Non-Flammable Epoxy Composition Based on Epoxy Resin DER-331 and 4-(β-Carboxyethenyl)phenoxy-phenoxycyclotriphosphazenes with Increased Adhesion to Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
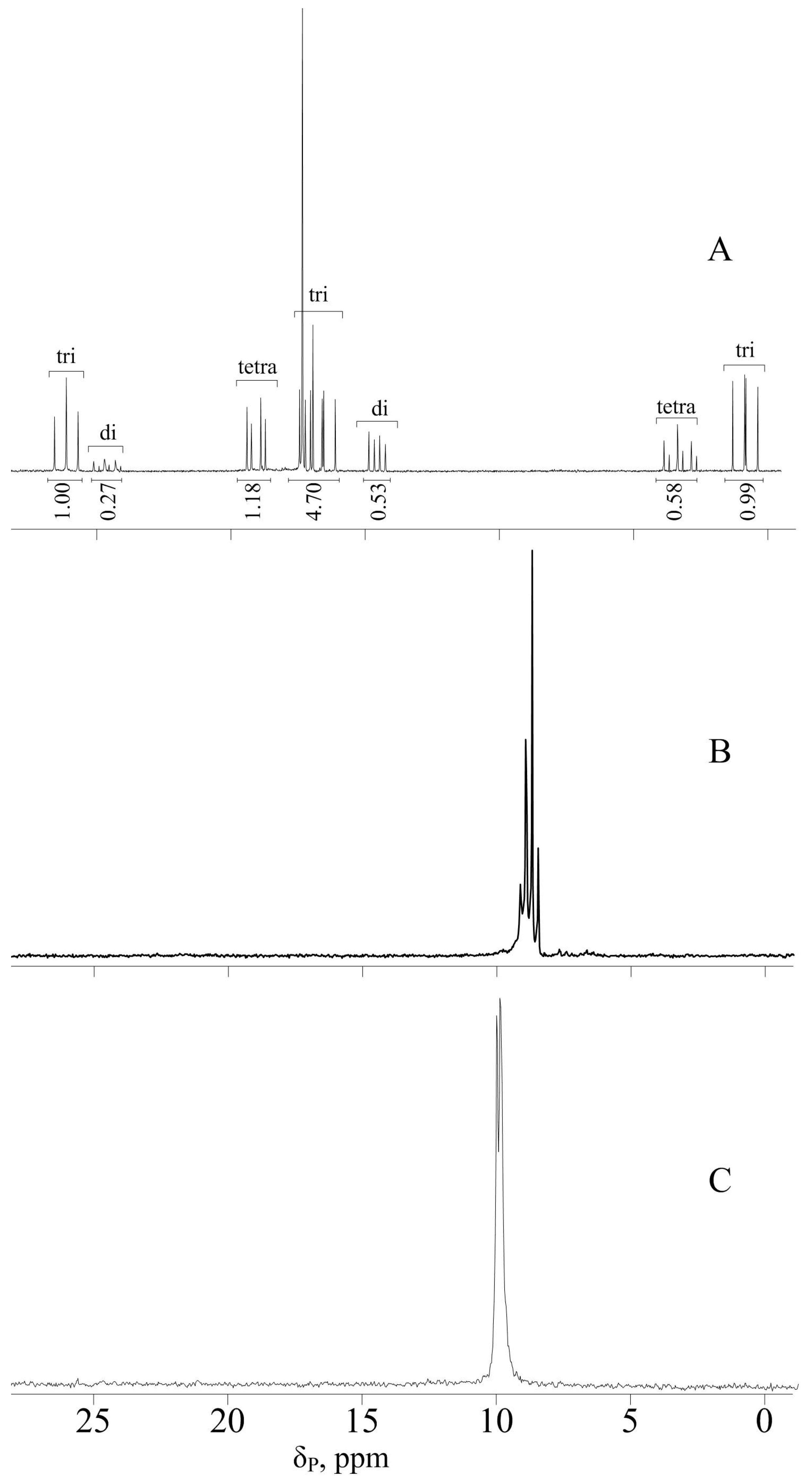
2.3. Synthesis of 4-Formylphenoxy-Phenoxycyclotriphosphazene (FPPP)
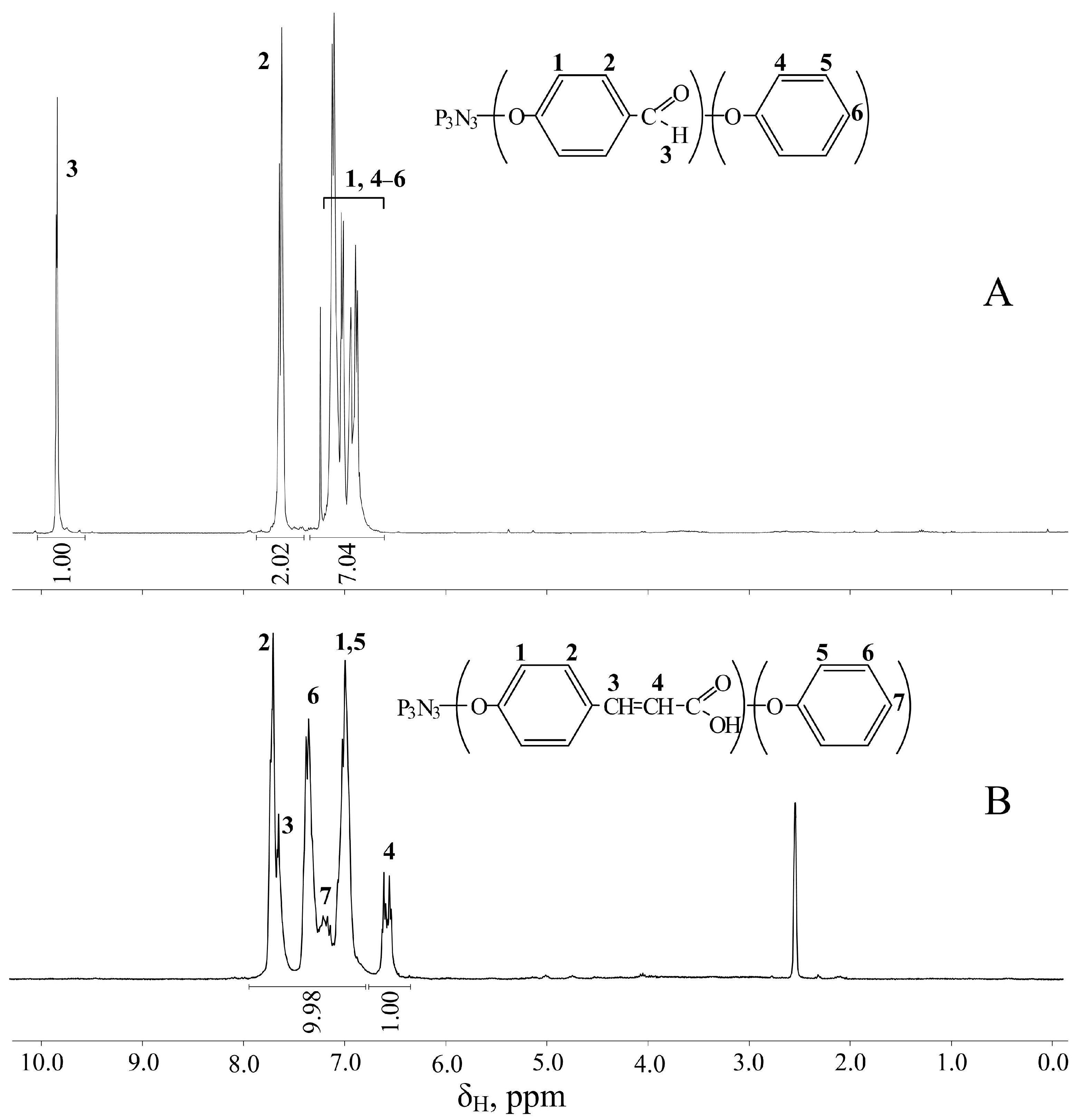
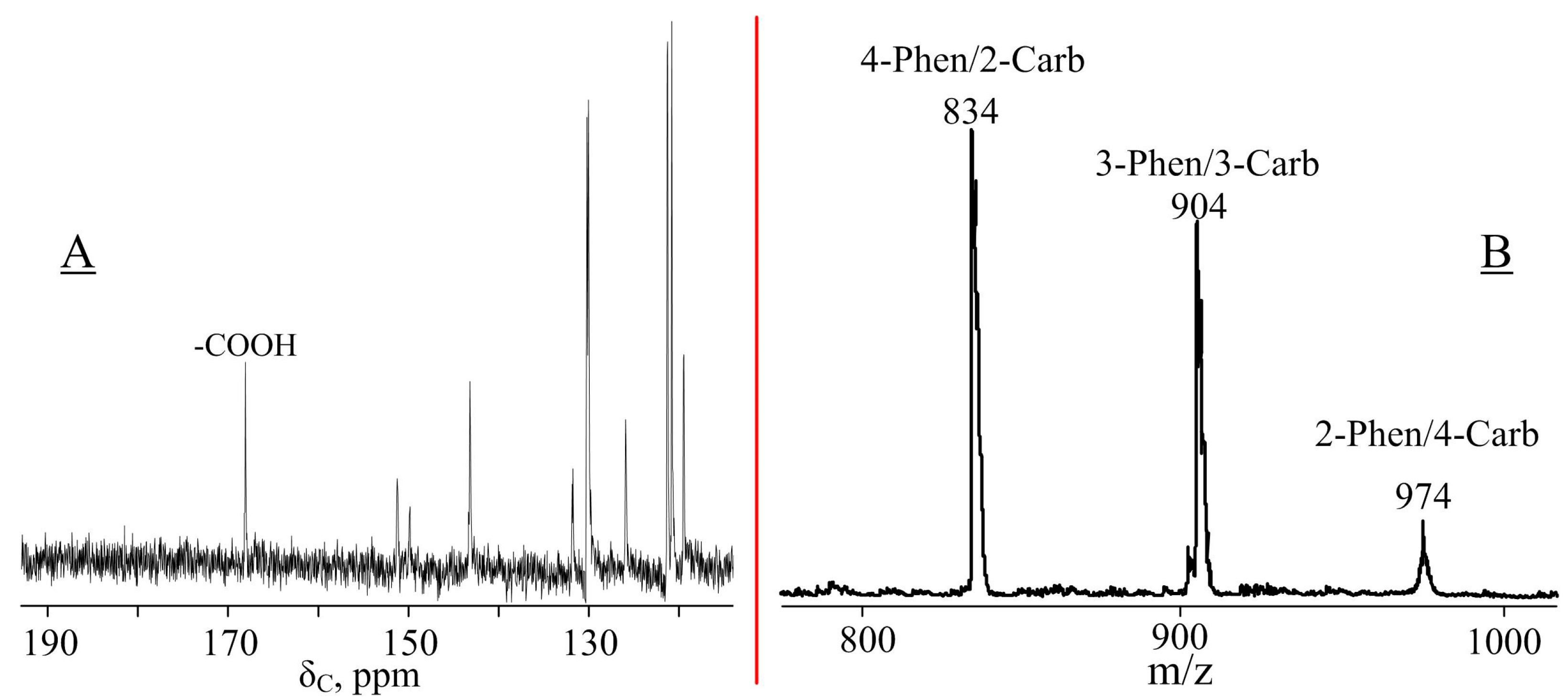
2.4. Synthesis of 4-(β-Carboxyethenyl)Phenoxy-Phenoxycyclotriphosphazene (CPPP)
2.5. Preparation of Composition and Test Samples Based on CPPP and DER-331
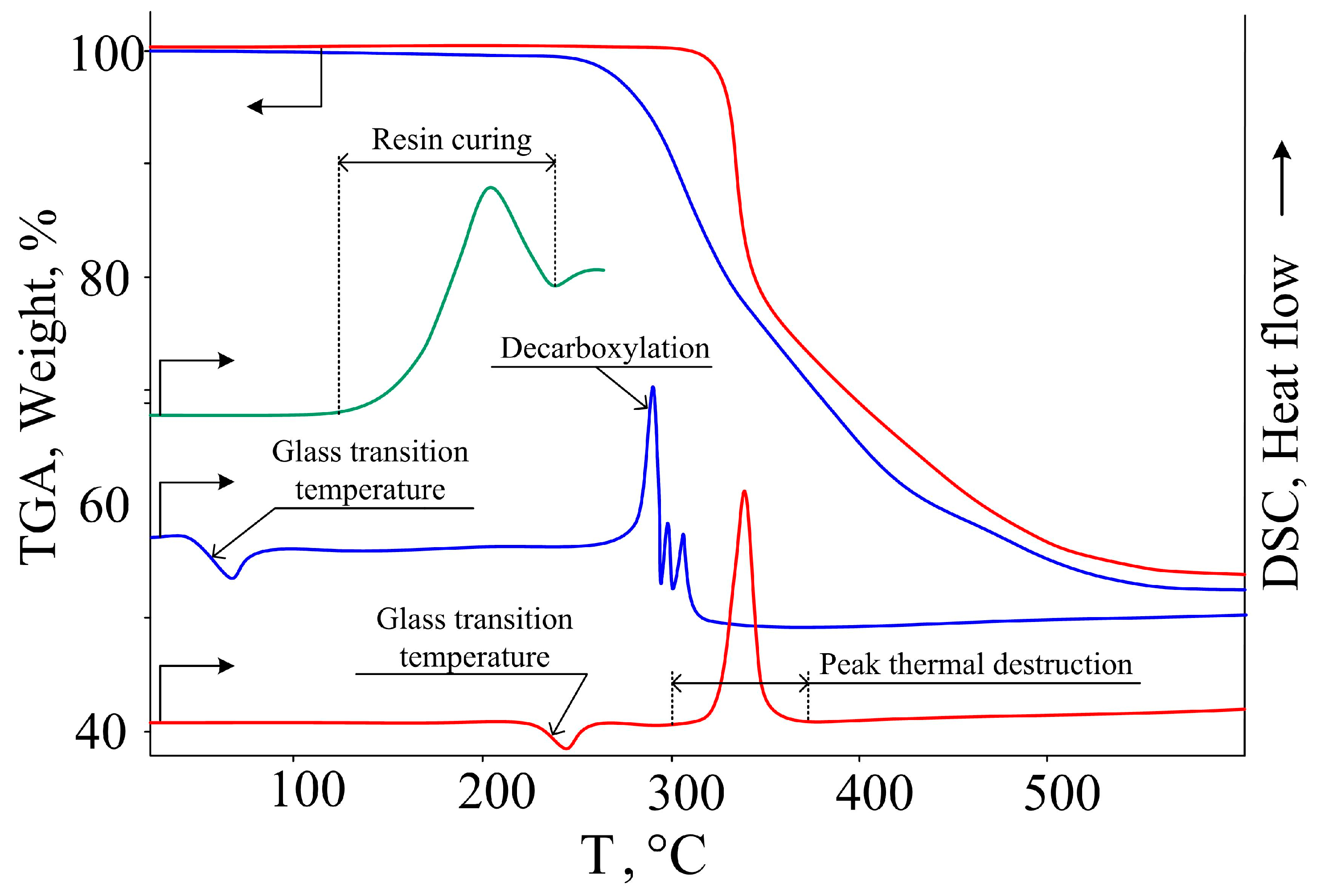
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopes, P.E.; Moura, D.; Hilliou, L.; Krause, B.; Pötschke, P.; Figueiredo, H.; Alves, R.; Lepleux, E.; Pacheco, L.; Paiva, M.C. Mixed carbon nanomaterial/epoxy resin for electrically conductive adhesives. J. Compos. Sci. 2020, 4, 105. [Google Scholar] [CrossRef]
- Li, S.; Cui, H.; Wang, H.; Wang, W.; Sui, Y.; Dong, L.; Wang, J. Preparation and performance investigation of epoxy resin-based permeable concrete containing ceramsite. Polymers 2023, 15, 4704. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, X.; Wang, Z.; Zhang, Z.; Liu, Z. A mini-review of ultra-low dielectric constant intrinsic epoxy resins: Mechanism, preparation and application. Polym. Adv. Tecnol. 2024, 35, 6241. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Wang, Y.; Li, W.; Nie, X. Eco-friendly epoxy-terminated polyurethane-modified epoxy resin with efficient enhancement in toughness. Polymers 2023, 15, 2803. [Google Scholar] [CrossRef]
- Siahtiri, S.; Sahraei, A.A.; Mokarizadeh, A.H.; Baghani, M.; Bodaghi, M.; Baniassadi, M. Influence of curing agents molecular structures on interfacial characteristics of graphene/epoxy nanocomposites: A molecular dynamics framework. Macromol. Mater. Eng. 2023, 308, 2300030. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Dai, J.; Zhang, X.; Liu, X.; Liu, X.; Yi, X. Preparation and application of a multifunctional interfacial modifier for ramie fiber/epoxy resin composites. Polymers 2023, 15, 3800. [Google Scholar] [CrossRef] [PubMed]
- Akin, E.; Çakir, M.; Demirer, H. Multi-featured epoxy composites filled with surface-modified PTFE powders treated by Na-naphthalenide system. J. Appl. Polym. Sci. 2024, 141, 54947. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Yoon, H. Fire-safe polymer composites: Flame-retardant effect of nanofillers. Polymers 2021, 13, 540. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Chen, J.; Zou, L.; Xing, X.; Zhang, K.; Liu, J.; Liu, X. Flame-retardant thermoplastic polyether ester/aluminum butylmethylphosphinate/phenolphthalein composites with enhanced mechanical properties and antidripping. Polymers 2024, 16, 552. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Z.; Zhu, J. Synergistic flame retardant effect of aluminum hydroxide and ammonium polyphosphate on epoxy resin. J. Appl. Polym. Sci. 2022, 139, 53168. [Google Scholar] [CrossRef]
- Shi, C.; Wan, M.; Qian, X.; Jing, J.; Zhou, K. Zinc Hydroxystannate/carbon nanotube hybrids as flame retardant and smoke suppressant for epoxy resins. Molecules 2023, 28, 6820. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.F.; Thomas, W.; Chalk, R.; Singamneni, S. Flame retardant polymeric materials for additive manufacturing. Mater. Today Proc. 2020, 33, 5720–5724. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Xie, J. Synthesis and curing properties of fluorinated curing agent for epoxy resin based on natural soybean isoflavones. React. Funct. Polym. 2023, 184, 105511. [Google Scholar] [CrossRef]
- Benmammar, R.K.; Mundlapati, V.R.; Bouberka, Z.; Barrera, A.; Staelens, J.-N.; Tahon, J.-F.; Ziskind, M.; Carpentier, Y.; Focsa, C.; Supiot, P.; et al. Electron beam processing as a promising tool to decontaminate polymers containing brominated flame retardants. Molecules 2023, 28, 7753. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wan, Y.; Liu, J.; Yang, N.; Guo, J.; Li, J.; Zou, Y.; Zhan, J.; Zhan, H.; Wang, M. Preparation and performance analysis of methyl-silicone resin-modified epoxy resin-based intumescent flame retardant thermal insulation coating. J. Micromechanics Mol. Phys. 2023, 8, 61–82. [Google Scholar] [CrossRef]
- Tavakoli, M.; Mazela, B.; Grześkowiak, W.; Proch, J.; Mleczek, M.; Perdoch, W. The strength and fire properties of paper sheets made of phosphorylated cellulose fibers. Molecules 2024, 29, 133. [Google Scholar] [CrossRef]
- Dou, Y.; Zhong, Z.; Huang, J.; Ju, A.; Yao, W.; Zhang, C.; Guan, D. A new phosphorous/nitrogen-containing flame-retardant film with high adhesion for jute fiber composites. Polymers 2023, 15, 1920. [Google Scholar] [CrossRef]
- Davidson, D.J.; McKay, A.P.; Cordes, D.B.; Woollins, J.D.; Westwood, N.J. The covalent linking of organophosphorus heterocycles to date palm wood-derived lignin: Hunting for new materials with flame-retardant potential. Molecules 2023, 28, 7885. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Li, W.; Wan, S.; Liu, Z.; Zhang, Y.; Zhang, Y.; Zhang, J.; Kong, Q. Amino phenyl copper phosphate-bridged reactive phosphaphenanthrene to intensify fire safety of epoxy resins. Molecules 2023, 28, 623. [Google Scholar] [CrossRef]
- Ma, X.; Kang, N.; Zhang, Y.; Min, Y.; Yang, J.; Ban, D.; Zhao, W. Enhancing flame retardancy and smoke suppression in epoxy resin composites with sulfur–phosphorous reactive flame retardant. Molecules 2024, 29, 227. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Wang, C.; Xu, C.; Ni, A. The flame retardant and mechanical properties of the epoxy modified by an efficient DOPO-based flame retardant. Polymers 2024, 16, 631. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Li, H.; Liu, Y. Synthesis of phosphorus-nitrogen hybrid flame retardant and investigation of its efficient flame-retardant behavior in PA6/PA66. J. Appl. Polym. Sci. 2023, 140, 53536. [Google Scholar] [CrossRef]
- Li, K.; Gao, Y.; Li, X.; Zhang, Y.; Zhu, B.; Zhang, Q. Fragmentation pathway of organophosphorus flame retardants by liquid chromatography–orbitrap-based high-resolution mass spectrometry. Molecules 2024, 29, 680. [Google Scholar] [CrossRef] [PubMed]
- Akbaş, H.; Şenocak, A.; Kılıç, Z.; Tayhan, S.E.; Bilgin, S.; Yıldırım, A.; Hökelek, T. Syntheses of tetrachloro and tetraamino (2-furanylmethyl) spiro (N/N) cyclotriphosphazenes: Chemical, structural elucidation, antiproliferative and antimigratory activity studies. J. Mol. Struct. 2023, 1282, 135209. [Google Scholar] [CrossRef]
- Koran, K.; Çalışkan, E.; Öztürk, D.A.; Çapan, İ.; Tekin, S.; Sandal, S.; Görgülü, A.O. The first peptide derivatives of dioxybiphenyl-bridged spiro cyclotriphosphazenes: In vitro cytotoxicity activities and DNA damage studies. Bioorg. Chem. 2023, 132, 106338. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Vlad-Bubulac, T.; Macsim, A.M.; Bǎlan, V. Design and synthesis of amphiphilic graft polyphosphazene micelles for docetaxel delivery. Pharmaceutics 2023, 15, 1564. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.; Zhang, S.; Hassan, M.; Ma, C.; Liu, Z.; Gong, W. Synthesis of pillar[5]arene-and phosphazene-linked porous organic polymers for highly efficient adsorption of uranium. Molecules 2023, 28, 1029. [Google Scholar] [CrossRef] [PubMed]
- Piskun, Y.A.; Ksendzov, E.A.; Resko, A.V.; Soldatov, M.A.; Timashev, P.; Liu, H.; Vasilenko, I.V.; Kostjuk, S.V. Phosphazene functionalized silsesquioxane-based porous polymer as thermally stable and reusable catalyst for bulk ring-opening polymerization of ε-caprolactone. Polymers 2023, 15, 1291. [Google Scholar] [CrossRef]
- Dagdag, O.; Kim, H. Progress in the field of cyclophosphazenes: Preparation, properties, and applications. Polymers 2023, 16, 122. [Google Scholar] [CrossRef]
- Bao, D.M.; Wang, J.H.; Hou, Z.M.; Xu, Z.Y.; Ye, X.L.; Qi, Y.Z.; Wen, Z. Synthesis of a novel flame retardant with phosphaphenanthrene and phosphazene double functional groups and flame retardancy of poly (lactic acid) composites. Front. Mater. 2022, 9, 951515. [Google Scholar] [CrossRef]
- Mayer-Gall, T.; Plohl, D.; Derksen, L.; Lauer, D.; Neldner, P.; Ali, W.; Fuchs, S.; Gutmann, J.S.; Opwis, K. A green water-soluble cyclophosphazene as a flame retardant finish for textiles. Molecules 2019, 24, 3100. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zheng, Q.; Zeng, H.; Wang, X.; Cui, J.; Yang, B.; Guo, J.; Mu, B.; Bao, X.; Wan, X.; et al. Synthesis of multi-element hybrid polyphosphonitrile microspheres and their flame retardant application in epoxy resins. J. Appl. Polym. Sci. 2023, 140, 54668. [Google Scholar] [CrossRef]
- Xu, B.; Wu, M.; Liu, Y.; Wei, S. Study on flame retardancy behavior of epoxy resin with phosphaphenanthrene triazine compound and organic zinc complexes based on phosphonitrile. Molecules 2023, 28, 3069. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.; Liu, X.; Dong, Y. Gas-phase flame-retardant effects of a bi-group compound based on phosphaphenanthrene and triazine-trione groups in epoxy resin. Polym. Degrad. Stab. 2016, 133, 350–357. [Google Scholar] [CrossRef]
- Waldin, N.A.; Jamain, Z. The effect of alkyl terminal chain length of Schiff-based cyclotriphosphazene derivatives towards epoxy resins on flame retardancy and mechanical properties. Polymers 2023, 15, 1431. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, E.M.; Panfilova, D.V.; Kireev, V.V.; Volkov, V.V.; Bobrov, M.F. Synthesis and properties of hexakis-(β-carboxyethenylphenoxy)cyclotriphosphazene. J. Mol. Struct. 2017, 1148, 1–6. [Google Scholar] [CrossRef]
- ISO 4587:1979; Adhesives—Determination of Tensile Lap-Shear Strength of High Strength Adhesive Bonds. ISO: Geneva, Switzerland, 1979.
- ISO 62:2008; Plastics—Determination of Water Absorption. ISO: Geneva, Switzerland, 2008.
- ISO 175:2010; Plastics—Methods of Test for the Determination of the Effects of Immersion in Liquid Chemicals. ISO: Geneva, Switzerland, 2010.
- Wang, T.P.; Shen, L.; Forrester, M.; Lee, T.H.; Torres, S.; Pearson, C.; Cochran, E. Shelf-stable Bingham plastic polyurethane thermosets for additive manufacturing. ACS Mater. Lett. 2024, 6, 1077–1085. [Google Scholar] [CrossRef]
- Shen, L.; Wang, T.P.; Lee, T.H.; Forrester, M.; Becker, A.; Torres, S.; Cochran, E.W. 3D printable all-polymer epoxy composites. ACS Appl. Polym. Mater. 2021, 3, 5559–5567. [Google Scholar] [CrossRef]

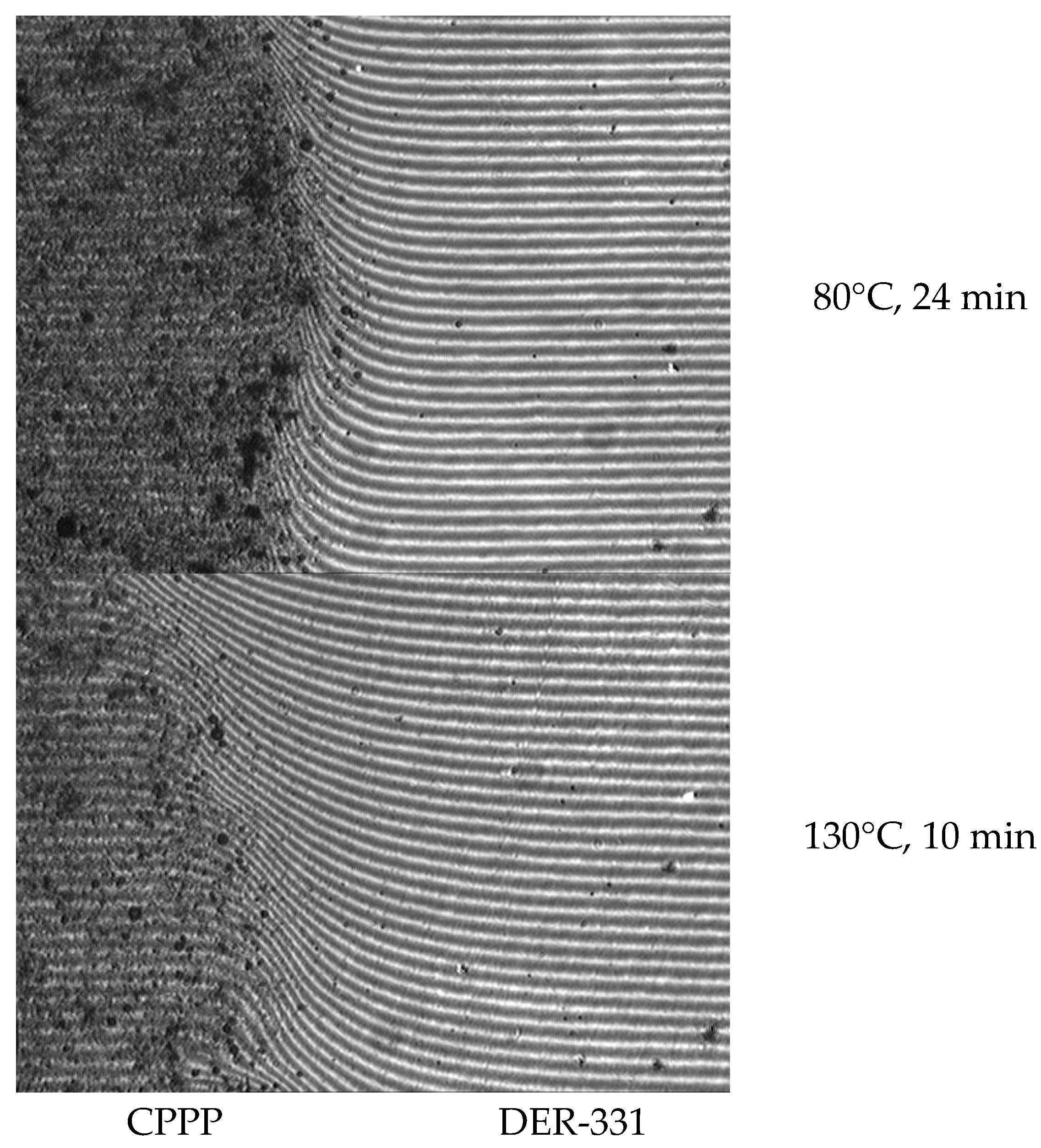
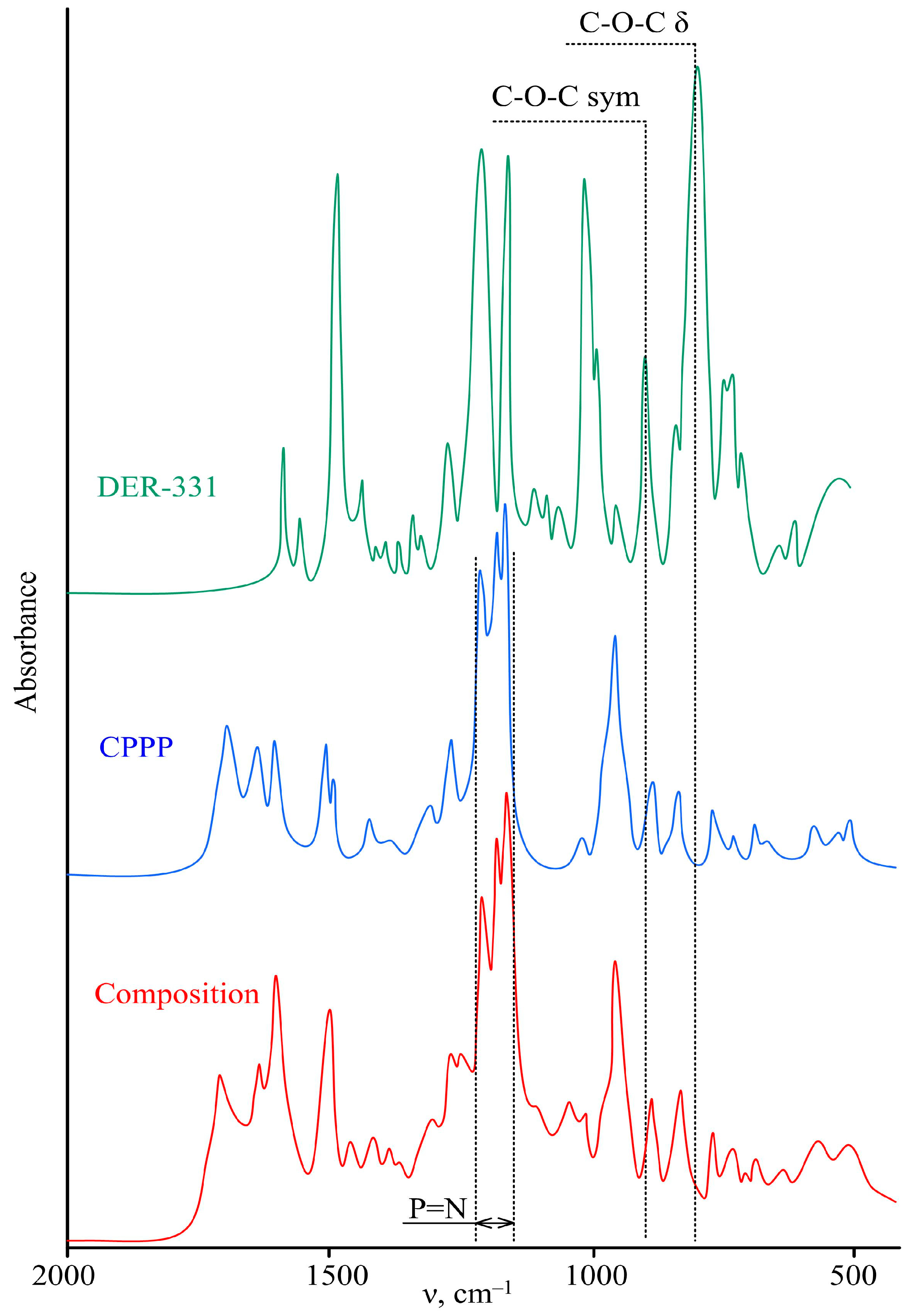
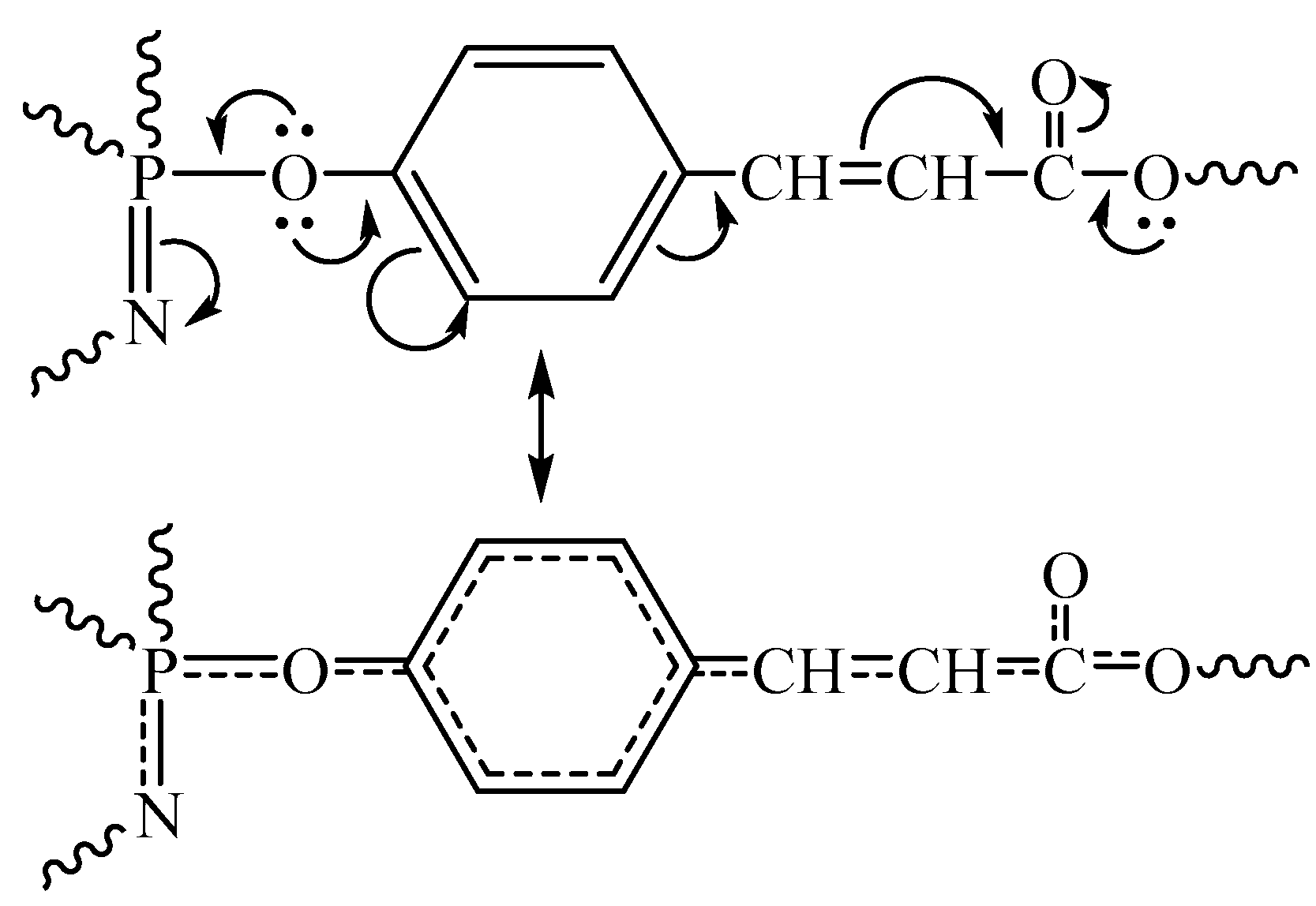

| Contained in CPPP Derivative | Diameter of Sphere, nm |
|---|---|
| Nongem-cis-bis[4-(β-carboxyethenyl)phenoxy]-tetraphenoxycyclotriphosphazene | 1.34 |
| Nongem-trans-bis[4-(β-carboxyethenyl)phenoxy]-tetraphenoxycyclotriphosphazene | 1.98 |
| Nongem-cis-tris[4-(β-carboxyethenyl)phenoxy]-triphenoxycyclotriphosphazene | 1.68 |
| Nongem-trans-tris[4-(β-carboxyethenyl)phenoxy]-triphenoxycyclotriphosphazene | 1.90 |
| Gem-tris[4-(β-carboxyethenyl)phenoxy]-triphenoxycyclotriphosphazene | 1.91 |
| Nongem-cis-tetrakis[4-(β-carboxyethenyl)phenoxy]-diphenoxycyclotriphosphazene | 1.93 |
| Nongem-trans-tetrakis[4-(β-carboxyethenyl)phenoxy]-diphenoxycyclotriphosphazene | 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinova, A.; Yudaev, P.; Shapagin, A.; Panfilova, D.; Palamarchuk, A.; Chistyakov, E. Non-Flammable Epoxy Composition Based on Epoxy Resin DER-331 and 4-(β-Carboxyethenyl)phenoxy-phenoxycyclotriphosphazenes with Increased Adhesion to Metals. Sci 2024, 6, 30. https://doi.org/10.3390/sci6020030
Konstantinova A, Yudaev P, Shapagin A, Panfilova D, Palamarchuk A, Chistyakov E. Non-Flammable Epoxy Composition Based on Epoxy Resin DER-331 and 4-(β-Carboxyethenyl)phenoxy-phenoxycyclotriphosphazenes with Increased Adhesion to Metals. Sci. 2024; 6(2):30. https://doi.org/10.3390/sci6020030
Chicago/Turabian StyleKonstantinova, Anastasia, Pavel Yudaev, Aleksey Shapagin, Darya Panfilova, Aleksandr Palamarchuk, and Evgeniy Chistyakov. 2024. "Non-Flammable Epoxy Composition Based on Epoxy Resin DER-331 and 4-(β-Carboxyethenyl)phenoxy-phenoxycyclotriphosphazenes with Increased Adhesion to Metals" Sci 6, no. 2: 30. https://doi.org/10.3390/sci6020030
APA StyleKonstantinova, A., Yudaev, P., Shapagin, A., Panfilova, D., Palamarchuk, A., & Chistyakov, E. (2024). Non-Flammable Epoxy Composition Based on Epoxy Resin DER-331 and 4-(β-Carboxyethenyl)phenoxy-phenoxycyclotriphosphazenes with Increased Adhesion to Metals. Sci, 6(2), 30. https://doi.org/10.3390/sci6020030









