Kinetic Analysis of Nitrite Reduction Reactions by Nitrite Reductase Derived from Spinach in the Presence of One-Electron Reduced Riboflavin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Methods
2.2.1. Preparation of Nitrite Reductase in a Spinach Plant Sample
2.2.2. Activity Test of Nitrite Reductase Derived from Spinach with One-Electron Reduced Riboflavin as Electron Donor
3. Results and Discussions
3.1. Temperature Dependence of Nitrite-Reducing Catalytic Activity
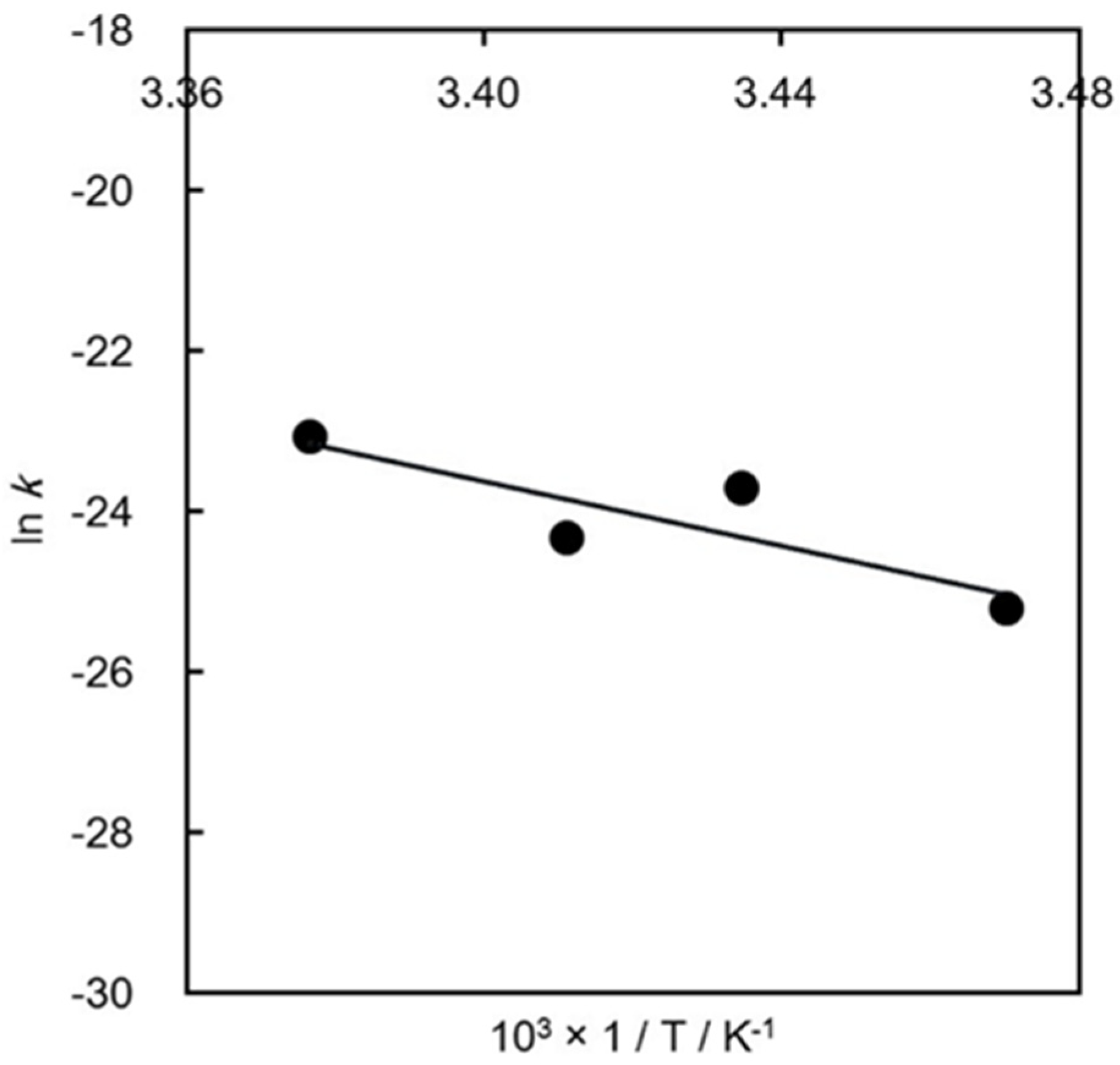
3.2. Calculation of the Activation Energy in Ammonium Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| T: Reaction Temperature K | v: Ammonium Production Rate μM·min−1 |
|---|---|
| 288.15 | 27.7 |
| 291.15 | 52.0 |
| 293.15 | 43.4 |
| 296.15 | 62.0 |
| 301.15 | 44.2 |
| 303.15 | 36.8 |
| 1/T K−1 | k: Rate Constant 10−12 × M2 | ln k |
|---|---|---|
| 3.47 | 10.8 | −25.2 |
| 3.43 | 72.2 | −23.7 |
| 3.41 | 41.5 | −24.3 |
| 3.38 | 123.0 | −23.1 |
References
- Stevens, C.J. Nitrogen in the environment. Science 2019, 363, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.; Gu, B. Urban rivers as hotspots of regional nitrogen pollution. Environ. Pollut. 2015, 205, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R. Fertilizer nitrogen, food security, health and the environment. World 2013, 16, 14–16. [Google Scholar]
- Choudhury, A.T.M.A.; Kennedy, I.R. Nitrogen fertilizer losses from rice soils and control of environmental pollution problems. Commun. Soil. Sci. Plant Anal. 2005, 36, 1625–1639. [Google Scholar] [CrossRef]
- Schullehner, J.; Hansen, B.; Thygesen, M.; Pedersen, C.B.; Sigsgaard, T. Nitrate in drinking water and colorectal cancer risk: A nationwide population-based cohort study. Int. J. Cancer 2018, 143, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, A.; Cusimano, G.; Favara, R. Groundwater nitrate risk assessment using intrinsic vulnerability methods: A comparative study of environmental impact by intensive farming in the Mediterranean region of Sicily, Italy. J. Geochem. Explor. 2015, 156, 89–100. [Google Scholar] [CrossRef]
- Archer, M.C. Hazards of Nitrote, Nitrite, ond N-Nitroso Compounds in Human Nutrition. Nutr. Toxicol. V1 2012, 1, 327. [Google Scholar]
- Kroupova, H.; Machova, J.; Svobodova, Z. Nitrite influence on fish: A review. Vet. Med.-Praha- 2005, 50, 461–471. [Google Scholar] [CrossRef]
- Rezvani, F.; Sarrafzadeh, M.H.; Ebrahimi, S.; Oh, H.M. Nitrate removal from drinking water with a focus on biological methods: A review. Environ. Sci. Pollut. Res. 2019, 26, 1124–1141. [Google Scholar] [CrossRef]
- Gayle, B.P.; Boardman, G.D.; Sherrard, J.H.; Benoit, R.E. Biological denitrification of water. J. Environ. Chem. Eng. 1989, 115, 930–943. [Google Scholar] [CrossRef]
- Wuhrmann, K. Nitrogen removal in sewage treatment processes: Int. Ver. Theor. Angew. Limnol. 1964, 15, 580–596. [Google Scholar]
- Liang, J.; Deng, B.; Liu, Q.; Wen, G.; Liu, Q.; Li, T.; Sun, X. High-efficiency electrochemical nitrite reduction to ammonium using a Cu3P nanowire array under ambient conditions. Green Chem. 2021, 23, 5487–5493. [Google Scholar] [CrossRef]
- Clark, C.A.; Reddy, C.P.; Xu, H.; Heck, K.N.; Luo, G.; Senftle, T.P.; Wong, M.S. Mechanistic insights into pH-controlled nitrite reduction to ammonia and hydrazine over rhodium. ACS Catal. 2019, 10, 494–509. [Google Scholar] [CrossRef]
- Guo, Y.; Stroka, J.R.; Kandemir, B.; Dickerson, C.E.; Bren, K.L. Cobalt metallopeptide electrocatalyst for the selective reduction of nitrite to ammonium. J. Am. Chem. Soc. 2018, 140, 16888–16892. [Google Scholar] [CrossRef]
- Sachdeva, V.; Hooda, V. Immobilization of nitrate reductase onto epoxy affixed silver nanoparticles for determination of soil nitrates. Int. J. Biol. Macromol. 2015, 79, 240–247. [Google Scholar] [CrossRef]
- Sachdeva, V.; Hooda, V. Effect of changing the nanoscale environment on activity and stability of nitrate reductase. Enzyme Microb. Technol. 2016, 89, 52–62. [Google Scholar] [CrossRef]
- Andoralov, V.; Shleev, S.; Dergousova, N.; Kulikova, O.; Popov, V.; Tikhonova, T. Octaheme nitrite reductase: The mechanism of intramolecular electron transfer and kinetics of nitrite bioelectroreduction. Bioelectrochemistry 2021, 138, 107699. [Google Scholar] [CrossRef]
- Einsle, O.; Messerschmidt, A.; Huber, R.; Kroneck, P.M.; Neese, F. Mechanism of the six-electron reduction of nitrite to ammonia by cytochrome c nitrite reductase. J. Am. Chem. Soc. 2002, 124, 11737–11745. [Google Scholar] [CrossRef]
- Einsle, O.; Messerschmidt, A.; Stach, P.; Bourenkov, G.P.; Bartunik, H.D.; Huber, R.; Kroneck, P.M. Structure of cytochrome c nitrite reductase. Nature 1999, 400, 476–480. [Google Scholar] [CrossRef]
- Wang, X.; Tamiev, D.; Alagurajan, J.; DiSpirito, A.A.; Phillips, G.J.; Hargrove, M.S. The role of the NADH-dependent nitrite reductase, Nir, from Escherichia coli in fermentative ammonification. Arch. Microbiol. 2019, 201, 519–530. [Google Scholar] [CrossRef]
- Kuznetsova, S.; Knaff, D.B.; Hirasawa, M.; Lagoutte, B.; Sétif, P. Mechanism of spinach chloroplast ferredoxin-dependent nitrite reductase: Spectroscopic evidence for intermediate states. Biochemistry 2004, 43, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Xuejiang, W.; Dzyadevych, S.V.; Chovelon, J.M.; Renault, N.J.; Ling, C.; Siqing, X.; Jianfu, Z. Conductometric nitrate biosensor based on methyl viologen/Nafion®/nitrate reductase interdigitated electrodes. Talanta 2006, 69, 450–455. [Google Scholar] [CrossRef]
- Ida, S.; Morita, Y. Purification and general properties of spinach leaf nitrite reductase. Plant Cell Physiol. 1973, 14, 661–671. [Google Scholar]
- Bourne, W.F.; Miflin, B.J. Studies on nitrite reductase in barley. Planta 1973, 111, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Willner, B. Artificial photosynthetic transformations through biocatalysis and biomimetic systems. Adv. Photochem. 1995, 20, 217–290. [Google Scholar]
- Willner, I.; Lapidot, N.; Riklin, A. Photoinduced enzyme-catalyzed reduction of nitrate (NO3−) and nitrite (NO2−) to ammonia (NH3). J. Am. Chem. Soc. 1989, 111, 1883–1884. [Google Scholar] [CrossRef]
- Ramirez, J.M.; Del Campo, F.F.; Paneque, A.; Losada, M. Ferredoxin-nitrite reductase from spinach. Biochim. Biophys. Acta. 1966, 118, 58–71. [Google Scholar] [CrossRef]
- Hirasawa-Soga, M.; Tamura, G. Some properties of ferredoxin-nitrite reductase from Spinacia oleracea. Agric. Biol. Chem. 1981, 45, 1615–1620. [Google Scholar] [CrossRef][Green Version]
- Tabe, H.; Oshima, H.; Ikeyama, S.; Amao, Y.; Yamada, Y. Enhanced catalytic stability of acid phosphatase immobilized in the mesospaces of a SiO2-nanoparticles assembly for catalytic hydrolysis of organophosphates. Mol. Catal. 2021, 510, 111669. [Google Scholar] [CrossRef]
- Vega, J.M.; Kamin, H. Spinach nitrite reductase. Purification and properties of a siroheme-containing iron-sulfur enzyme. J. Biol. Chem. 1977, 252, 896–909. [Google Scholar] [CrossRef]
- Bykov, D.; Neese, F. Six-electron reduction of nitrite to ammonia by cytochrome c nitrite reductase: Insights from density functional theory studies. Inorg. Chem. 2015, 54, 9303–9316. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeyama, S.; Tabe, H. Kinetic Analysis of Nitrite Reduction Reactions by Nitrite Reductase Derived from Spinach in the Presence of One-Electron Reduced Riboflavin. Sci 2022, 4, 13. https://doi.org/10.3390/sci4010013
Ikeyama S, Tabe H. Kinetic Analysis of Nitrite Reduction Reactions by Nitrite Reductase Derived from Spinach in the Presence of One-Electron Reduced Riboflavin. Sci. 2022; 4(1):13. https://doi.org/10.3390/sci4010013
Chicago/Turabian StyleIkeyama, Shusaku, and Hiroyasu Tabe. 2022. "Kinetic Analysis of Nitrite Reduction Reactions by Nitrite Reductase Derived from Spinach in the Presence of One-Electron Reduced Riboflavin" Sci 4, no. 1: 13. https://doi.org/10.3390/sci4010013
APA StyleIkeyama, S., & Tabe, H. (2022). Kinetic Analysis of Nitrite Reduction Reactions by Nitrite Reductase Derived from Spinach in the Presence of One-Electron Reduced Riboflavin. Sci, 4(1), 13. https://doi.org/10.3390/sci4010013






