How Could Nanomedicine Improve the Safety of Contrast Agents for MRI during Pregnancy?
Abstract
:1. Introduction
2. Magnetic Resonance Imaging on Pregnant Women: Clinical Indications and Safety Considerations
2.1. Indications for MRI during Pregnancy
2.1.1. Non-Obstetric Indications
2.1.2. Obstetric and Gynecological Indications
- Placenta accreta, in which placental villi adhere to the myometrium.
- Placenta increta, where placental villi invade the myometrium.
- Placenta percreta, in which the myometrium is entirely penetrated by the placental villi, that extend even deeper in other uterine tissues [24].
2.1.3. Fetal Indications
2.2. Safety Issues of Non-Contrast-Enhanced MRI during Pregnancy
- It is not prudent to delay the examination until the completion of pregnancy;
- The information needed cannot be obtained through other non-ionizing techniques;
- The data gained may influence the management of the patient or the fetus.
2.2.1. Static Magnetic Fields and Teratogenicity
2.2.2. Gradient Fields and Hearing Damage
2.2.3. Radiofrequency Pulses and Tissue Heating
2.3. Safety Issues of Contrast-Enhanced MRI during Pregnancy
2.3.1. Safety of Contrast Agents Containing Gadolinium
Teratogenicity
Nephrogenic Systemic Fibrosis
Bioaccumulation
3. Nanostructures as Potential MRI Contrast Agents
3.1. Advantages of Nanostructured MRI Contrast Agents over Conventional Molecular Chelates
3.2. Nanoparticles’ Characteristics Affecting Contrast Agent Efficiency
3.2.1. Size
3.2.2. Surface Properties
- Low thickness.
- High hydration.
- Fast water exchange rate.
- Presence of π-electrons.
- Stability and biocompatibility in biologically relevant media [7].
3.3. Examples of Nanostructured MRI Contrast Agents
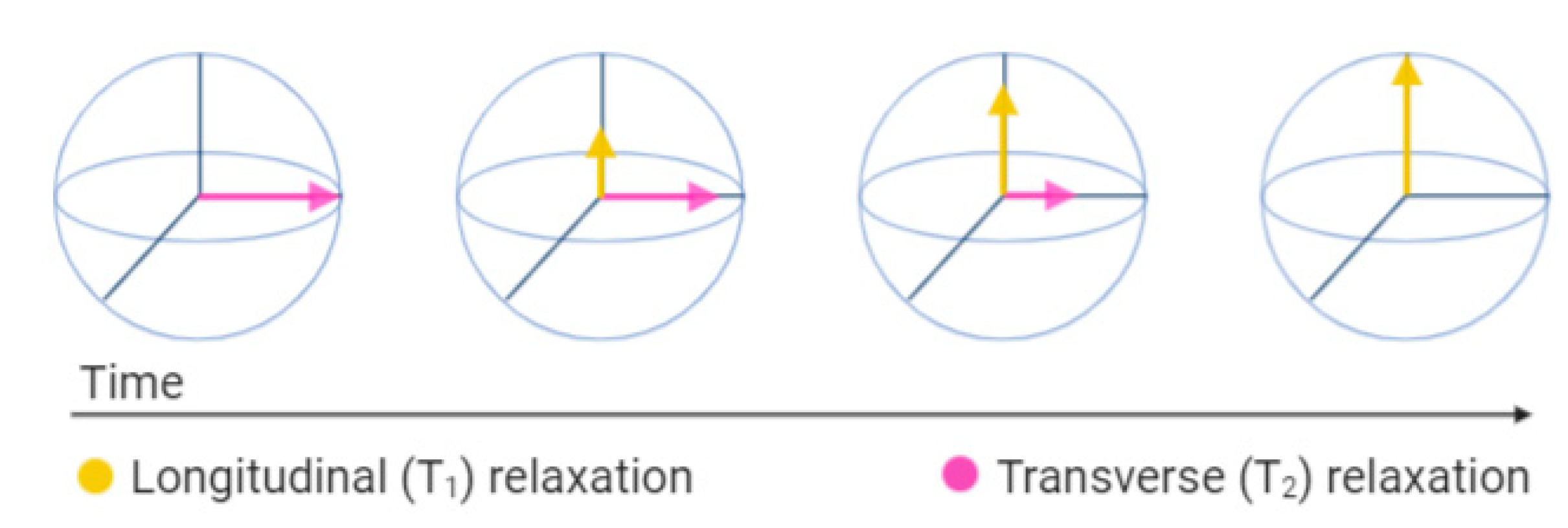
3.3.1. T1 Contrast Agents
Gadolinium
Manganese
| Type of CA | Nanostructured CA | Composition | Mean Diameter (nm) | Magnetic Field (T) | Relaxivity (mM−1·s−1) | Ref. |
|---|---|---|---|---|---|---|
| T1 | Gd-liposomes | DSPG/Gd-DOTA-DSPE/DSPG-PEG-Succinyl/Cholesterol | 156.0 | 14 | 12.3 | [81] |
| Gd-MSNs | Gd-DOTA in porous silica NPs | 138.9 | 3 | 39.3 | [82] | |
| Gd2O3-GO sheets | Gd2O3-decorated GO | 268.9 | 7 | 34.5 | [78] | |
| GlcA-Gd2O3 | (D-glucuronic acid)-coated Gd2O3 | 4.0 | 1.5 | 9.9 | [85] | |
| Mn-polymeric NPs | PEG-coated, Mn-containing, DOPA reverse micelles | 75.8 | 3 | 11.6 | [84] | |
| Mn-ferrite cubes | Mn-doped iron oxide | 16.4 | 0.5 | 57.8 | [83] | |
| USPIONs | PEG-coated iron oxide | 10.0 | 1.4 | 7.3 | [86] | |
| T2 | MLPs | DOPC/Cholesterol/DSPE-PEG2000 MLPs | 156.0 | 9.4 | 575 | [45] |
| UMLs | DPPC/DSPC/DSPE-PEG2000 MLPs | 230.0 | 7 | 225 | [87] | |
| Zn-ferrite | (Zn0.4Fe0.6) Fe2O4 | 15.0 | 4.5 | 687.0 | [88] | |
| Mn-ferrite | PEGylated Mn-Fe2O4 | 68.8 | 1.5 | 217.1 | [89] | |
| SWCNT· | Cylindrical (IO-NPs)-conjugated CNTs | 4–5 (IO-NPs d.) 200–300 (CNTs l.) | 4.7 | 231.3 | [90] | |
| HoF3 NPs | Rhomboidal HoF3 | 110 × 50 | 9.4 | 608.4 | [91] | |
| DyF3 NPs | Rhomboidal DyF3 | 110 × 50 | 9.4 | 380.4 | [91] | |
| T1-T2 | MnFe2O4@SiO2@Gd2O(CO3)2 NPs | Mn-ferrite-silica-Gd2O(CO3)2 (20 nm silica) | 36.5 | 4.7 | r1 = 33.1 r2 = 213 | [72] |
| Gadolinium and Dysprosium oxide NPs | (D-glucuronic acid)-coated (Gd-Dy) oxide | 1.0 | 1.5 | r1 = 6.0 r2 = 40.0 | [92] |
Iron
3.3.2. T2 Contrast Agents
SPIONs and USPIONs
Ferrites
Dysprosium and Holmium Derivates
3.3.3. Dual-Mode T1-T2 Contrast Agents
4. Potential of Nanostructured Contrasts to Improve the Safety of CE-MRI in Pregnancy
4.1. Possible Advantages of Nanostructured Contrast Agents for MRI on Pregnant Women
4.2. Examples of Evaluation of Potential Nanostructured Contrast Agents on Pregnant Animal Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith-Bindman, R.; Kwan, M.L.; Marlow, E.C.; Theis, M.K.; Bolch, W.; Cheng, S.Y.; Bowles, E.J.A.; Duncan, J.R.; Greenlee, R.T.; Kushi, L.H.; et al. Trends in Use of Medical Imaging in US Health Care Systems and in Ontario, Canada, 2000–2016. JAMA-J. Am. Med. Assoc. 2019, 322, 843–856. [Google Scholar] [CrossRef]
- ACOG. ACOG Guidelines for Diagnostic Imaging During Pregnancy and Lactation; ACOG: Washington, DC, USA, 2017. [Google Scholar]
- Bourgioti, C.; Konidari, M.; Gourtsoyianni, S.; Moulopoulos, L.A. Imaging during pregnancy: What the radiologist needs to know. Diagn. Interv. Imaging 2021, 102, 593–603. [Google Scholar] [CrossRef]
- Tirada, N.; Dreizin, D.; Khati, N.J.; Akin, E.A.; Zeman, R.K. Imaging pregnant and lactating patients. Radiographics 2015, 35, 1751–1765. [Google Scholar] [CrossRef]
- Eastwood, K.; Mohan, A.R. Imaging in pregnancy. Obstet. Gynaecol. 2019, 21, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Marasini, R.; Thanh Nguyen, T.D.; Aryal, S. Integration of gadolinium in nanostructure for contrast enhanced-magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1580. [Google Scholar] [CrossRef]
- Kostevšek, N. A Review on the Optimal Design of Magnetic Nanoparticle-Based T2 MRI Contrast Agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Pellico, J.; Ellis, C.M.; Davis, J.J. Nanoparticle-Based Paramagnetic Contrast Agents for Magnetic Resonance Imaging. Contrast Media Mol. Imaging 2019, 2019, 1845637. [Google Scholar] [CrossRef]
- Ramdass, S.; Adam, S.; Lockhat, Z.; Masenge, A.; Suleman, F.E. Foetal magnetic resonance imaging: A necessity or adjunct? A modality comparison of in-utero ultrasound and ultrafast foetal magnetic resonance imaging. S. Afr. J. Radiol. 2021, 25, 6. [Google Scholar] [CrossRef]
- Ladd, M.E.; Bachert, P.; Meyerspeer, M.; Moser, E.; Nagel, A.M.; Norris, D.G.; Schmitter, S.; Speck, O.; Straub, S.; Zaiss, M. Pros and cons of ultra-high-field MRI/MRS for human application. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 109, 1–50. [Google Scholar] [CrossRef]
- Smith, F.; Adam, A.; Philipps, W. NMR Imaging in Pregnancy. Lancet 1983, 321, 61–62. [Google Scholar] [CrossRef]
- Lum, M.; Tsiouris, A.J. MRI safety considerations during pregnancy. Clin. Imaging 2020, 62, 69–75. [Google Scholar] [CrossRef]
- Mervak, B.M.; Altun, E.; McGinty, K.A.; Hyslop, W.B.; Semelka, R.C.; Burke, L.M. MRI in pregnancy: Indications and practical considerations. J. Magn. Reson. Imaging 2019, 49, 621–631. [Google Scholar] [CrossRef]
- Lim, P.S.; Mackey, A.M.; Huang, M.L. MRI of the Placenta and the Pregnant Patient. In Imaging of the Pelvis, Musculoskeletal System, and Special Applications to CAD; Saba, L., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 145–160. ISBN 978-1-4822-1622-6. [Google Scholar]
- Furey, E.A.; Bailey, A.A.; Pedrosa, I. Magnetic Resonance Imaging of Acute Abdominal and Pelvic Pain in Pregnancy. Top. Magn. Reson. Imaging 2014, 23, 225–242. [Google Scholar] [CrossRef]
- Horowitz, J.M.; Hotalen, I.M.; Miller, E.S.; Barber, E.L.; Shahabi, S.; Miller, F.H. How Can Pelvic MRI with Diffusion-Weighted Imaging Help My Pregnant Patient? Am. J. Perinatol. 2020, 37, 577–588. [Google Scholar] [CrossRef]
- Kave, M.; Parooie, F.; Salarzaei, M. Pregnancy and appendicitis: A systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J. Emerg. Surg. 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Gui, B.; Cambi, F.; Micco, M.; Sbarra, M.; Petta, F.; Autorino, R.; De Vincenzo, R.; Valentini, V.; Scambia, G.; Manfredi, R. MRI in pregnant patients with suspected abdominal and pelvic cancer: A practical guide for radiologists. Diagn. Interv. Radiol. 2020, 26, 183–192. [Google Scholar] [CrossRef]
- Vandecaveye, V.; Amant, F.; Lecouvet, F.; Van Calsteren, K.; Dresen, R.C. Imaging modalities in pregnant cancer patients. Int. J. Gynecol. Cancer 2021, 31, 423–431. [Google Scholar] [CrossRef]
- Ishiguro, T.; Nishikawa, N.; Ishii, S.; Yoshihara, K.; Haino, K.; Yamaguchi, M.; Adachi, S.; Watanabe, T.; Soeda, S.; Enomoto, T. PET/MR imaging for the evaluation of cervical cancer during pregnancy. BMC Pregnancy Childbirth 2021, 21, 288. [Google Scholar] [CrossRef]
- Masselli, G. (Ed.) MRI of Fetal and Maternal Diseases in Pregnancy; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-21428-3. [Google Scholar]
- Windram, J.; Grewal, J. Cardiovascular Imaging and Pregnancy. Can. J. Cardiol. 2021, 37, 2080–2082. [Google Scholar] [CrossRef]
- Josephs, S.C. Obstetric and Gynecologic Emergencies: A Review of Indications and Interventional Techniques. Semin. Interv. Radiol. 2008, 25, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Masselli, G.; Derme, M. Mistakes in Emergency Imaging of Pregnant Patients. In Errors in Emergency and Trauma Radiology; Patlas, M.N., Katz, D.S., Scaglione, M., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 195–206. [Google Scholar]
- Guo, P.; Wu, Y.; Yuan, X.; Wan, Z. Clinical diagnostic value and analysis of MRI combined with ultrasound in prenatal pernicious placenta previa with placenta accreta. Ann. Palliat. Med. 2021, 10, 6753–6759. [Google Scholar] [CrossRef]
- Brown, B.P.; Meyers, M.L. Placental magnetic resonance imaging Part II: Placenta accreta spectrum. Pediatr. Radiol. 2020, 50, 275–284. [Google Scholar] [CrossRef]
- Nagenthran, G.; Rangasami, R.; Chandrasekharan, A.; Soundararajan, P.; Godla, U.R. Role of magnetic resonance imaging in pregnancy-associated obstetric and gynecological complications. Egypt. J. Radiol. Nucl. Med. 2019, 50, 1–7. [Google Scholar] [CrossRef]
- Bulas, D.; Egloff, A. Benefits and risks of MRI in pregnancy. Semin. Perinatol. 2013, 37, 301–304. [Google Scholar] [CrossRef]
- Sakuma, J.; Nakata, M.; Takano, M.; Nagasaki, S.; Hayata, E.; Maemura, T.; Ohtsu, M.; Morita, M. Prenatal evaluation of functional pulmonary hypoplasia via fetal magnetic resonance imaging. J. Obstet. Gynaecol. Res. 2021, 47, 3100–3106. [Google Scholar] [CrossRef]
- Berger, A. Magnetic Resonance Imaging. BMJ 2002, 324, 7328–7335. [Google Scholar] [CrossRef]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, L.; Magalhães, R.; Navas, D.; Moraes, S.; Redondo, C.; Morales, R.; Araújo, J.P.; Sousa, C.T. Magnetic nanostructures for emerging biomedical applications. Appl. Phys. Rev. 2020, 7, 011310. [Google Scholar] [CrossRef]
- ACR Committee on MR Safety. ACR Manual on MR Safety Version 1.0; ACR Committee on MR Safety: Reston, VA, USA, 2020. [Google Scholar]
- Kanal, E.; Barkovich, A.J.; Bell, C.; Borgstede, J.P.; Bradley, W.G.; Froelich, J.W.; Gimbel, J.R.; Gosbee, J.W.; Kuhni-Kaminski, E.; Larson, P.A.; et al. ACR guidance document on MR safe practices: 2013. J. Magn. Reson. Imaging 2013, 37, 501–530. [Google Scholar] [CrossRef] [Green Version]
- Tsai, L.L.; Grant, A.K.; Mortele, K.J.; Kung, J.W.; Smith, M.P. A practical guide to MR imaging safety: What radiologists need to know. Radiographics 2015, 35, 1722–1737. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Lunov, O.; Dejneka, A. How a High-Gradient Magnetic Field Could Affect Cell Life. Sci. Rep. 2016, 6, 37407. [Google Scholar] [CrossRef]
- American Academy of Pediatrics—Committee on Environmental Health. Noise: A Hazard for the Fetus and Newborn. Pediatrics 1997, 100, 724–727. [Google Scholar] [CrossRef] [Green Version]
- Ciet, P.; Litmanovich, D.E. MR Safety Issues Particular to Women. Magn. Reson. Imaging Clin. N. Am. 2015, 23, 59–67. [Google Scholar] [CrossRef]
- Myers, C.; Duncan, K.R.; Gowland, P.A.; Johnson, I.R.; Baker, P.N. Failure to detect intrauterine growth restriction following in utero exposure to MRI. Br. J. Radiol. 2014, 71, 549–551. [Google Scholar] [CrossRef]
- Kok, R.D.; De Vries, M.M.; Heerschap, A.; Van Den Berg, P.P. Absence of harmful effects of magnetic resonance exposure at 1.5 T in utero during the third trimester of pregnancy: A follow-up study. Magn. Reson. Imaging 2004, 22, 851–854. [Google Scholar] [CrossRef]
- Chartier, A.L.; Bouvier, M.J.; McPherson, D.R.; Stepenosky, J.E.; Taysom, D.A.; Marks, R.M. The Safety of Maternal and Fetal MRI at 3 T. Am. J. Roentgenol. 2019, 213, 1170–1173. [Google Scholar] [CrossRef]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, H.S.; Webb, J.A. (Eds.) Contrast Media—Safety Issues and ESUR Guidelines, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2014; (eBook); ISBN 978-3-642-36724-3. [Google Scholar]
- Bisso, S.; Leroux, J.C. Nanopharmaceuticals: A focus on their clinical translatability. Int. J. Pharm. 2020, 578, 119098. [Google Scholar] [CrossRef]
- Kostevšek, N.; Cheung, C.C.L.; Serša, I.; Kreft, M.E.; Monaco, I.; Franchini, M.C.; Vidmar, J.; Al-Jamal, W.T. Magneto-liposomes as MRI contrast agents: A systematic study of different liposomal formulations. Nanomaterials 2020, 10, 889. [Google Scholar] [CrossRef]
- Do, C.; DeAguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360 2020, 1, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Puac, P.; Rodríguez, A.; Vallejo, C.; Zamora, C.A.; Castillo, M. Safety of Contrast Material Use During Pregnancy and Lactation. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 787–797. [Google Scholar] [CrossRef]
- Fraum, T.J.; Ludwig, D.R.; Bashir, M.R.; Fowler, K.J. Gadolinium-based contrast agents: A comprehensive risk assessment. J. Magn. Reson. Imaging 2017, 46, 338–353. [Google Scholar] [CrossRef]
- Prola-Netto, J.; Woods, M.; Roberts, V.H.J.; Sullivan, E.L.; Miller, C.A.; Frias, A.E.; Oh, K.Y. Gadolinium chelate safety in pregnancy: Barely detectable gadolinium levels in the juvenile nonhuman primate after in utero exposure. Radiology 2018, 286, 122–128. [Google Scholar] [CrossRef]
- Oh, K.Y.; Roberts, V.H.J.; Schabel, M.C.; Grove, K.L.; Woods, M.; Frias, A.E. Gadolinium chelate contrast material in pregnancy: Fetal biodistribution in the nonhuman primate. Radiology 2015, 276, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Ray, J.G.; Vermeulen, M.J.; Bharatha, A.; Montanera, W.J.; Park, A.L. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA—J. Am. Med. Assoc. 2016, 316, 952–961. [Google Scholar] [CrossRef]
- Thomsen, H.S. ESUR Guidelines on Contrast Agents; Version 10.0; ESUR: Lisbon, Portugal, 2018. [Google Scholar]
- Do, H.D.; Couillaud, B.M.; Doan, B.T.; Corvis, Y.; Mignet, N. Advances on non-invasive physically triggered nucleic acid delivery from nanocarriers. Adv. Drug Deliv. Rev. 2019, 138, 3–17. [Google Scholar] [CrossRef]
- Carradori, D.; Gaudin, A.; Brambilla, D.; Andrieux, K. Application of Nanomedicine to the CNS Diseases. Int. Rev. Neurobiol. 2016, 130, 73–113. [Google Scholar] [CrossRef]
- Lu, J.; Ni, C.; Huang, J.; Liu, Y.; Tao, Y.; Hu, P.; Wang, Y.; Zheng, S.; Shi, M. Biocompatible Mesoporous Silica–Polydopamine Nanocomplexes as MR/Fluorescence Imaging Agent for Light-Activated Photothermal–Photodynamic Cancer Therapy In Vivo. Front. Bioeng. Biotechnol. 2021, 9, 1029. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Ma, X.; He, W.; Liu, C.; Liu, Z. Functional micro/nanobubbles for ultrasound medicine and visualizable guidance. Sci. China Chem. 2021, 64, 899–914. [Google Scholar] [CrossRef]
- Li, J.; Xi, A.; Qiao, H.; Liu, Z. Ultrasound-mediated diagnostic imaging and advanced treatment with multifunctional micro/nanobubbles. Cancer Lett. 2020, 475, 92–98. [Google Scholar] [CrossRef]
- Yuan, H.; Wilks, M.Q.; Normandin, M.D.; El Fakhri, G.; Kaittanis, C.; Josephson, L. Heat-induced radiolabeling and fluorescence labeling of Feraheme nanoparticles for PET/SPECT imaging and flow cytometry. Nat. Protoc. 2018, 13, 392–412. [Google Scholar] [CrossRef] [Green Version]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef]
- Barani, M.; Mukhtar, M.; Rahdar, A.; Sargazi, S.; Pandey, S.; Kang, M. Recent Advances in Nanotechnology-Based Diagnosis and Treatments of Human Osteosarcoma. Biosensors 2021, 11, 55. [Google Scholar] [CrossRef]
- Gaurav, C.; Saurav, B.; Goutam, R.; Goyal, A. Nano-Systems for Advanced Therapeutics and Diagnosis of Atherosclerosis. Curr. Pharm. Des. 2015, 21, 4498–4508. [Google Scholar] [CrossRef]
- Brusini, R.; Varna, M.; Couvreur, P. Advanced nanomedicines for the treatment of inflammatory diseases. Adv. Drug Deliv. Rev. 2020, 157, 161. [Google Scholar] [CrossRef]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef]
- Jacob, L.J.; Deigner, H.P. Nanoparticles and Nanosized Structures in Diagnostics and Therapy. In Precision Medicine: Tools and Quantitative Approaches; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 229–252. ISBN 9780128054338. [Google Scholar]
- Lee, S.H.; Kim, B.H.; Na, H.B.; Hyeon, T. Paramagnetic inorganic nanoparticles as T1 MRI contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 196–209. [Google Scholar] [CrossRef]
- Yadollahpour, A.; Asl, H.M.; Rashidi, S. Applications of nanoparticles in magnetic resonance imaging: A comprehensive review. Asian J. Pharm. 2017, 11, S7–S13. [Google Scholar]
- Makino, A.; Harada, H.; Okada, T.; Kimura, H.; Amano, H.; Saji, H.; Hiraoka, M.; Kimura, S. Effective encapsulation of a new cationic gadolinium chelate into apoferritin and its evaluation as an MRI contrast agent. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 638–646. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Mekuria, S.L.; Debele, T.A.; Tsai, H.C. Encapsulation of Gadolinium Oxide Nanoparticle (Gd2O3) Contrasting Agents in PAMAM Dendrimer Templates for Enhanced Magnetic Resonance Imaging in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 6782–6795. [Google Scholar] [CrossRef]
- Zhang, B.; Liang, R.; Zheng, M.; Cai, L.; Fan, X. Surface-functionalized nanoparticles as efficient tools in targeted therapy of pregnancy complications. Int. J. Mol. Sci. 2019, 20, 3642. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Tan, L.; Yu, Y.; Wang, B.; Chen, Z.; Han, J.; Li, M.; Chen, J.; Xiao, T.; Ambati, B.K.; et al. Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice. Theranostics 2018, 8, 2765–2781. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.; Shin, T.; Song, H.; Kim, E.Y. Self-Confirming “AND” Logic Nanoparticles for Fault-Free MRI. JACS 2010, 132, 11015–11017. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure–Relaxivity Relationships of Magnetic Nanoparticles for Magnetic Resonance Imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as MRI Contrast Agents. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Shen, Y.; Goerner, F.L.; Snyder, C.; Morelli, J.N.; Hao, D.; Hu, D.; Li, X.; Runge, V.M. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7T. Investig. Radiol. 2015, 50, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Peng, E.; Zheng, B.; Li, S.F.Y.; Xue, J.M. Synthesis of Water-Dispersible Gd2O3/GO Nanocomposites with Enhanced MRI T1 Relaxivity. J. Phys. Chem. C 2015, 119, 23735–23742. [Google Scholar] [CrossRef]
- Ulanova, M.; Poljak, A.; Wen, W.; Bongers, A.; Gloag, L.; Gooding, J.; Tilley, R.; Sachdev, P.; Braidy, N. Nanoparticles as contrast agents for the diagnosis of Alzheimer’s disease: A systematic review. Nanomedicine 2020, 15, 725–743. [Google Scholar] [CrossRef]
- Aouidat, F.; Boumati, S.; Khan, M.; Tielens, F.; Doan, B.T.; Spadavecchia, J. Design and synthesis of gold-gadolinium-coreshell nanoparticles as contrast agent: A smart way to future nanomaterials for nanomedicine applications. Int. J. Nanomed. 2019, 14, 9309–9324. [Google Scholar] [CrossRef] [Green Version]
- Pitchaimani, A.; Thanh Nguyen, T.D.; Wang, H.; Bossmann, S.H.; Aryal, S. Design and characterization of gadolinium infused theranostic liposomes. RSC Adv. 2016, 6, 36898–36905. [Google Scholar] [CrossRef]
- Davis, J.J.; Huang, W.; Davies, G. Location-tuned relaxivity in Gd-doped mesoporous silica nanoparticles. J. Mater. Chem. 2012, 22, 22848–22850. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Sun, C.; Bao, J.; Yang, L.; Wei, R.; Cheng, J.; Lin, H.; Gao, J. Surface manganese substitution in magnetite nanocrystals enhances: T1 contrast ability by increasing electron spin relaxation. J. Mater. Chem. B 2018, 6, 401–413. [Google Scholar] [CrossRef]
- Liu, D.; He, C.; Poon, C.; Lin, W. Theranostic nanoscale coordination polymers for magnetic resonance imaging and bisphosphonate delivery. J. Mater. Chem. B 2014, 2, 8249–8255. [Google Scholar] [CrossRef]
- Park, J.Y.; Baek, M.J.; Choi, E.S.; Woo, S.; Kim, J.H.; Kim, T.J.; Jung, J.C.; Chae, K.S.; Chang, Y.; Lee, G.H. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T 1 MRI contrast agent: Account for large longitudinal relaxivity, optimal particle diameter, and in Vivo T1 MR images. ACS Nano 2009, 3, 3663–3669. [Google Scholar] [CrossRef]
- Tromsdorf, U.I.; Bruns, O.T.; Salmen, S.C.; Beisiegel, U.; Weller, H. A Highly Effective, Nontoxic T 1 MR Contrast Agent Based on Ultrasmall PEGylated Iron Oxide Nanoparticles. Nano Lett. 2009, 9, 4434–4440. [Google Scholar] [CrossRef]
- Thébault, C.J.; Ramniceanu, G.; Boumati, S.; Michel, A.; Seguin, J.; Larrat, B.; Mignet, N.; Ménager, C.; Doan, B.T. Theranostic MRI liposomes for magnetic targeting and ultrasound triggered release of the antivascular CA4P. J. Control. Release 2020, 322, 137–148. [Google Scholar] [CrossRef]
- Jang, J.; Nah, H.; Lee, J.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical Enhancements of MRI Contrast and Hyperthermic Effects by Dopant-Controlled Magnetic Nanoparticles. Angew. Chem. 2009, 4, 1234–1238. [Google Scholar] [CrossRef]
- Kang, B.; Lim, J.; Son, H.; Choi, Y.; Kang, T.; Jung, J.; Huh, Y.-M.; Haam, S.; Lim, E.-K. PEGylated Magnetic Nano-Assemblies as Contrast Agents for Effective T2-Weighted MR Imaging. Nanomaterials 2019, 9, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Faraj, A.; Shaik, A.P.; Shaik, A.S. Magnetic single-walled carbon nanotubes as efficient drug delivery nanocarriers in breast cancer murine model: Noninvasive monitoring using diffusion-weighted magnetic resonance imaging as sensitive imaging biomarker. Int. J. Nanomed. 2014, 10, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Mancebo, D.; Becerro, A.I.; Rojas, T.C.; García-martín, M.L.; Fuente, J.M.; De Ocaña, M. HoF3 and DyF3 Nanoparticles as Contrast Agents for High-Field Magnetic Resonance Imaging. Part. Part. Syst. Charact. 2017, 34, 1–10. [Google Scholar] [CrossRef]
- Tegafaw, T.; Xu, W.; Ahmad, M.W.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Kim, T.J.; Lee, G.H. Dual-mode T1 and T2 magnetic resonance imaging contrast agent based on ultrasmall mixed gadolinium-dysprosium oxide nanoparticles: Synthesis, characterization, and in vivo application. Nanotechnology 2015, 26, 365102. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J.; Kwong, R.Y. Contrast Agents in Cardiovascular Magnetic Resonance Imaging. In Cardiovascular Magnetic Resonance Imaging. Contemporary Cardiology; Kwong, R.Y., Ed.; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2019; pp. 127–143. ISBN 978-1-4939-8841-9. [Google Scholar]
- Issa, B.; Obaidat, I.M. Magnetic Nanoparticles as MRI Contrast Agents. In Magnetic Resonance Imaging; Manchev, L., Ed.; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-83880-173-1. [Google Scholar]
- Shetty, A.N.; Pautler, R.; Ghagahda, K.; Rendon, D.; Gao, H.; Starosolski, Z.; Bhavane, R.; Patel, C.; Annapragada, A.; Yallampalli, C.; et al. A liposomal Gd contrast agent does not cross the mouse placental barrier. Sci. Rep. 2016, 6, 27863. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.T.; Gelperin, K.; Sahin, L.; Bleich, K.B.; Fazio-Eynullayeva, E.; Woods, C.; Radden, E.; Greene, P.; McCloskey, C.; Johnson, T.; et al. First-trimester exposure to gadolinium-based contrast agents: A utilization study of 4.6 Million U.S. Pregnancies. Radiology 2019, 293, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lee, N.; Choi, S.H.; An, K.; Yu, S.H.; Kim, J.H.; Kwon, S.H.; Kim, D.; Kim, H.; Baek, S.I.; et al. Large-scale synthesis of ultrathin manganese oxide nanoplates and their applications to T1 MRI contrast agents. Chem. Mater. 2011, 23, 3318–3324. [Google Scholar] [CrossRef]
- Guo, C.; Hu, J.; Bains, A.; Pan, D.; Luo, K.; Li, N.; Gu, Z. The potential of peptide dendron functionalized and gadolinium loaded mesoporous silica nanoparticles as magnetic resonance imaging contrast agents. J. Mater. Chem. B 2016, 4, 2322–2331. [Google Scholar] [CrossRef]
- Keelan, J.A.; Leong, J.W.; Ho, D.; Iyer, K.S. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in pregnancy. Nanomedicine 2015, 10, 2229–2247. [Google Scholar] [CrossRef] [PubMed]
- Ghaghada, K.B.; Starosolski, Z.A.; Bhayana, S.; Stupin, I.; Patel, C.V.; Bhavane, R.C.; Gao, H.; Bednov, A.; Yallampalli, C.; Belfort, M.; et al. Pre-clinical evaluation of a nanoparticle-based blood-pool contrast agent for MR imaging of the placenta. Placenta 2017, 57, 60–70. [Google Scholar] [CrossRef]
- Badachhape, A.A.; Kumar, A.; Ghaghada, K.B.; Stupin, I.V.; Srivastava, M.; Devkota, L.; Starosolski, Z.; Tanifum, E.A.; George, V.; Fox, K.A.; et al. Pre-clinical magnetic resonance imaging of retroplacental clear space throughout gestation. Placenta 2019, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Valero, L.; Alhareth, K.; Gil, S.; Lecarpentier, E.; Tsatsaris, V.; Mignet, N.; Fournier, T.; Andrieux, K. Nanomedicine as a potential approach to empower the new strategies for the treatment of preeclampsia. Drug Discov. Today 2018, 23, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M. Transplacental transport of nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, K.R.; Xu, Y.; Ramirez, P.A.; DeLaine, J.; Parker, C.; Bao, Y.; Rasco, J.F. Surface charge and dosage dependent potential developmental toxicity and biodistribution of iron oxide nanoparticles in pregnant CD-1 mice. Reprod. Toxicol. 2014, 50, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Valero, L.; Alhareth, K.; Gil, S.; Simasotchi, C.; Roques, C.; Scherman, D.; Mignet, N.; Fournier, T.; Andrieux, K. Assessment of dually labelled PEGylated liposomes transplacental passage and placental penetration using a combination of two ex-vivo human models: The dually perfused placenta and the suspended villous explants. Int. J. Pharm. 2017, 532, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhaliq, A.; Van Der Zande, M.; Peters, R.J.B.; Bouwmeester, H. Combination of the BeWo b30 placental transport model and the embryonic stem cell test to assess the potential developmental toxicity of silver nanoparticles. Part. Fibre Toxicol. 2020, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Aengenheister, L.; Dugershaw, B.B.; Manser, P.; Wichser, A.; Schoenenberger, R.; Wick, P.; Hesler, M.; Kohl, Y.; Straskraba, S.; Suter, M.J.F.; et al. Investigating the accumulation and translocation of titanium dioxide nanoparticles with different surface modifications in static and dynamic human placental transfer models. Eur. J. Pharm. Biopharm. 2019, 142, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Correia Carreira, S.; Walker, L.; Paul, K.; Saunders, M. The toxicity, transport and uptake of nanoparticles in the in vitro BeWo b30 placental cell barrier model used within NanoTEST. Nanotoxicology 2015, 9, 66–78. [Google Scholar] [CrossRef]
- Pereira, K.V.; Giacomeli, R.; Gomes de Gomes, M.; Haas, S.E. The challenge of using nanotherapy during pregnancy: Technological aspects and biomedical implications. Placenta 2020, 100, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Šimečková, P.; Hubatka, F.; Kotouček, J.; Turánek Knötigová, P.; Mašek, J.; Slavík, J.; Kováč, O.; Neča, J.; Kulich, P.; Hrebík, D.; et al. Gadolinium labelled nanoliposomes as the platform for MRI theranostics: In vitro safety study in liver cells and macrophages. Sci. Rep. 2020, 10, 4780. [Google Scholar] [CrossRef]
- Alfaifi, A.A.; Heyder, R.S.; Bielski, E.R.; Almuqbil, R.M.; Kavdia, M.; Gerk, P.M.; da Rocha, S.R.P. Megalin-targeting liposomes for placental drug delivery. J. Control. Release 2020, 324, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Renshall, L.J.; Beards, F.; Evangelinos, A.; Greenwood, S.L.; Brownbill, P.; Stevens, A.; Sibley, C.P.; Aplin, J.D.; Johnstone, E.D.; Teesalu, T.; et al. Targeted Delivery of Epidermal Growth Factor to the Human Placenta to Treat Fetal Growth Restriction. Pharmaceutics 2021, 13, 1778. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Difonzo, M.; Fliedel, L.; Mignet, N.; Andrieux, K.; Alhareth, K. How Could Nanomedicine Improve the Safety of Contrast Agents for MRI during Pregnancy? Sci 2022, 4, 11. https://doi.org/10.3390/sci4010011
Difonzo M, Fliedel L, Mignet N, Andrieux K, Alhareth K. How Could Nanomedicine Improve the Safety of Contrast Agents for MRI during Pregnancy? Sci. 2022; 4(1):11. https://doi.org/10.3390/sci4010011
Chicago/Turabian StyleDifonzo, Marinella, Louise Fliedel, Nathalie Mignet, Karine Andrieux, and Khair Alhareth. 2022. "How Could Nanomedicine Improve the Safety of Contrast Agents for MRI during Pregnancy?" Sci 4, no. 1: 11. https://doi.org/10.3390/sci4010011
APA StyleDifonzo, M., Fliedel, L., Mignet, N., Andrieux, K., & Alhareth, K. (2022). How Could Nanomedicine Improve the Safety of Contrast Agents for MRI during Pregnancy? Sci, 4(1), 11. https://doi.org/10.3390/sci4010011











