poly-L-lactic acid films by modification of surface properties with ion-beam irradiation. Surf. Coat. Tech. 2013, 218, 162–166. [Google Scholar] [CrossRef]
- van Kan, J.A.; Malar, P.; Wang, Y.H. Resist materials for proton beam writing: A review. Appl. Surf. Sci. 2014, 310, 100–111. [Google Scholar] [CrossRef]
- Jin, H.; Turaga, S.P.; Vanga, S.K.; Bettiol, A.A. Single-mode light guiding in diamond waveguides directly written by a focused proton beam. Opt. Lett. 2018, 43, 2648–2651. [Google Scholar] [CrossRef]
- Kaneko, Y.; Hayashi, H.; Ishii, Y.; Kada, W.; Nishikawa, H. Refractive index change and thermo-optic effect in polydimethylsiloxane nanocomposites with oxide nanoparticles induced by proton beam writing. Nucl. Instrum. Methods B 2019, 459, 94–97. [Google Scholar] [CrossRef]
- Huszank, R.; Rajta, I.; Cserháti, C. Proton beam lithography in negative tone liquid phase PDMS polymer resist. Nucl. Instrum. Methods B 2015, 348, 213–217. [Google Scholar] [CrossRef]
- Huszank, R.; Szilágyi, E.; Szoboszlai, Z.; Szikszaia, Z. Investigation of chemical changes in PMMA induced by 1.6 MeV He+ irradiation by ion beam analytical methods (RBS-ERDA) and infrared spectroscopy (ATR-FTIR). Nucl. Instrum. Methods B 2019, 450, 364–368. [Google Scholar] [CrossRef]
- Huszank, R.; Bonyár, A.; Kámán, J.; Furua, E. Wide range control in the elastic properties of PDMS polymer by ion beam (H+) irradiation. Polym. Degrad. Stab. 2018, 152, 253–258. [Google Scholar] [CrossRef]
- Watt, F.; Breese, M.B.H.; Bettiol, A.A.; van Kan, J.A. Proton beam writing. Mater. Today 2007, 10, 20–29. [Google Scholar] [CrossRef]
- Liu, H.; Jian, R.; Chen, H.; Tian, X.; Sun, C.; Zhu, J. Application of Biodegradable and Biocompatible Nanocomposites in Electronics: Current Status and Future Directions. Nanomaterials 2019, 9, 950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomatter. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, B.; Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of biodegradable cellulose derivatives. I. Radiation-induced crosslinking of CMC. J. Appl. Polym. Sci. 2000, 78, 278–283. [Google Scholar] [CrossRef]
- Wach, R.A.; Mitomo, H.; Nagasawa, N.; Yoshii, F. Radiation crosslinking of carboxymethylcellulose of various degree of substitution at high concentration in aqueous solutions of natural pH. Radiat. Phys. Chem. 2003, 68, 771–779. [Google Scholar] [CrossRef]
- Yoshii, F.; Zhao, L.; Wach, R.A.; Nagasawa, N.; Mitomo, H.; Kume, T. Hydrogels of polysaccharide derivatives crosslinked with irradiation at paste-like condition. Nucl. Instrum. Methods Phys. Res. B 2003, 208, 320–324. [Google Scholar] [CrossRef]
- Nagasawa, N.; Kimura, A.; Idesaki, A.; Yamada, N.; Koka, M.; Satoh, T.; Ishii, Y.; Taguchi, M. Microfabrication of biocompatible hydrogels by proton beam writing. Nucl. Instrum. Methods B 2017, 409, 102–106. [Google Scholar] [CrossRef]
- Miyoshi, H.; Adachi, T. Topography design concept of a tissue engineering scaffold for controlling cell function and fate through actin cytoskeletal modulation. Tissue Eng. Part B Rev. 2014, 20, 609–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, V.; Samadian, H.; Jaymand, M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haema, K.; Oyama, T.G.; Kimura, A.; Taguchi, M. Radiation stability and modification of gelatin for biological and medical applications. Radiat. Phys. Chem. 2014, 103, 126–130. [Google Scholar] [CrossRef]
- Riedel, S.; Bela, K.; Wisotzki, E.I.; Suckfüll, C.; Zajadacz, J.; Mayr, S.G. Reagent-free mechanical patterning of gelatin surfaces by two-step electron irradiation treatment. Mater. Des. 2018, 153, 80–85. [Google Scholar] [CrossRef]
- Tadsen, M.; Friedrich, R.P.; Riedel, S.; Alexiou, C.; Mayr, S.G. Contact guidance by microstructured gelatin hydrogels for prospective tissue engineering applications. ACS Appl. Mater. Interfaces 2019, 11, 7450–7458. [Google Scholar] [CrossRef]
- Riedel, S.; Hietschold, P.; Krömmelbein, C.; Kunschmann, T.; Konieczny, R.; Knolle, W.; Mierke, C.T.; Zink, M.; Mayr, S.G. Design of biomimetic collagen matrices by reagent-free electron beam induced crosslinking: Structure-property relationships and cellular response. Mater. Des. 2019, 168, 107606. [Google Scholar] [CrossRef]
- Oyama, T.G.; Oyama, K.; Kimura, A.; Yoshida, F.; Ishida, R.; Yamazaki, M.; Miyoshi, H.; Taguchi, M. Elasticity and topography-controlled collagen hydrogels mimicking native cellular milieus. BioRXiv 2019, 706962. [Google Scholar] [CrossRef] [Green Version]
- Piruska, A.; Nikcevic, I.; Lee, S.H.; Ahn, C.; Heineman, W.R.; Limbach, P.A.; Seliskar, C.J. The autofluorescence of plastic materials and chips measured under laser irradiation. Lab Chip 2005, 5, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Khodakov, D.A.; Ellis, A.V.; Voelcker, N.H. Surface modification for PDMS-based microfluidic devices. Electrophoresis 2012, 33, 89–104. [Google Scholar] [CrossRef]
- Tóth, A.; Bertóti, I.; Blazsó, M.; Bánhegyi, G.; Bognar, A.; Szaplonczay, P. Oxidative damage and recovery of silicone rubber surfaces. I. X-ray photoelectron spectroscopic study. J. Appl. Polym. Sci. 1994, 52, 1293–1307. [Google Scholar] [CrossRef]
- Hillborg, H.; Ankner, J.F.; Gedde, U.W.; Smith, G.D.; Yasuda, H.K.; Wikström, K. Crosslinked polydimethylsiloxane exposed to oxygen plasma studied by neutron reflectometry and other surface specific techniques. Polymer 2000, 41, 6851–6863. [Google Scholar] [CrossRef]
- Kim, J.; Chaudhury, M.K.; Owen, M.J.; Orbeck, T. The mechanisms of hydrophobic recovery of polydimethylsiloxane elastomers exposed to partial electrical discharges. J. Colloid. Interface Sci. 2001, 244, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Hillborg, H.; Sandelin, M.; Gedde, U.W. Hydrophobic recovery of polydimethylsiloxane after exposure to partial discharges as a function of crosslink density. Polymer 2001, 42, 7349–7362. [Google Scholar] [CrossRef]
- Oyama, T.G.; Barba, B.J.D.; Hosaka, Y.; Taguchi, M. Single-step fabrication of polydimethylsiloxane microwell arrays with long-lasting hydrophilic inner surfaces. Appl. Phys. Lett. 2018, 112, 213704. [Google Scholar] [CrossRef]
- Kamiya, T.; Suda, T.; Tanaka, R. Submicron microbeam apparatus using a single-ended accelerator with very high voltage stability. Nucl. Instrum. Methods Phys. Res. B 1995, 104, 43–48. [Google Scholar] [CrossRef]
- Niita, K.; Sato, T.; Iwase, H.; Nose, H.; Nakashima, H.; Sihver, L. Particle and heavy ion transport code system; PHITS. Radiat. Meas. 2006, 41, 1080–1090. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyama, T.G.; Kimura, A.; Nagasawa, N.; Oyama, K.; Taguchi, M. Development of Advanced Biodevices Using Quantum Beam Microfabrication Technology. Quantum Beam Sci. 2020, 4, 14. https://doi.org/10.3390/qubs4010014
Oyama TG, Kimura A, Nagasawa N, Oyama K, Taguchi M. Development of Advanced Biodevices Using Quantum Beam Microfabrication Technology. Quantum Beam Science. 2020; 4(1):14. https://doi.org/10.3390/qubs4010014
Chicago/Turabian StyleOyama, Tomoko G., Atsushi Kimura, Naotsugu Nagasawa, Kotaro Oyama, and Mitsumasa Taguchi. 2020. "Development of Advanced Biodevices Using Quantum Beam Microfabrication Technology" Quantum Beam Science 4, no. 1: 14. https://doi.org/10.3390/qubs4010014
APA StyleOyama, T. G., Kimura, A., Nagasawa, N., Oyama, K., & Taguchi, M. (2020). Development of Advanced Biodevices Using Quantum Beam Microfabrication Technology. Quantum Beam Science, 4(1), 14. https://doi.org/10.3390/qubs4010014





