Optimization of DD-110 Neutron Generator Output for Boron Neutron Capture Therapy Using Monte Carlo Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. DD-110 Neutron Generator
2.2. Beam-Shaping Assembly
2.3. Clinical Parameters and Dose Calculation
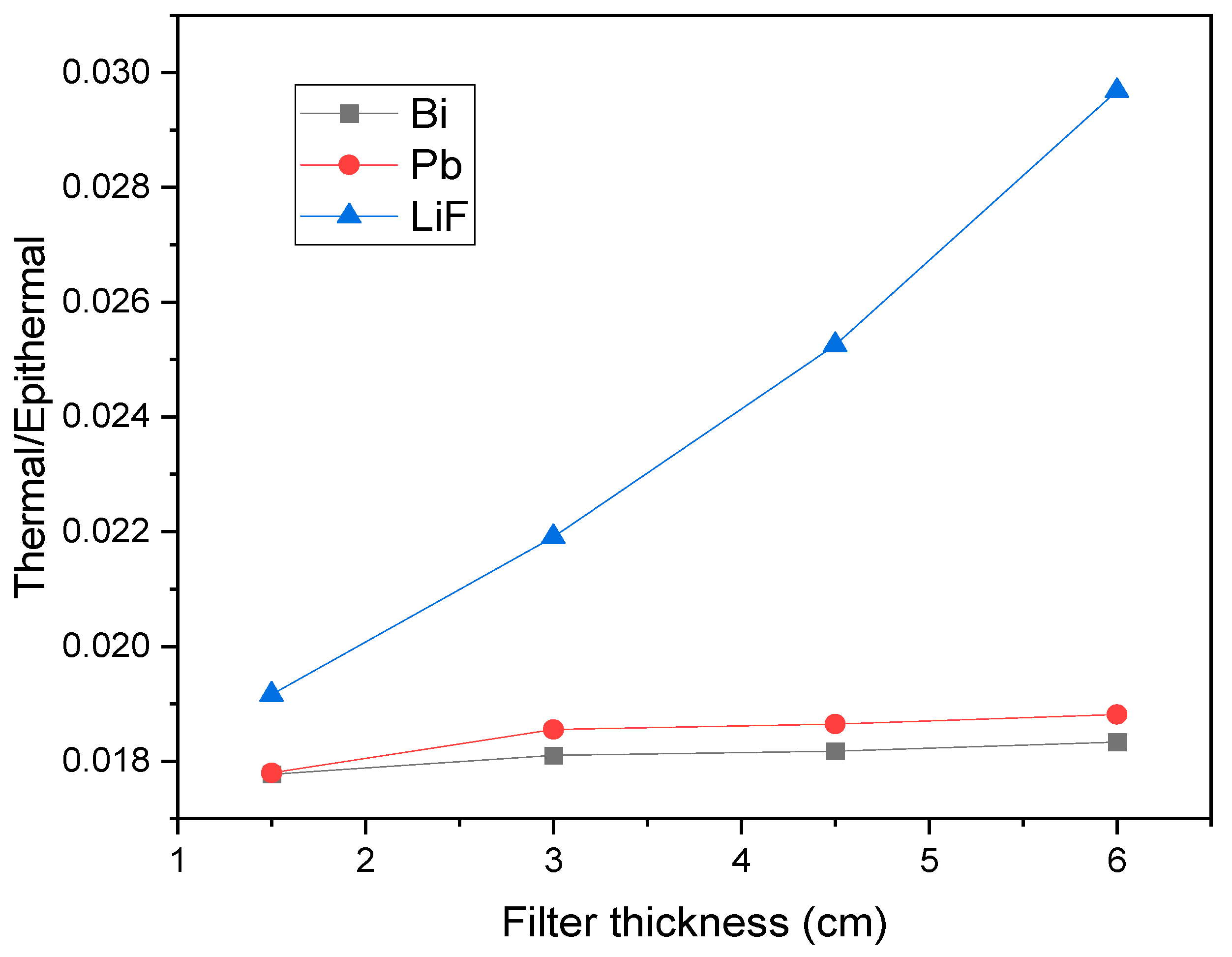
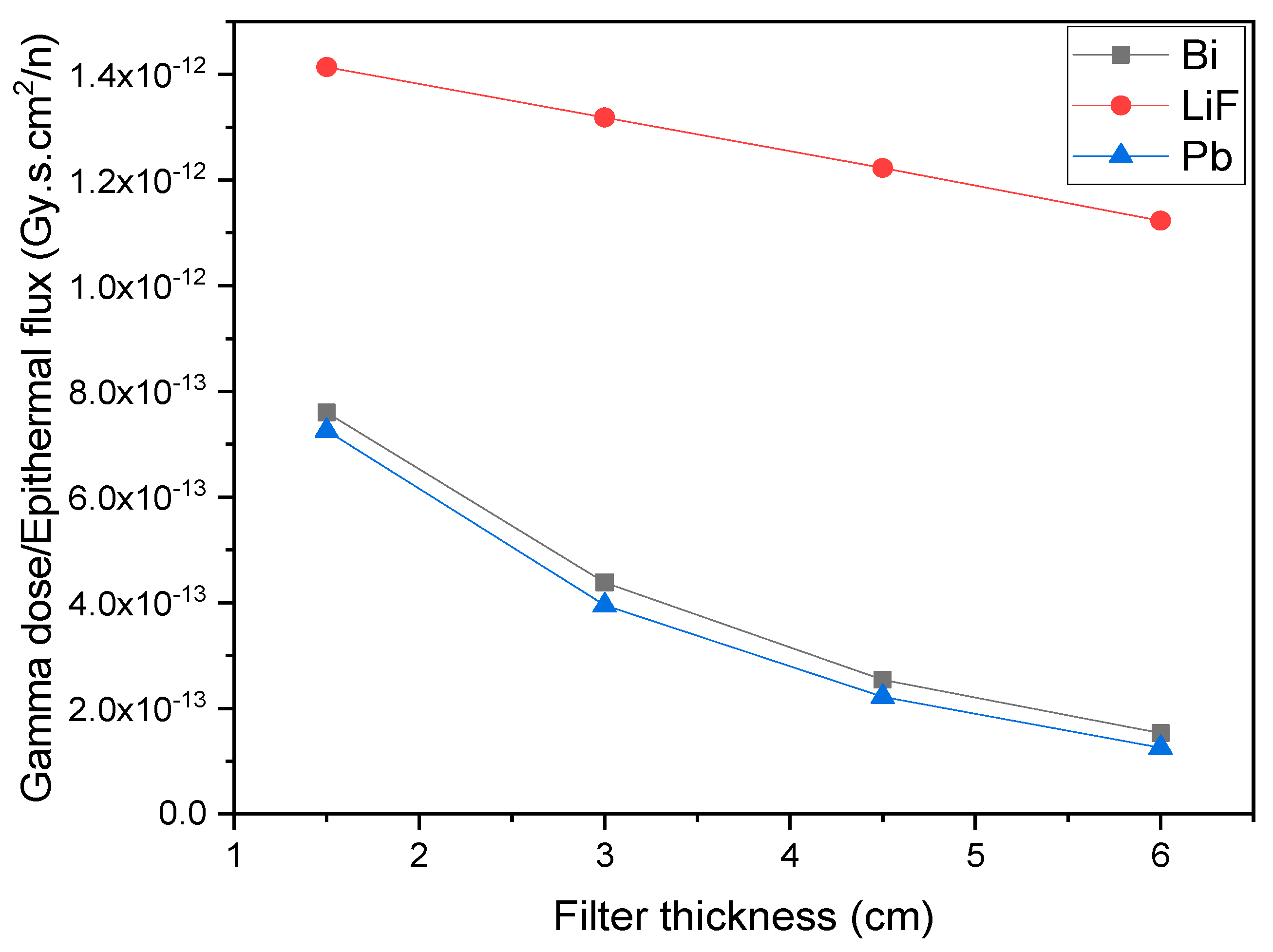
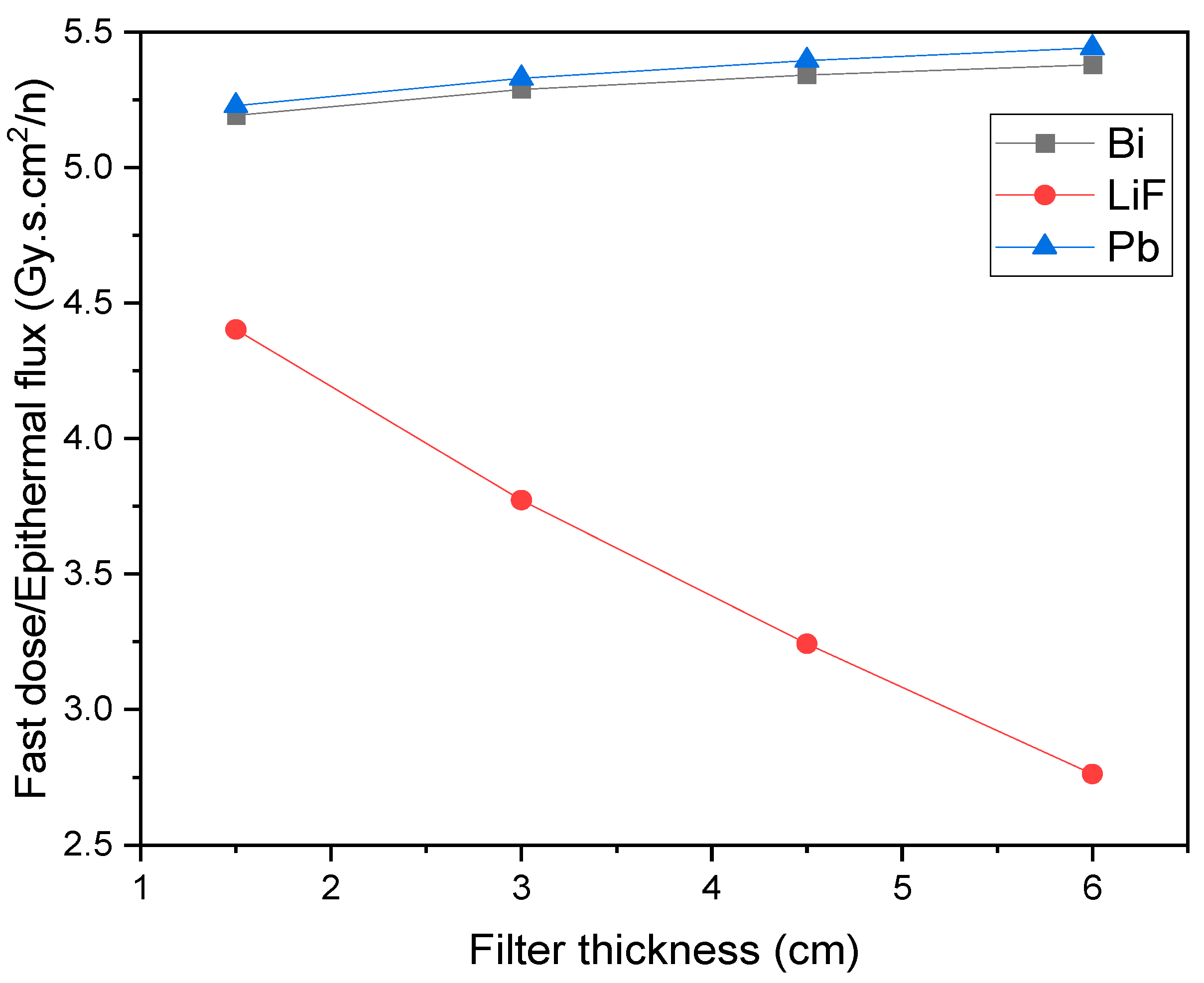
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sathishkumar, K.; Chaturvedi, M.; Das, P.; Stephen, S.; Mathur, P. Cancer incidence estimates for 2022 & projection for 2025: Result from National Cancer Registry Programme, India. Indian J. Med. Res. 2022, 156, 598–607. [Google Scholar] [PubMed]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and other primary brain malignancies in adults: A review. Jama 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Alsharif, A.; Alsharif, M.T.; Samman, M.; Binmadi, N.; Kassim, S.; Mourad, S.; Warnakulasuriya, S. Forecasting Head and Neck Cancer Trends in GCC Countries: Implications for Public Health Policy and Strategy. Risk Manag. Healthc. Policy 2023, 16, 2943–2952. [Google Scholar] [CrossRef]
- Meram, A.T.; Woo, B.M. Oral Cancer Management. In Peterson’s Principles of Oral and Maxillofacial Surgery; Springer: Cham, Switzerland, 2022; pp. 1009–1055. [Google Scholar]
- Matsumoto, Y.; Fukumitsu, N.; Ishikawa, H.; Nakai, K.; Sakurai, H. A critical review of radiation therapy: From particle beam therapy (proton, carbon, and BNCT) to beyond. J. Pers. Med. 2021, 11, 825. [Google Scholar] [CrossRef]
- Kanno, H.; Nagata, H.; Ishiguro, A.; Tsuzuranuki, S.; Nakano, S.; Nonaka, T.; Kiyohara, K.; Kimura, T.; Sugawara, A.; Okazaki, Y.; et al. Designation products: Boron neutron capture therapy for head and neck carcinoma. Oncologist 2021, 26, e1250–e1255. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Song, Z.; Zhou, Y.; Liu, T.; Zhang, Z.; Zhao, Y.; Chen, Y.; Jin, C.; Chen, X.; Lu, J.; et al. Boron neutron capture therapy for malignant melanoma: First clinical case report in China. Chin. J. Cancer Res. 2016, 28, 634. [Google Scholar] [CrossRef]
- Coderre, J.A.; Morris, G.M. The radiation biology of boron neutron capture therapy. Radiat. Res. 1999, 151, 1–18. [Google Scholar] [CrossRef]
- He, H.; Li, J.; Jiang, P.; Tian, S.; Wang, H.; Fan, R.; Liu, J.; Yang, Y.; Liu, Z.; Wang, J. The basis and advances in clinical application of boron neutron capture therapy. Radiat. Oncol. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Karaoglu, A.; Arce, P.; Obradors, D.; Lagares, J.I.; Unak, P. Calculation by GAMOS/Geant4 simulation of cellular energy distributions from alpha and lithium-7 particles created by BNCT. Appl. Radiat. Isot. 2018, 132, 206–211. [Google Scholar] [CrossRef]
- Valentin, J. Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (wR): ICRP Publication 92. Ann. ICRP 2003, 33, 1–121. [Google Scholar]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron neutron capture therapy: A review of clinical applications. Front. Oncol. 2021, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Locher, G.L. Biological effects and therapeutic possibilities of neutrons. Am. J. Roentgenol. 1936, 36, 1–13. [Google Scholar]
- Taylor, H.; Goldhaber, M. Detection of nuclear disintegration in a photographic emulsion. Nature 1935, 135, 341. [Google Scholar] [CrossRef]
- Jin, W.H.; Seldon, C.; Butkus, M.; Sauerwein, W.; Giap, H.B. A review of boron neutron capture therapy: Its history and current challenges. Int. J. Part. Ther. 2022, 9, 71–82. [Google Scholar] [CrossRef]
- Lamba, M.; Goswami, A.; Bandyopadhyay, A. A periodic development of BPA and BSH based derivatives in boron neutron capture therapy (BNCT). Chem. Commun. 2021, 57, 827–839. [Google Scholar] [CrossRef]
- Sakurai, Y.; Tanaka, H.; Takata, T.; Fujimoto, N.; Suzuki, M.; Masunaga, S.; Kinashi, Y.; Kondo, N.; Narabayashi, M.; Nakagawa, Y.; et al. Advances in boron neutron capture therapy (BNCT) at kyoto university-From reactor-based BNCT to accelerator-based BNCT. J. Korean Phys. Soc. 2015, 67, 76–81. [Google Scholar] [CrossRef]
- Ahmed, M.; Bedogni, R.; Chen, Y.; Dagrosa, A.; Green, S. Advances in Boron Neutron Capture Therapy; International Atomic Energy Agency: Vienna, Austria, 2023. [Google Scholar]
- Donya, H.; Seniwal, B.; Darwesh, R.; Fonseca, T.C.J.T. Prospective Monte Carlo simulation for choosing high efficient detectors for small-field dosimetry. In Theory, Application, and Implementation of Monte Carlo Method in Science and Technology; IntechOpen: Rijeka, Croatia, 2019; p. 39. [Google Scholar]
- Donya, H. Pencil-beam fluence evaluation based on Monte Carlo simulations algorithm of high energetic treatment photons. J. Med. Signals Sens. 2018, 8, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Alem-Bezoubiri, A.; Bezoubiri, F.; Speiser, M.; Suleiman, S.A.; Donya, H.; Chami, A.C. Monte Carlo study of organ doses and related secondary cancer risk estimations for patients undergoing prostate radiotherapy: Algerian Population-Based Study. Appl. Radiat. Isot. 2024, 216, 111595. [Google Scholar] [CrossRef]
- Robalino, L.E.C.; Fernández, G.F.G.; Gallego, E.; Guzmán-García, K.A.; Vega-Carrillo, H.R. Study by Monte Carlo methods of an explosives detection system made up with a DD neutron generator and NaI (Tl) gamma detectors. Appl. Radiat. Isot. 2018, 141, 167–175. [Google Scholar]
- Rinard, P. Neutron interactions with matter. In Passiv. Nondestruct. Assay Nucl. Mater.; Reilly, D., Ensslin, N., Smith, H., Eds.; Los Alamos National Laboratory Report LA-UR-90-732; Los Alamos National Laboratory: Los Alamos, NM, USA, 1991; pp. 375–377. [Google Scholar]
- Obayashi, S.; Kato, I.; Ono, K.; Masunaga, S.-I.; Suzuki, M.; Nagata, K.; Sakurai, Y.; Yura, Y. Delivery of 10boron to oral squamous cell carcinoma using boronophenylalanine and borocaptate sodium for boron neutron capture therapy. Oral Oncol. 2004, 40, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.; Liu, Y.; Mostafaei, F.; Poulson, J.M.; Nie, L.H. A feasibility study of a deuterium–deuterium neutron generator-based boron neutron capture therapy system for treatment of brain tumors. Med. Phys. 2017, 44, 637–643. [Google Scholar] [CrossRef] [PubMed]
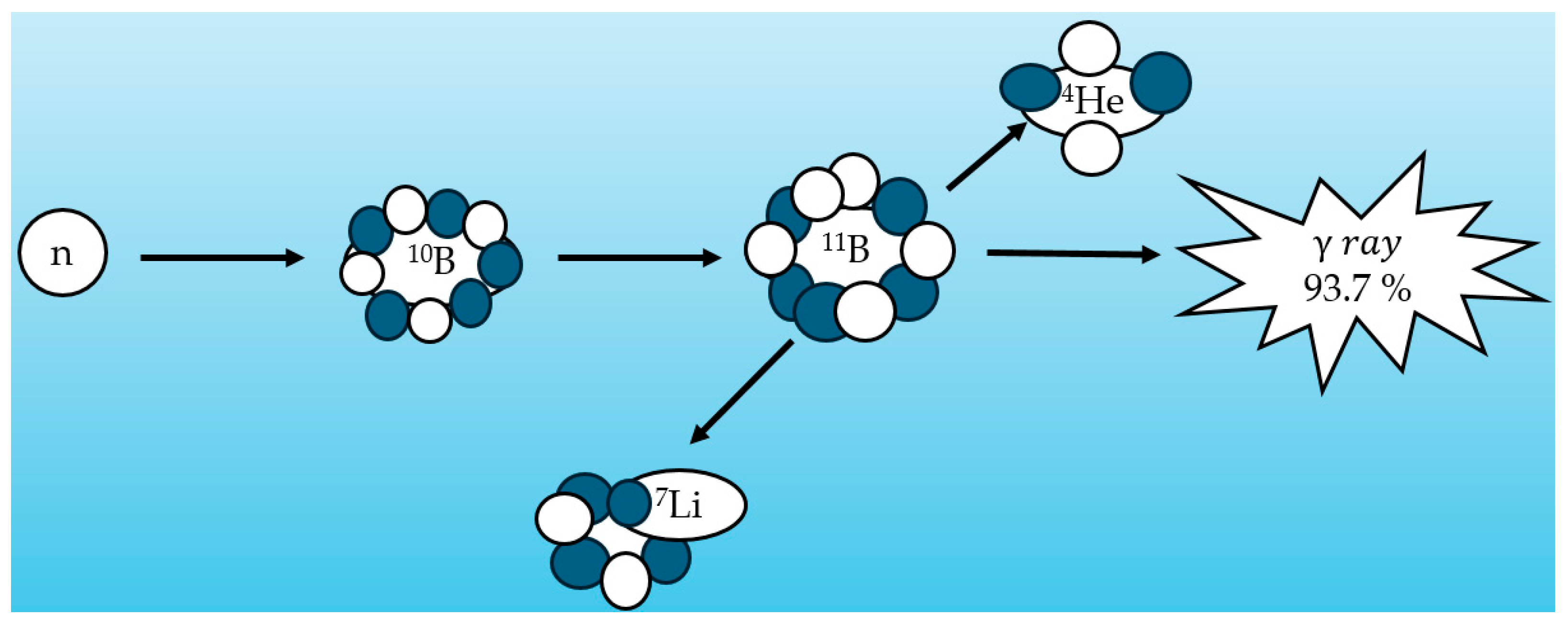
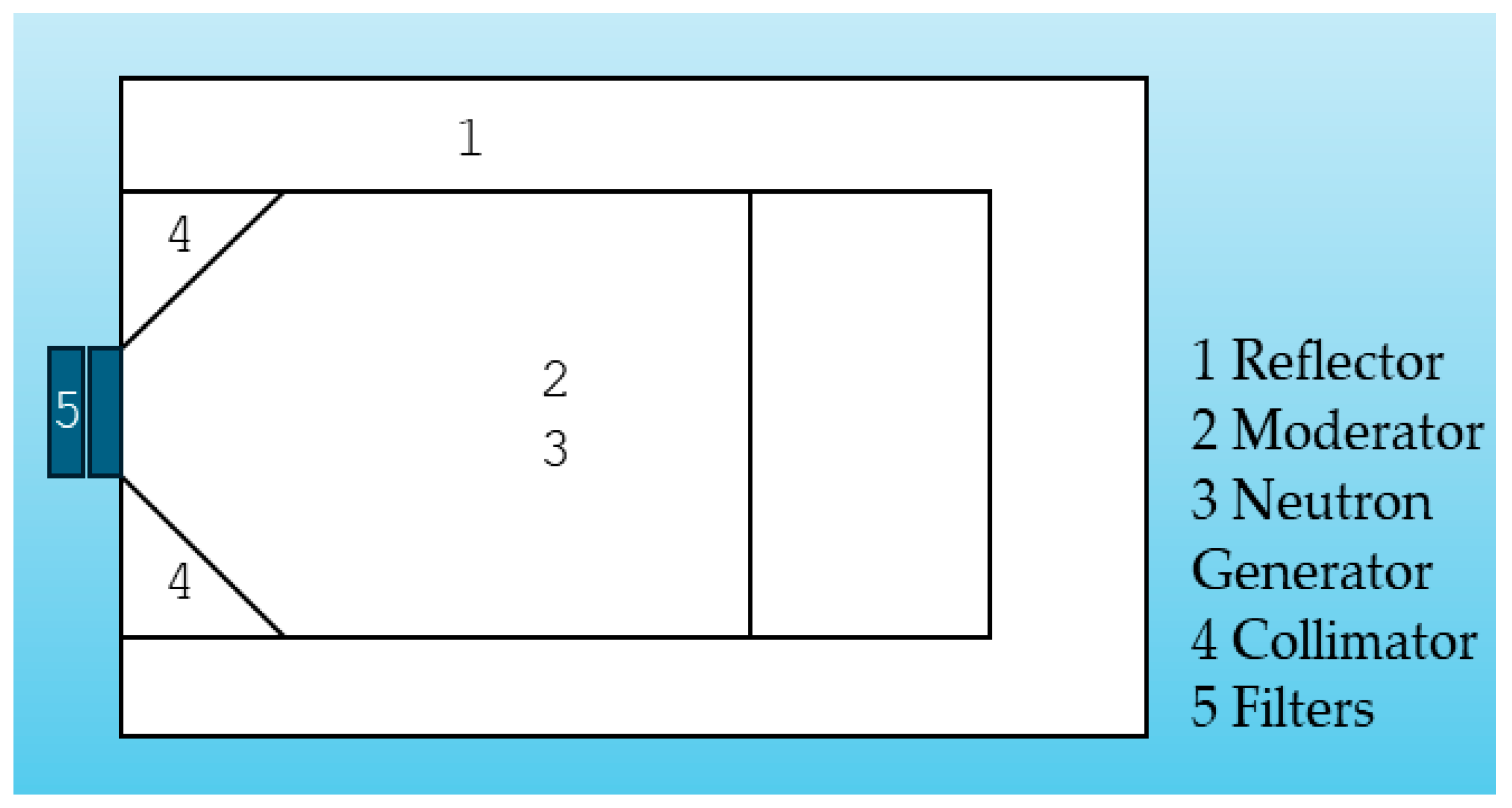

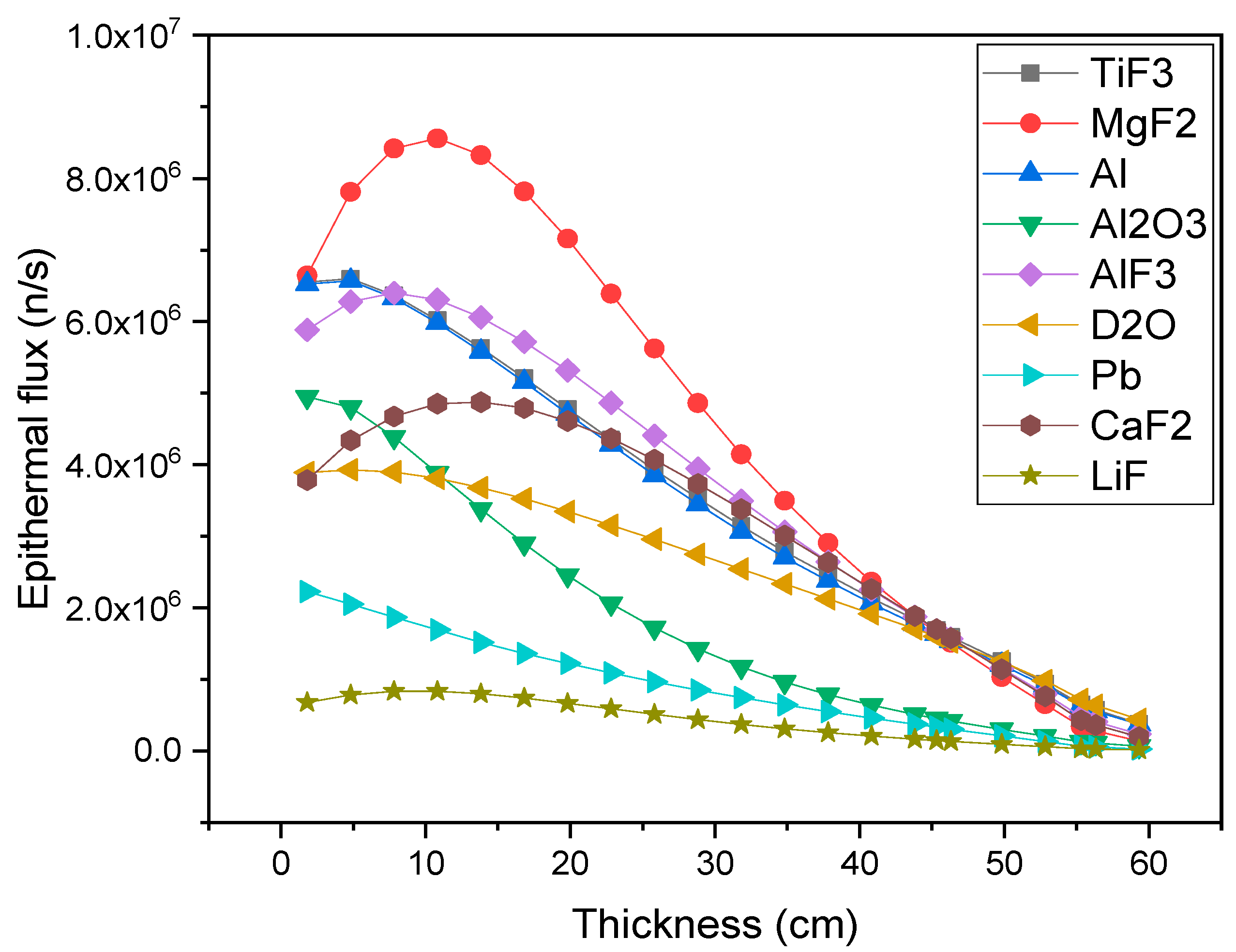
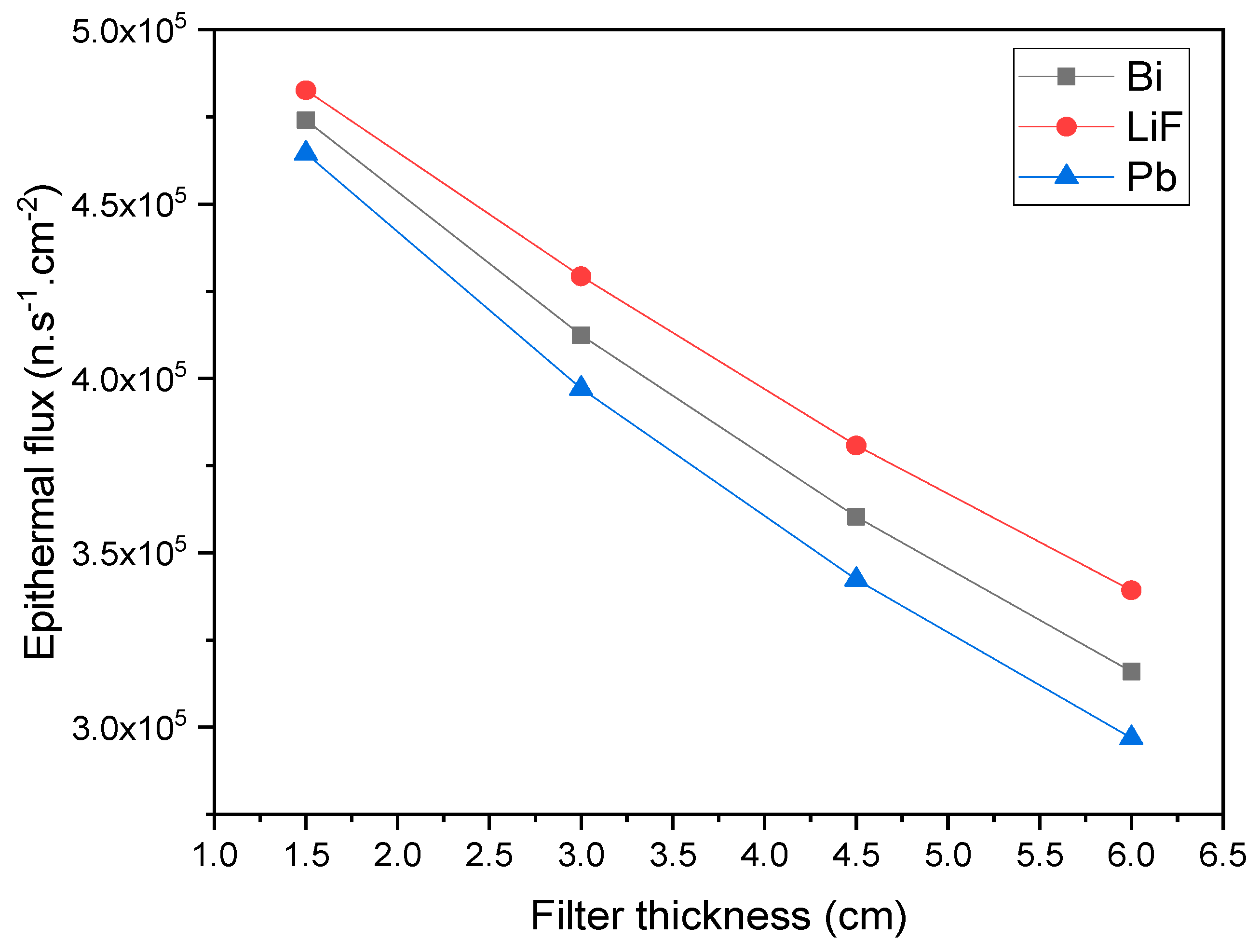


| Neutron Type | Energy Range |
|---|---|
| Thermal neutron | <0.5 eV |
| Epithermal neutron | 1eV–10 keV |
| Fast neutron | >10 keV |
| Parameter | Recommended Value |
|---|---|
| Thermal neutron | <0.5 eV |
| ) | ≥5 × 108 nepith.cm−2s−1 |
| ) | ≤0.05 |
| ) | ≥0.7 |
| ) | ≤2 × 10−13 Gy.cm2 |
| ) | ≤2 × 10−13 Gy.cm2 |
| ) | ≥5 × 108 nepith.cm−2s−1 |
| ) | ≤0.05 |
| ) | ≥0.7 |
| ) | ≤7 × 10−13 Gy.cm2 |
| ) | ≤2 × 10−13 Gy.cm2 |
| (Normal Tissue) | (Tumor) | |||
|---|---|---|---|---|
| 1.35 | 3.8 | 3 | 3 | 1 |
| Pb (cm) | LiF (cm) | Bi (cm) | nepith.cm−2s−1 | (Gy.cm2) | (Gy.cm2) | |
|---|---|---|---|---|---|---|
| 0.52 | 0 | 0.5 | 6.01 × 105 | 1.79 × 10−2 | 4.17 × 10−12 | 6.71 × 10−13 |
| 1.52 | 0 | 0.52 | 5.46 × 105 | 1.86 × 10−2 | 4.19 × 10−12 | 4.46 × 10−13 |
| 3 | 0 | 1.52 | 4.36 × 105 | 1.88 × 10−2 | 4.19 × 10−12 | 1.71 × 10−13 |
| 3 | 1.52 | 0 | 4.72 × 105 | 2.41 × 10−2 | 3.87 × 10−12 | 4.82 × 10−13 |
| Generator Model | (nepith.cm−2s−1) | (Gy.cm2) | (Gy.cm2) | |
|---|---|---|---|---|
| DD-109 [26] | 1 × 105 | 0.05 | 5.5 × 10−13 | 2.49 × 10−13 |
| DD-110 (proposed generator) | 4.36 × 105 | 0.02 | 4.19 × 10−12 | 1.71 × 10−13 |
| IAEA recommendations | ≥5 × 108 | ≤0.05 | ≤2 × 10−13 | ≤2 × 10−13 |
| Generator Model | AD (cm) | AR | ADDR (Gy.Eq/min) | TT (min) |
|---|---|---|---|---|
| DD-109 [26] | 12.1 | 3.7 | 0.31 | 40 |
| DD-110 (proposed generator) | 5.5 | 2.29 | 0.47 | 30 |
| IAEA recommendations | No recommended value | ≤60 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donya, H.; Umer, M. Optimization of DD-110 Neutron Generator Output for Boron Neutron Capture Therapy Using Monte Carlo Simulation. Quantum Beam Sci. 2025, 9, 12. https://doi.org/10.3390/qubs9020012
Donya H, Umer M. Optimization of DD-110 Neutron Generator Output for Boron Neutron Capture Therapy Using Monte Carlo Simulation. Quantum Beam Science. 2025; 9(2):12. https://doi.org/10.3390/qubs9020012
Chicago/Turabian StyleDonya, Hossam, and Muhammed Umer. 2025. "Optimization of DD-110 Neutron Generator Output for Boron Neutron Capture Therapy Using Monte Carlo Simulation" Quantum Beam Science 9, no. 2: 12. https://doi.org/10.3390/qubs9020012
APA StyleDonya, H., & Umer, M. (2025). Optimization of DD-110 Neutron Generator Output for Boron Neutron Capture Therapy Using Monte Carlo Simulation. Quantum Beam Science, 9(2), 12. https://doi.org/10.3390/qubs9020012








