Abstract
Cassia alata, a traditional herb with a global presence, is renowned for its anti-inflammatory, antibacterial, and antifungal properties, making it a go-to remedy for skin ailments. While it has demonstrated wound healing capabilities in both in vitro and in vivo studies, the precise mechanisms remain elusive. This review aims to highlight its key phytochemicals, their effects, and the mechanism of action. The compounds that have been reviewed and discussed include kaempferol, apigenin, quercetin, rhein, and rutin. These polyphenols play important roles in normal and impaired wound healing processes, encompassing hemostasis, inflammation, proliferation, and tissue remodeling.
Keywords:
wound healing; Cassia alata; kaempferol; apigenin; quercetin; rhein; rutin; antioxidant; antimicrobial; inflammation 1. Introduction
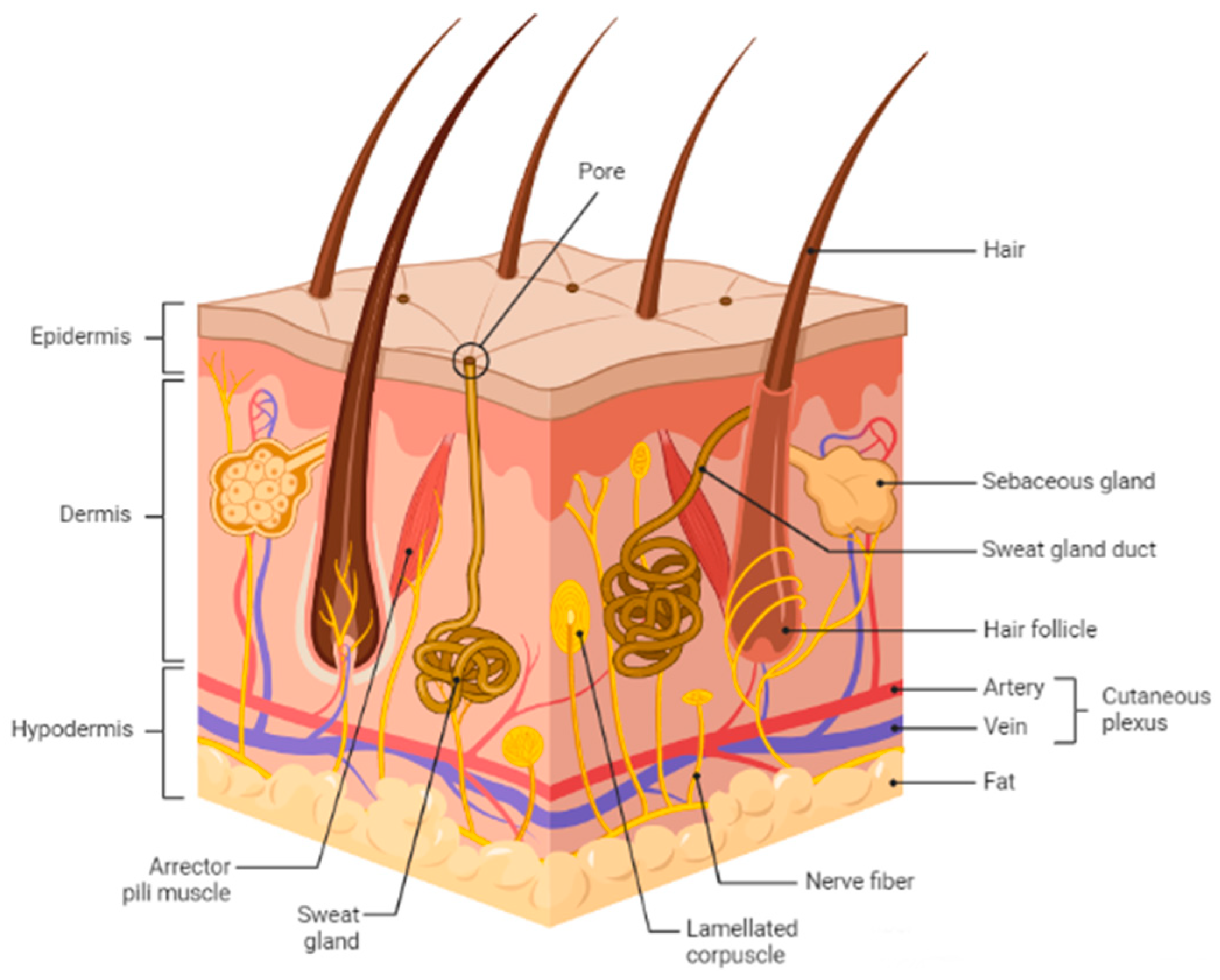
Skin is the body’s largest organ and serves as a protective barrier against external factors like bacteria, chemicals, temperature, and ultraviolet light. Additionally, it plays a crucial role in regulating the body’s homeostasis and preventing excessive water loss. The skin comprises three layers: the outermost layer is the epidermis, followed by the dermis, and finally, the hypodermis (Figure 1). When the skin is wounded, the protective epithelial barrier is restored through cell-cell interactions [1]. Every wound healing cycle takes about 4 to 6 weeks to complete, involving four highly programmed phases: hemostasis, inflammation, proliferation, and remodeling [2].
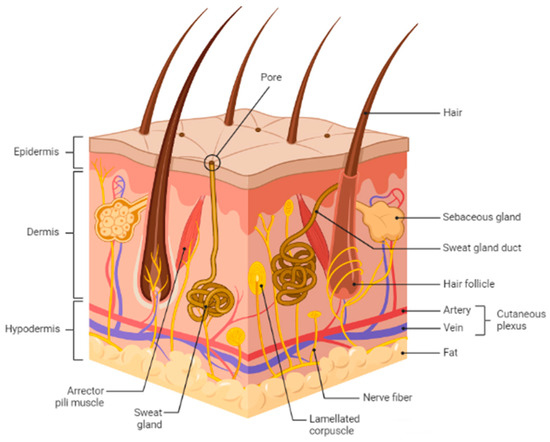
Figure 1.
Schematic representation of basic human skin anatomy depicting different skin layers and their components. The figure is adapted from Kolami et al. [3] under the Creative Commons Attribution License.
1.1. Phase 1: Hemostasis
Primary hemostasis results in the formation of a platelet plug at the injury site, followed by the coagulation cascade that stabilizes the plug and stops bleeding. A fibrin clot is formed to restore the skin barrier temporarily; the clot is a network of fibrin fibers containing red blood cells and platelets and acts as a matrix for cell migration [2].
1.2. Phase 2: Inflammation
Local immune cells, including mast cells, Langerhans cells, and macrophages, are activated by damage-associated molecular patterns (DAMPs) from damaged cells and pathogen-associated molecular patterns (PAMPs) from bacteria. These cells release proinflammatory cytokines and chemokines, recruiting neutrophils and macrophages through the release of chemoattractants, including interleukin 1 (IL-1), tumor necrosis factor-alpha (TNF-α), endotoxins, and pro-inflammatory signals [4]. Neutrophils produce proinflammatory cytokines (i.e., IL-1, interleukin 6 [IL-6], and TNF-α) and antipathogenic compounds (i.e., reactive oxygen species (ROS), proteases, and cationic peptides), adhere to the wound tissue, and phagocytize cellular and bacterial debris in the wound. Chemotactic signaling initiate the macrophages’ formation from monocytes in neighboring blood vessels [2].
During the inflammatory phase, two subsets of macrophages are produced [5]: the classically activated M1 macrophages and alternatively activated M2 macrophages. M1 macrophages promote inflammation through the release of ROS, pro-inflammatory cytokines, and growth factors, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), as well as clean up the wound debris [2,4]. VEGF and PDGF are essential in promoting re-epithelialization, fibroplasia, and angiogenesis. The growth of new blood vessels increases the supply of oxygen and nutrients to the wound area for wound repair. In addition, PDGF stimulates the migration of cells to the site of injury and regulates cell proliferation and differentiation in the wound healing process [4].
M2 macrophages are responsible for inflammation resolution by releasing immunoregulatory cytokines such as interleukin 4 (IL-4), interleukin 10 (IL-10), and interleukin 13 (IL-13) with anti-inflammatory properties and may either be newly recruited or transitioned from M1 macrophages [4]. In addition, arginase expression is upregulated to increase the production of proline and polyamines, supporting the proliferative phase and matrix remodeling process [6]. Prolonged inflammation in the wound area has been associated with delayed wound healing that can result in delayed re-epithelialization, abnormal extracellular matrix (ECM) deposition, damage to surrounding tissue, and formation of scars [7,8,9]. This prolonged inflammation has also been linked with increased oxidative stress in the wound that can result in lipid peroxidation as indicated by the presence of thiobarbituric acid reactive substances (TBARs) as well as protein oxidative damage as indicated by the presence of carbonyls and sulfhydryls [10], thus delaying wound healing [11,12]. The presence of bacterial infection in wounds has also been linked to prolonged inflammation and increased oxidative stress [13]. Antioxidative and antibacterial compounds such as in bergamot and elderberry may thus contribute to the resolution of inflammation [10,14].
1.3. Phase 3: Proliferation
Tissue repair is initiated during the proliferation phase with the synthesis of granulation tissue, comprising blood vessels, fibroblasts, and the ECM, including collagen, elastin, and proteoglycans. In the re-epithelialization process, keratinocytes at the wound edge become migratory and invasive, moving across the wound to form the epidermis. This migration is facilitated by matrix metalloproteinase 1 (MMP-1) and MMP-9 enzymes, aiding in the reconstruction of the basement membrane. As the gap closes, keratinocytes eventually adhere to the ECM, contributing to the rebuilding of the epidermis [4]. Fibroblasts are then differentiated into myofibroblasts in the matrix, which express smooth muscle actin (SMA) and also deposit ECM proteins such as collagen and fibronectin. SMA promotes wound contraction and wound closure. VEGF and PDGF stimulate growth and differentiation, while angiogenin, transforming growth factor alpha (TGF-α), and transforming growth factor beta (TGF-β) induce the migration and proliferation of endothelial cells in angiogenesis [2].
1.4. Phase 4: Tissue Remodeling
New tissue is remodeled and strengthened to restore its normal structure and function. Fibroblasts assist in re-epithelialization and rebuilding the ECM; they are signaled into the wound bed, followed by collagen protein deposition and reorganization [15]. The existing more elastic collagen with thinner strands (collagen III) is degraded by matrix metalloproteinases (MMPs) to allow the deposition and reorganization of new collagen (collagen I) at the wound area, which strengthens the wound structure [4,16].
It was notable that despite the importance of ECM rebuilding and remodeling, excessive ECM production can result in scarring. Keloid and hypertrophic scars often exhibit disorganized and excessive ECM deposition due to the imbalance in deposition and degradation of ECM components [17]. Fibroblasts from hypertrophic scars show increased expression and production of types I and III collagen compared to normal skin [7], while keloid scars also have increased but disorganized collagen and dysregulated MMPs production that contribute to the migratory and invasive properties of keloid fibroblasts into surrounding tissue [8,9].
1.5. Botanical Description
Cassia alata is a traditional herb belonging to the Fabaceae family (Figure 2). It has various local names, such as candlebush, gelenggeng or daun kurap (Malay), Ath thora or Eth thora (Sri Lanka), dad mardan (India), Roman Candle tress (Fiji), and 翅莢決明 (China) [18]. Various parts of the plant have been used traditionally to treat skin diseases (including eczema, ringworm, and itching), hepatitis, jaundice, and gastrointestinal issues (including constipation, food poisoning, and dysentery) [19,20]. Its medicinal properties include antifungal, anti-inflammatory, analgesic, laxative, and anthelmintic effects. In addition, it also exhibits antidiabetic effects in vivo [21].

Figure 2.
Cassia alata plant.
C. alata has been traditionally used for treating skin diseases and has shown remarkable potential as a wound healing agent. Despite its lesser-known role in wound care, numerous studies have highlighted its effectiveness in wound healing, suggesting it is a promising candidate for development. This review aims to discuss and highlight the relevant wound healing mechanisms exhibited by C. alata and its phytochemicals. Several polyphenols are highlighted, and their contribution to the wound healing process is thoroughly discussed.
The role and effectiveness of C. alata as a wound healing agent have been reported [22,23,24,25,26]. It has demonstrated highly promising effects on wound recovery in both in vitro and in vivo models. These include fibroblast and keratinocyte models, as well as rodent excision and burn wound models (Table 1). However, its wound healing mechanism has yet to be fully elucidated.

Table 1.
Wound healing effects of C. alata studied using different models.
2. Chemical Constituents and Their Wound Healing Properties
C. alata contains various classes of phytochemicals that contribute to its biological activities (Table 2). These phytochemicals may be used as biomarkers in samples through the use of attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy, which has been used to identify and quantify other drugs such as metformin as well [27,28]. They also possess activities, including antibacterial, antifungal, anti-inflammatory, and antioxidant activities, and other properties that contribute to wound healing [29,30,31,32].
The leaves contain flavonoids including kaempferol and its derivatives, isoflavones, and rutin [33,34]. Anthraquinones, such as emodin and rhein, along with terpenes, cardiac glycosides, cyanogenic glycosides, phenolic compounds, alkaloids, coumarins, saponins, resins, and other compounds are also present [35]. Additionally, stigmasterol, β-sitosterol, and taraxerol have been identified, along with vitamin E and methyl 2,4,6-trihydroxybenzoate [34,36,37].
The flower of C. alata contains various compounds, including unsaturated fatty acids, flavonoids, alkaloids, saponins, tannins, phenolic acids, and antinutrient compounds, such as phytates, cyanide, and oxalates [38,39]. Several plant sterols and cyclosiloxanes are also present [40]. Additionally, the seed of C. alata is rich in fatty acids, including palmitic acid, stearic acid, oleic acid, linoleic acid, arachidic acid, and behenic acid [41]. Other metabolites, such as tannins, saponins, flavonoids, phenols, and alkaloids, as well as antinutrient constituents, including oxalate, cyanide, and phytate, have also been discovered [38].
The root of C. alata contains alkaloids, carbohydrates, tannins, saponins, phenol, flavonoids, anthraquinone, and cardiac glycosides [36,42]. Studies by Fernand et al. and Chatsiriwej et al. revealed the presence of rhein, kaempferol, aloe-emodin, emodin, chrysophanol, and physcion using high-pressure liquid chromatography (HPLC) [43,44]. In addition, other compounds were also detected using gas chromatography and mass spectroscopy (GC-MS): 1,2,3-propanetriol; α-D-glucopyranoside; β-D-mannofuranoside; n-hexadecenoic acid; 1,3-dihydroxy-2-propanone; oleic acid; and 6-deoxy-L-mannose [36].
It was interesting to note that analytical studies on the same species of plants might yield different chemical components in varying compositions. This can be explained in terms of climate and environmental variations as well as the methods or solvents used to produce the plant extract [33].

Table 2.
Chemical constituents that have been found in different parts of C. alata.
Table 2.
Chemical constituents that have been found in different parts of C. alata.
| Chemical Class | Compounds | Refs. |
|---|---|---|
| Flavonoids | 2,5,7,4′-Tetrahydroxy isoflavone 3,5,7,4’-Tetrahydroxy flavone Apigenin Epigenin Kaempferol Kaempferol 3-O-gentiobioside Kaempferol-3,7-diglucoside Kaempferol-3-O-gentiobioside Kaempferol-3-O-glucoside Kaempferol-3-O-ß-D-glucopyranoside Kaempferol-O-diglucoside Kaempferol-O-glucoside Quercetin-O-glucoside Rutin Syringone | [33,34,43,45,46,47,48,49] |
| Phenolics | Caffeic acid (-)Epiafzelechin Gallic acid | [34,50,51] |
| Anthraquinones | Alanonal Aloe-emodin Chrysophanol Danthron Emodin Physcion Rhein | [33,37,43,46,47,52] |
| Others | 1,3-Dihydroxy-2-propanone 6-Deoxy-l-mannose Methyl 2,4,6-trihydroxybenzoate Vitamin E Cyclotrisiloxane and its derivatives Thiophene, tocopherol Β-carotene | [34,36,40] |
While many phytochemicals have been detected in C. alata, this review specifically focuses on selected wound healing flavonoids and anthraquinones, namely rutin, kaempferol, quercetin, aloe-emodin, and rhein. These compounds are highlighted because they have demonstrated promising effects in various wound healing models [29,53,54,55,56]. Their molecular mechanisms, which contribute to the overall wound healing efficacy, are also discussed.
2.1. Flavonoids
Flavonoids have been detected in C. alata. They belong to a large class of phenolic phytochemical compounds that can be further classified into anthocyanidins, flavones, flavanols, flavanones, coumarins, aurones, chalcones, biflavones, and other types based on their chemical structure and side chains [57,58]. This class of compounds has been associated with various biological properties, including antioxidant and pro-oxidant activities [59], anti-inflammatory effects, antiproliferative effects, antidiabetic properties [60], and wound healing [61]. Kaempferol and its derivatives, which are detected in C. alata, have shown promising wound healing activity [33,62,63].
2.1.1. Kaempferol and Its Derivatives
Kaempferol (Figure 3) and its derivatives [64], such as kaempferol 3-O-gentiobioside [65], kaempferol-3-O-glucoside [34], kaempferol 3-O-sophoroside [66], kaempferol-O-diglucoside [47], and kaempferol 3-O-β-glucopyranoside [45], have been reported in C. alata. Various studies have shown that these compounds exhibit wound healing properties, and their mechanisms of action have been described (Table 3).
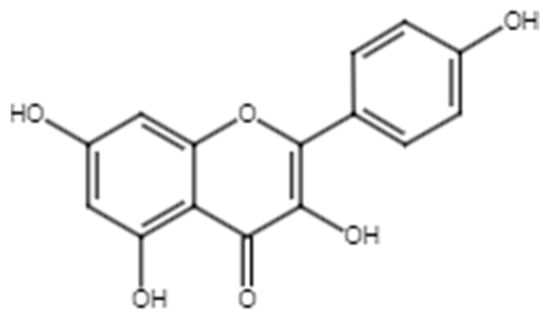
Figure 3.
Chemical structure of kaempferol.

Table 3.
Relevant studies on the wound healing properties of kaempferol and derivatives.
In addition to promoting wound healing, kaempferol and its derivatives have shown promising anti-inflammatory effects. These effects indirectly promote wound healing by suppressing the expression of MMP-9 [73]. Cui et al. demonstrated that the release of the pro-inflammatory cytokine TNF-α in wounds induces the production of MMP-9 in HaCaT cells [74], leading to inflammation. High levels of MMP-9 reduce collagen deposition and inhibit the formation of ECM [75], resulting in slower healing. Kaempferol and its derivatives bind to the active sites of MMP-1 and MMP-9, inhibiting their activities. This contributes to their anti-inflammatory effect and promotes tissue growth and the regeneration of the ECM [73,76]. Moreover, kaempferol has been reported to downregulate the gene expression of proteins involved in inflammatory and immune responses, including the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway by downregulating peroxisome proliferator-activated receptors (PPARs) [77]. NF-κB is a crucial pathway in inflammation, as it regulates the release of inflammatory cytokines such as TNF-α, interleukin 1 beta (IL-1β), IL-6, and interleukin 8 (IL-8); T-cell activation and differentiation; and the regulation of inflammasomes [78]. Thus, the downregulation of NF-κB and other inflammatory pathways by kaempferol may result in anti-inflammatory activity.
Blood vessel formation, known as angiogenesis, plays a crucial role in wound healing. Angiogenesis ensures the proper supply of oxygen, nutrients, and immune cells to the site of injury, supporting the regeneration and repair of damaged tissues. VEGF promotes blood vessel formation by increasing the proliferation and sprouting of endothelial cells [79] resulting in neovascularization during the proliferative phase of wound healing [80]. VEGF also increases the permeability of blood vessels and promotes epidermal regeneration, thus aiding re-epithelialization [69,81,82]. The presence of kaempferol in C. alata extract increases angiogenesis at low concentrations (1–10 µM) by binding with VEGF. Hu et al. found that kaempferol’s binding with VEGF promotes wound healing processes, facilitating the migration of monocytes, endothelial cells, and keratinocytes, as well as signaling for MMP-2 and 9, which promote angiogenesis [69].
Chronic wounds can result from increased inflammation and hyperglycemic conditions [83,84,85]. Inflammation slows down wound healing, delays re-epithelialization, and hinders the formation of scar granulation tissue. This delay in wound healing processes is particularly evident in diabetic wounds, where inflammation and hyperglycemia prolong the healing process. Ozay et al. [53] discovered that kaempferol is effective in diabetic wound healing. It enhances wound contraction and re-epithelialization while increasing the tensile strength of the wound. Additionally, it promotes collagen synthesis in both diabetic and nondiabetic wounds. To facilitate wound closure, kaempferol scavenges free radicals generated in the wound, thereby reducing oxidative stress and decreasing inflammation at the site of injury [86].
Uncontrolled inflammation in wounds is also associated with the formation of hypertrophic and keloidal scars [83,84]. Keloids and hypertrophic scars form due to disorganized and uncontrolled ECM deposition. Kaempferol can prevent the formation of these scars by inhibiting collagen synthesis and regulating the proliferation of hypertrophic fibroblasts and keloid fibroblasts [70,87]. It also regulates excessive inflammation [88].
Several studies have demonstrated the effectiveness of kaempferol derivatives in wound healing. For instance, kaempferol-3-O-glucoside and kaempferol-3-O-rutinoside induce cell migration through focal adhesion kinase (FAK)/Akt activation, leading to the formation of filopodia and lamellipodia through Rac1 activation during the proliferative phase. Nicotiflorin and juglanin have also been shown to induce fibroblast migration in scratch assays [89]. Upon treatment, fibroblasts migrate to the wound tissue and secrete proteases that break down the existing ECM. This process releases growth factors that stimulate new growth and the production of ECM proteins [4].
To sum up, kaempferol has shown a significant role in different phases of wound healing, with the ability to regulate angiogenesis in the proliferative phase, inflammation, and ECM synthesis. The ability of kaempferol to increase angiogenesis despite its anti-angiogenic mechanism in studies reported by Chin et al. [90] and Liang et al. [91] is notable with the same pathways involved in both its pro-angiogenic and anti-angiogenic properties. With the concentration used in the anti-angiogenic studies [90,91] much higher than that used in the pro-angiogenic study by Hu et al. [69], it is plausible that the concentration of a phytochemical in a plant can determine its biological properties; however, this remains to be further elucidated.
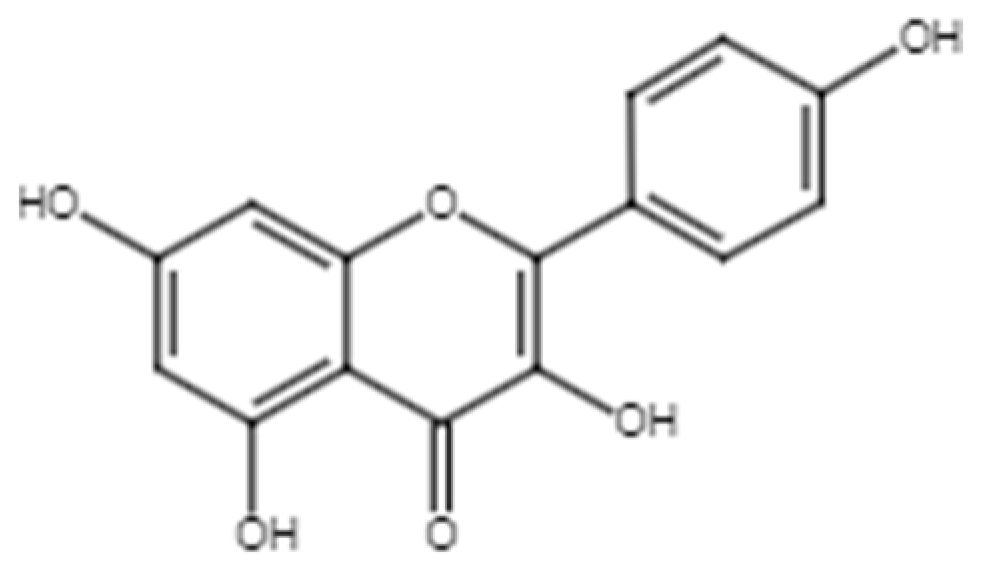
2.1.2. Apigenin
Apigenin (4′, 5, 7,-trihydroxyflavone) (Figure 4) has been identified in C. alata. A review by Zhou et al. summarized the various biological activities exhibited by apigenin, including anticancer, antioxidant, and anti-inflammatory effects, as well as antidiabetic and antidepressant effects [92].
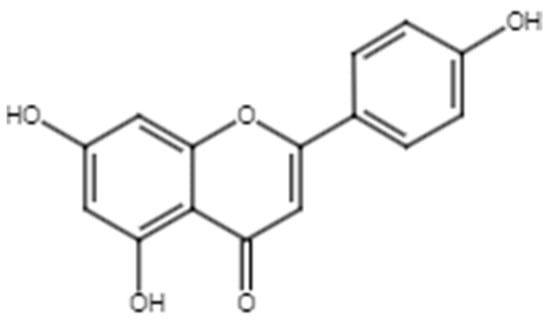
Figure 4.
Chemical structure of apigenin.
Several studies have reported on the wound healing activity of apigenin. Studies by Süntar et al. showed that topical application of apigenin promoted wound healing in rats with incisional and circular excision wounds [93], while Shukla et al. found that a hydrogel incorporating apigenin improved diabetic wound healing [94]. Table 4 summarizes the wound healing activity of apigenin.

Table 4.
Summary of wound healing studies on apigenin.
In wound healing models, it was found that apigenin exhibits three biological properties that facilitate wound healing: anti-inflammatory, antioxidant, and angiogenic properties [93,95]. Süntar et al. observed the anti-inflammatory activity of apigenin, reducing vascular permeability induced by acetic acid in rats using the Whittle method. This reduction in vascular permeability regulates the influx of immune cells, preventing an excessive inflammatory response that could cause tissue damage. Proper regulation of vascular permeability in this phase ensures an adequate blood supply to the developing tissue [93]. Ma et al. also noted that topical application could decrease the levels of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, at both high and low doses [97]. These cytokines mediate the inflammatory process. Increases in TNF-α and IL-1β causes the initiation and amplification of the inflammatory response, while an increase in IL-6 signifies the response to infection and tissue damage. The reduction in these pro-inflammatory cytokines indicates appropriate regulation of the inflammatory response, facilitating the wound recovery process.
In vitro studies have shown that apigenin regulates hyaluronidase and collagenase activity [93] and inhibits TNF-α-mediated MMP-9 expression [68]. It modulates the breakdown of ECM components by proteolytic enzymes while increasing the synthesis of collagen I and III by fibroblasts through the smad2/3 signaling pathway [97]. Proteolytic enzymes, such as MMPs, are produced by various skin cells, including fibroblasts, endothelial cells, wound-edge keratinocytes, macrophages, and neutrophils. These enzymes play important roles in ECM degradation during the proliferative and tissue remodeling processes, cell migration, and immune system defence during the inflammatory process [98].
Protease activity is regulated by protease inhibitors in interstitial and plasma fluids to prevent excessive proteolysis. Wound inflammation leads to increased levels of proteases during the early stage of wound healing. Excessive inflammation, as seen in chronic wounds, may overwhelm protective mechanisms such as the production of tissue inhibitors of metalloproteinases (TIMPs), leading to excessive breakdown of the ECM [99,100,101]. The anti-inflammatory property of apigenin may thus play a role in regulating proteolysis in addition to enzyme inhibition.
Apigenin could also promote wound healing by increasing angiogenesis. Tu et al. found that low concentrations of apigenin promote human umbilical vein endothelial cell (HUVEC) migration and proliferation through the VEGF and endothelial nitric oxide synthase (eNOS) pathways in ischemia–reperfusion (I/R) conditions [102]. I/R conditions increase wound inflammation [103]. However, the VEGF and eNOS pathways could be stimulated under hypoxic conditions, thus promoting angiogenesis during the initial stage of wound healing [104]. The induction of angiogenesis under such conditions may present another mechanism by which apigenin promotes wound healing in chronic wounds. It is worth noting that while low concentrations of apigenin promote angiogenesis [102], higher concentrations may inhibit this process, as observed in a study reported by Fang et al. [105]. This has also been noted in phytochemicals including kaempferol, with more studies required to justify the pro-angiogenic activity of apigenin in wound healing.
2.1.3. Rutin
Rutin (quercetin-3-O-rutinoside, Figure 5) is a flavonol known for its antioxidant, anti-inflammatory, and antibacterial activities [106] that are important in facilitating wound recovery (Table 5). Rutin has demonstrated its ability to improve wound healing in various models. For example, Shehab et al. observed enhanced wound closure in murine subjects treated with 5 mg/mL of rutin. The treatment group exhibited a significantly better wound healing effect than the positive control, valproic acid. This effect was characterized by reduced inflammation, reduced oxidation, improvement in tissue remodeling, re-epithelialization, and enhanced wound closure [107]. Additionally, Seo et al. also found that rutin may promote the migration of keratinocytes and human dermal fibroblasts via the β-catenin pathway [54]. The β-catenin pathway plays a significant role, particularly during the proliferative phase, as the β-catenin protein is a key component of the Wnt signaling pathway and is involved in various cellular processes, such as cell proliferation, differentiation, and tissue regeneration [108,109,110].
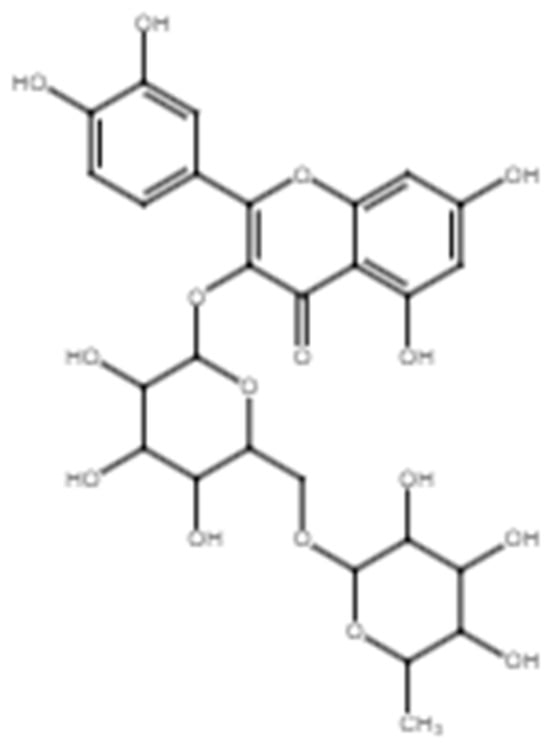
Figure 5.
Chemical structure of rutin.

Table 5.
Wound healing studies on rutin.
Rutin improves wound healing in diabetic animal models [111,114]. Notably, rutin-treated diabetic mice exhibited a significant reduction in inflammatory cells and inflammatory cytokines, TNF-α, and NF-κB. Simultaneously, the antioxidant enzymes superoxide dismutase 1 (SOD1) and glutathione peroxidase (GPx) showed significant increases in the treated wounds [111]. In an in silico study by Shehab et al., rutin was found to potentially inhibit the NF-κB inflammatory pathway by binding to IKKβ [107]. This binding reduced the phosphorylation of IKKβ, subsequently inhibiting the activation and migration of NF-κB to the nucleus, resulting in the downregulation of inflammation [115].
In addition to its anti-inflammatory effects, in silico studies by Selvaraj et al. and Taherkhani et al. explored rutin’s ability to bind to various metalloproteinases, including gelatinase A (MMP-2), stromelysin (MMP-3), collagenase (MMP-9), metalloelastase (MMP-12), collagenase-3 (MMP-13), and collagenase-2 (MMP-8) [116,117,118]. Rutin has demonstrated potential inhibition of MMP-2 and MMP-9 consistent with findings by Chen et al., who observed reduced MMP-2 and MMP-9 levels in a diabetic wound animal model upon rutin treatment [111]. Caley et al. reviewed the role of MMP enzymes in wound healing, showing that MMP enzymes play an important role in ECM degradation as well as modulating the chemokines and wound healing processes, facilitating cell migration and angiogenesis [119] through anti-inflammatory activities. High levels of MMPs have been observed in chronic wound fluid compared to serum and acute wound fluid, indicating that the high levels of proteolysis may be associated with slow wound recovery [120] with the breakdown of important healing factors as well as the ECM.
The wound healing properties of rutin have been utilized in various wound care applications. Almeida et al. developed a rutin-containing hydrogel that proved effective in wound healing, increasing catalase (CAT) and endogenous antioxidants as well as reducing glutathione (GSH) and oxidative stress markers in a rat model [112]. Additionally, Tran et al. demonstrated that rutin released from a chitosan hydrogel in the form of an injectable dressing significantly improved L929 fibroblast cell proliferation, promoted wound closure, and accelerated the healing process [113]. These findings suggest that rutin has the potential to be incorporated into conventional wound care dressings and products, as well as the possible role of antioxidants in the wound healing process.
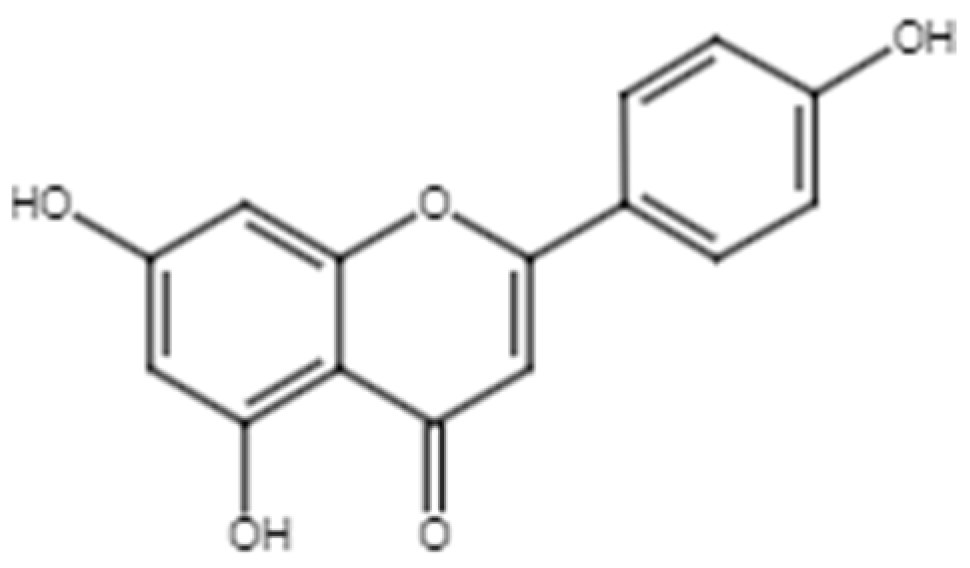
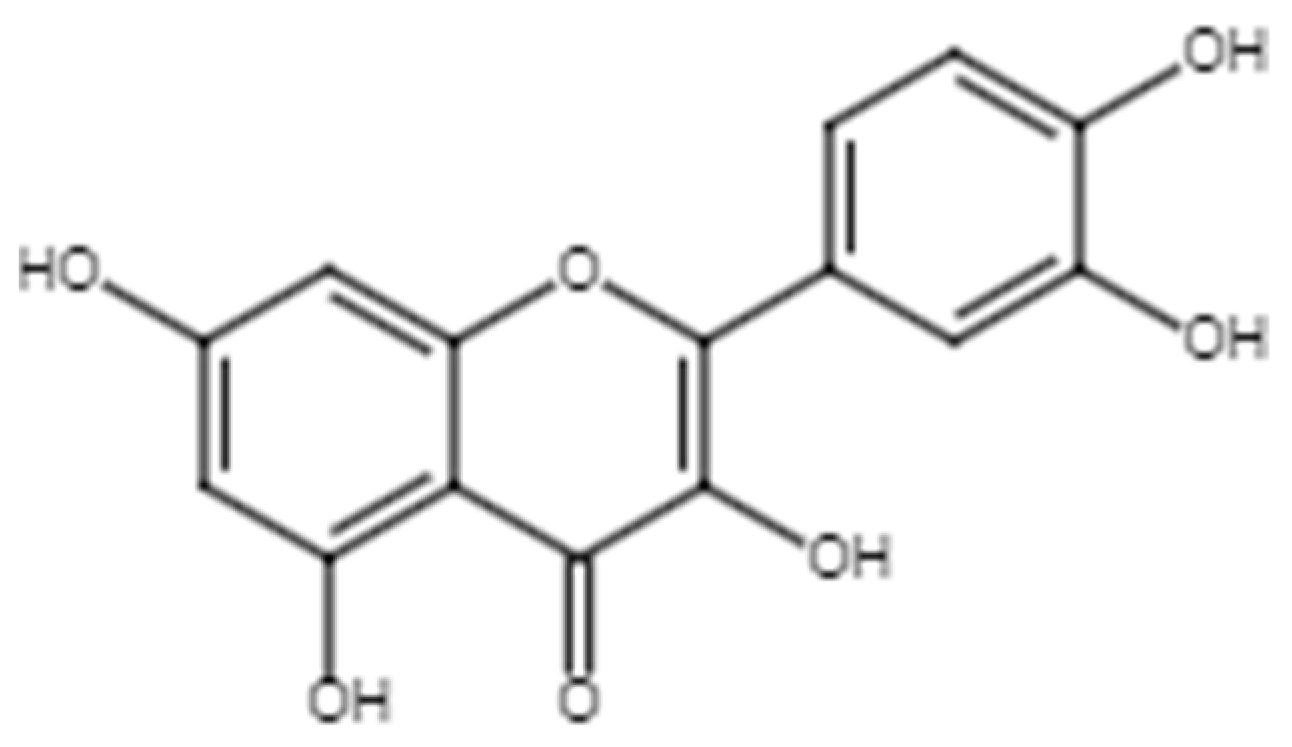
2.1.4. Quercetin
Various studies have reported that quercetin (Figure 6) promotes wound healing due to its effects on angiogenesis, wound remodeling, oxidative state, inflammation, and re-epithelialization [55,121,122,123,124,125]. Table 6 summarizes the relevant studies that highlight the wound healing activity of quercetin.
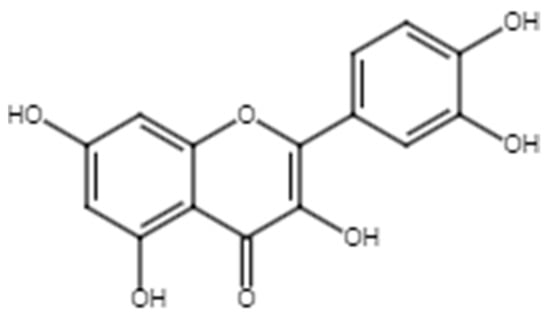
Figure 6.
Chemical structure of quercetin.

Table 6.
Summary of studies regarding wound healing effects of quercetin.
The wound healing activity of quercetin is closely linked to the Wnt/β-catenin pathway and telomerase (TERT) [121]. The Wnt/β-catenin pathway promotes cell proliferation, mediates migration, and encourages cell differentiation, contributing to tissue repair and regeneration [126,127]. TERT activity is associated with cellular regenerative potential, increasing the proliferation of keratinocytes [128]. Tissues with high regeneration capacity, such as basal keratinocytes [129], fibroblasts and endothelial cells in granulation tissue [130], have high expression of TERT. In chronic inflammation, decreased telomerase activity leads to slowed cell proliferation and migration, impairing the wound healing process [130].
The literature reports that quercetin may increase TERT expression and promote wound recovery [121]. Additionally, quercetin exhibits anti-inflammatory effects, reducing inflammatory factors while increasing the expression of growth factors and other proteins essential for wound repair [121]. Fu et al. and Ploeger et al. reported that quercetin reduces the expression of pro-inflammatory cytokines in both normal and impaired wound healing models. It regulates cytokine expression by targeting the mitogen-activated protein kinase (MAPK) and NF-κB pathways, both associated with inflammation and wound healing. The MAPK pathway activation promotes cell proliferation, migration to the wound site, immune cell recruitment, tissue matrix remodeling, and ECM regulation, ultimately promoting tissue repair. NF-κB initially promotes inflammation, then promotes the expression of anti-inflammatory molecules, facilitating the transition to the proliferative and remodeling phases of wound healing [131,132].
Inflammation can lead to scar formation due to impaired wound healing, resulting in scarring [83]. Quercetin could reduce scar formation by targeting surface αV integrin and β1 integrin. The former is related to the migration of fibroblasts, while β1 signaling is related to cell adherence to ECM and also potentially to the profibrotic activity of quercetin. This suggests that quercetin may improve the migration of fibroblasts and thus enhance wound healing. It has also shown less ECM deposition in the in vivo wound model, indicating that quercetin impacts ECM production [122].
In addition, the presence of antioxidant enzymes and endogenous antioxidants in the wound could also reduce ROS, which in turn improves the wound healing activity [133]. Similar to rutin, treatment with quercetin in in vivo wound models has shown improvement in wound healing activity due to the increased presence of antioxidants as well as reduced levels of radicals [55,134]. This is also evident in impaired in vitro atopic dermatitis wound model by Beken et al. [125]. Atopic dermatitis is an inflammatory skin disease with increased levels of inflammatory cytokines in the skin that could be resolved through the use of anti-inflammatory therapy, such as corticosteroids; thus, the anti-inflammatory property of quercetin may also be able to assist in the healing of such wounds [135].
2.2. Anthraquinones
Anthraquinones, a class of phytochemicals, exhibit a range of activities, including anti-tumor, anti-inflammatory, laxative, and antimalarial effects, among others. The interaction of certain anthraquinones with DNA has led to the development of anticancer drugs, such as daunorubicin [136]. Anthraquinones play a role in the primary metabolism of plants and can be synthesized in fungi [137] as well as several types of plants [136]. Anthraquinones, including danthron, emodin, aloe-emodin, and rhein, have been detected in C. alata and contribute to the biological effects important for wound healing [34,44,52].
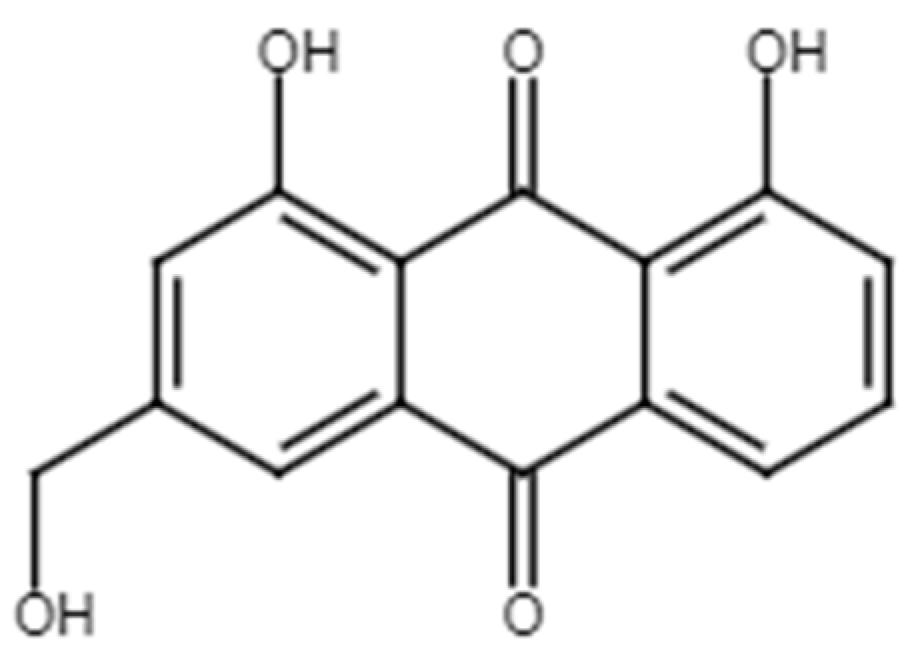
2.2.1. Aloe-Emodin
Aloe-emodin (Figure 7) is an anthraquinone that possesses anti-inflammatory and antimicrobial properties beneficial for wound recovery [138]. Aloe-emodin has demonstrated wound healing activity in both in vivo and in vitro studies (Table 7).
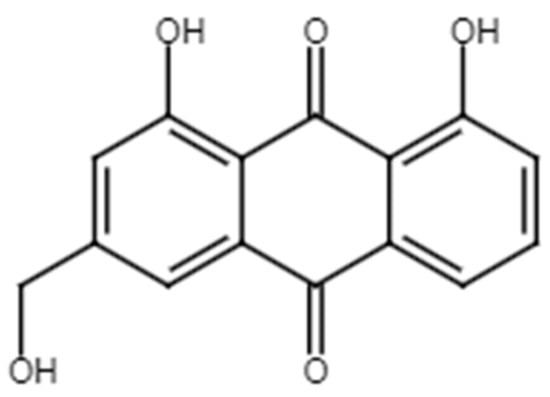
Figure 7.
Chemical structure of aloe-emodin.

Table 7.
Summary of wound healing studies involving aloe-emodin.
Aloe-emodin increases MCP-1, IL-1β, and VEGF in an animal burn wound model. MCP-1 is a chemoattractant that recruits macrophages to the wounds as part of the inflammatory process in early wound healing. A high level of IL-1β is produced by macrophages at the site of injury, and VEGF facilitates re-epithelialization and angiogenesis [138].
The wound healing effect exhibited by aloe-emodin is largely due to its anti-inflammatory properties. It downregulates the expression of inflammatory factors and markers such as inducible nitric oxide synthase (iNOS), TNF-α, IL-1β, and IL-6 through the NF-κB, MAPK, and phosphoinositide 3-kinase (PI3K) pathways in LPS-stimulated macrophages [139,140]. Aloe-emodin binds to and inhibits lipoxygenase (LOX) enzymes [141,142] that decrease inflammation and enhance wound healing [143]. Eicosanoids and leukotrienes are synthesized from arachidonic acid; the former is synthesized by COX and LOX enzymes, and the latter by the 5-lipoxygenase (5-LOX) pathway [144]. The inhibition of 5-LOX correlates with reduced inflammation and shorter wound healing duration in the knockout mice model, as reported by Ramalho et al. [145]. Thus, the inhibition of LOX by aloe-emodin may contribute to its ability to promote wound healing.
Aloe-emodin may also promote wound healing by inducing CCD-1079Sk fibroblast cell migration at low concentrations due to its high affinity for the MAPK pathways, specifically Jun N-terminal kinase (JNK) and p38 [56]. The migration of fibroblasts into the wound occurs during the proliferative phase, allowing for collagen deposition at the injury site during wound repair [146]. However, the authors noted that the effect on fibroblast migration was not concentration dependent, as lower concentrations of aloe-emodin promoted cell migration while the process was attenuated at higher concentrations. Proper regulation of JNK, p38, and extracellular signal-regulated kinase (ERK) in the MAPK pathway is crucial, as their activation varies during specific phases of healing. Dysregulation of JNK and p38 could lead to delayed or impaired healing. Further studies are needed to fully understand the wound healing properties of aloe-emodin.
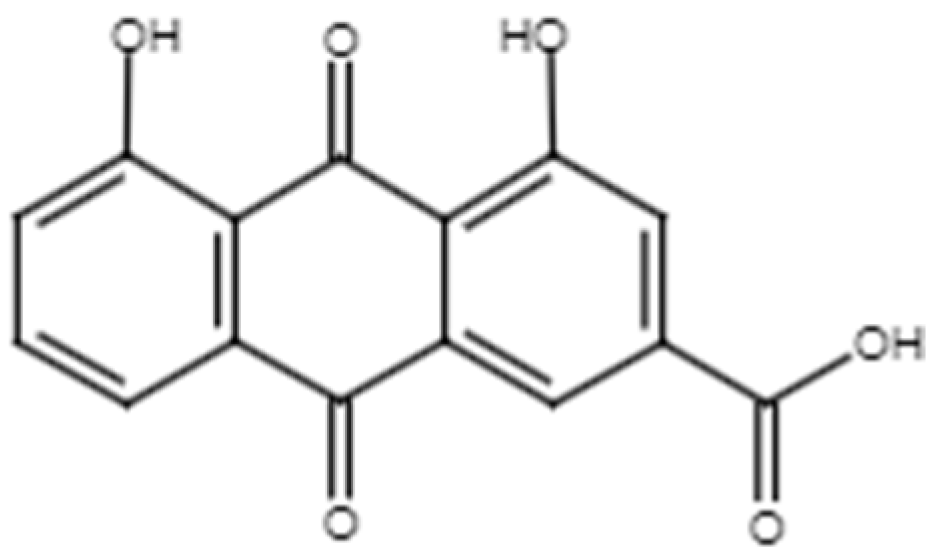
2.2.2. Rhein
Rhein (Figure 8) is an anthraquinone commonly found in Cassia sp. Rhein promotes wound recovery due to its anti-inflammatory activity. Lin et al. demonstrated that topical application of rhein reduced macrophages and neutrophils in imiquimod-induced psoriasiform lesions in mice [147]. Additional studies supported this observation, revealing rhein’s ability to modulate activity in LPS-stimulated macrophages [148]. Furthermore, it also downregulates the production of inflammatory cytokines in imiquimod-stimulated THP-1 macrophages and TNF-stimulated HaCaT cells through the MAPK and NF-κB pathways [147,149]. Rhein reduces the levels of inflammatory cytokines in both in vitro and in vivo studies [121,150], thus exhibiting anti-inflammatory activity that can facilitate the wound healing process.
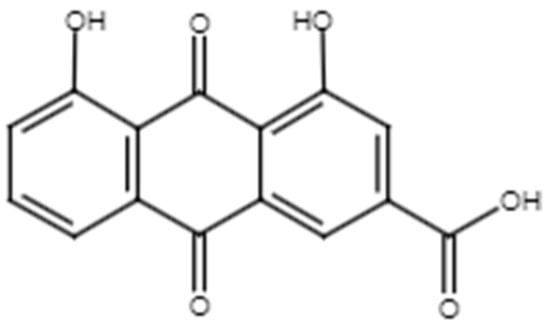
Figure 8.
Chemical structure of rhein.
In addition, rhein increases the proliferation of HaCaT cells by binding with the estrogen receptors in HaCaT cells during the proliferative phase (S phase) [151]. During the proliferative phase, re-epithelialization was observed in HaCaT cells at the wound edge, signifying the restoration of the epithelial layer in response to an injury. The proliferation of HaCaT cells contributes to the closure of the wound [87].
Hydrogels are considered excellent candidates for wound dressings due to their structural similarity to the ECM, high water retention enabling absorption of wound exudate, permeability allowing gaseous exchange, and optimal water content providing an ideal wound healing environment for dry wounds [152]. Rhein has been successfully incorporated into various delivery systems to improve wound healing. Yin et al. developed a silk fibroin hydrogel with rhein for S. aureus-infected burn wounds, resulting in reduced inflammation, improved angiogenesis, and increased formation of skin appendages, such as hair [30]. In addition, Li et al. developed a fibrous hydrogel reinforced with aramid nanofibers containing rhein that also showed excellent biocompatibility in skin tissue, mechanical strength, and water retention capacity as well as antibacterial activity against S. aureus. The hydrogel could also promote collagen and new blood vessel formation in burn wounds [29]. Zhang et al. also developed a hydrogel consisting of rhein, thiolated hyaluronate, gelatin, and silver ions. It has demonstrated skin regeneration properties in the murine full-thickness skin defect model by promoting angiogenesis as well as increasing the collagen deposition and M1 to M2 macrophage, a sign of reduced inflammation [31]. Another hydrogel formulation containing self-assembling rhein also reduces inflammation and oxidative stress in the diabetic full-thickness wound model, thus facilitating wound healing [153]. Additionally, delivery systems containing rhein such as phospholipid complexes have demonstrated good skin permeation and low skin irritation in vivo [154].
From the review of the current literature, further studies on the wound healing mechanism of rhein are lacking compared to the other compounds discussed in this review. However, its incorporation in wound dressings could promote wound healing in vivo [29,30,31,153]. With rhein exhibiting the ability to reduce inflammation and oxidative stress in tert-butyl hydroperoxide-induced HaCaT cells, LPS-stimulated RAW264.7 macrophages, and transgenic zebrafish [148,149,155] possibly by downregulation of inflammatory cytokines, it can be summarized that this mechanism may contribute to the healing properties of the rhein-incorporated dressings and support the hypothesis that regulation of the inflammatory phase could be beneficial in the context of wounds.
3. Conclusions
C. alata’s traditional use in treating skin diseases is backed by its potent anti-inflammatory, antibacterial, antifungal, and other medicinal properties. It contains diverse classes of phytochemicals that support the wound healing process across various stages: hemostasis, inflammation, proliferation, and tissue remodeling. The effectiveness of C. alata in wound healing can be attributed to the combined actions of these phytochemicals, which stimulate cell growth and movement, encourage new blood vessel formation, aid in the production of ECM, reduce oxidative stress, and alleviate inflammation. Studies have consistently shown that the synergistic or additive effects of these phytochemicals enhance wound healing compared to using a single compound. This may be due to the phytochemicals acting simultaneously on the same or different pathways; the chemical interaction between compounds including complexations, increasing each other’s bioavailability; and interactions with gut bacteria. While the compounds all possess wound healing activity, the research on this property of C. alata remains undeveloped. Thus, further research directions may include the further elucidation of the wound healing activity of C. alata by examining its ability to upregulate the expression of genes or proteins involved in wound healing, such as VEGF, TGF-β, and EGF; its ability to regulate the pathways implicated, such as the Akt pathway; as well as clinical trials to examine its wound healing properties in human models. Its ability to promote the different phases of wound healing such as angiogenesis and the tissue remodeling process could also be assessed in in vitro and in vivo of normal or impaired wound models. This will further build the case for C. alata to be utilized as a wound healing agent.
Author Contributions
Conceptualization: J.-W.K. and Y.-L.C.; Writing—Original Draft Preparation: J.-W.K. and S.-K.L. (Sue-Kei Lee); Writing—Review & Editing: K.-B.L., S.-K.L. (Siew-Keah Lee), S.-H.S., S.-C.C., S.-S.T., W.M.S.M.M., G.A.A., C.-W.M. and Y.-L.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the support of the Fundamental Research Grant Scheme (FRGS) (FRGS/1/2021/STG02/UCSI/02/1) from the Ministry of Higher Education Malaysia and UCSI University Research Excellence & Innovation Grant (REIG) (REIG-FPS-2023/037).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grubbs, H.; Manna, B. Wound Physiology. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Youssef, A.A.A.; Nyavanandi, D.; Dudhipala, N. A systemic review on development of mesoporous nanoparticles as a vehicle for transdermal drug delivery. Nanotheranostics 2023, 7, 70–89. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Chen, C.; Lin, Z.; Liu, W.; Hu, Q.; Wang, J.; Zhuang, X.; Guan, S.; Wu, X.; Hu, T.; Quan, S.; et al. Emodin accelerates diabetic wound healing by promoting anti-inflammatory macrophage polarization. Eur. J. Pharmacol. 2022, 936, 175329. [Google Scholar] [CrossRef] [PubMed]
- Szondi, D.C.; Wong, J.K.; Vardy, L.A.; Cruickshank, S.M. Arginase Signalling as a Key Player in Chronic Wound Pathophysiology and Healing. Front. Mol. Biosci. 2021, 8, 773866. [Google Scholar] [CrossRef]
- Zhang, K.; Garner, W.; Cohen, L.; Rodriguez, J.; Phan, S. Increased types I and III collagen and transforming growth factor-β1 mRNA and protein in hypertrophic burn scar. J. Investig. Dermatol. 1995, 104, 750–754. [Google Scholar] [CrossRef]
- Fujiwara, M.; Muragaki, Y.; Ooshima, A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br. J. Dermatol. 2005, 153, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.-S.; Xia, W.-S.; Yi, C.-G.; Wang, Y.-M.; Li, B.; Xia, W.; Liu, B.; Guo, S.-Z.; Sun, X.-D. Trichostatin A inhibits collagen synthesis and induces apoptosis in keloid fibroblasts. Arch. Dermatol. Res. 2011, 303, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Russo, M.; Cafeo, G.; Caruso, D.; Falliti, G.; Dugo, P.; Dossena, S.; et al. Mechanisms underlying the anti-aging activity of bergamot (Citrus bergamia) extract in human red blood cells. Front. Physiol. 2023, 14, 1225552. [Google Scholar] [CrossRef]
- Silva, A.C.C.; Eugênio, A.N.; Mariano, S.S.; Poletti, S.; Gaspi, F.G.; Bittencourt, J.V.S.; Casagrande, L.R.; Silveira, P.C.L.; Esquisatto, M.A.M.; Aro, A.A.; et al. Topical application of Azadirachta indica improves epidermal wound healing in hyperglycemic rats. Comp. Clin. Path. 2021, 30, 461–472. [Google Scholar] [CrossRef]
- Serarslan, G.; Altug, E.; Kontas, T.; Atik, E.; Avci, G. Caffeic acid phenethyl ester accelerates cutaneous wound healing in a rat model and decreases oxidative stress. Clin. Exp. Dermatol. 2007, 32, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, A.; Andersen, C.B.; Givskov, M.; Tolker-Nielsen, T. Quantitative analysis of the cellular inflammatory response against biofilm bacteria in chronic wounds. Wound Repair Regen. 2011, 19, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra extracts–natural antioxidants and antimicrobial compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Diller, R.B.; Tabor, A.J. The role of the extracellular matrix (ECM) in wound healing: A review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of collagen I and collagen III in tissue injury and regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Trowbridge, R.M.; Ayoub, N.T.; Agrawal, D.K. High-mobility group box protein-1, matrix metalloproteinases, and vitamin D in keloids and hypertrophic scars. Plast. Reconstr. Surg. Glob. Open 2015, 3, e425. [Google Scholar] [CrossRef] [PubMed]
- Globinmed. Senna alata (L.) Roxb. Available online: https://globinmed.com/medicinal_herbs/senna-alata-l-roxb-104893/ (accessed on 2 August 2023).
- Pieme, C.; Penlap, V.; Nkegoum, B.; Taziebou, P.; Tekwu, E.; Etoa, F.; Ngongang, J. Evaluation of acute and subacute toxicities of aqueous ethanolic extract of leaves of Senna alata (L.) Roxb (Ceasalpiniaceae). Afr. J. Biotechnol. 2006, 5, 283–289. [Google Scholar]
- Chew, Y.-L.; Khor, M.-A.; Xu, Z.; Lee, S.-K.; Keng, J.-W.; Sang, S.-H.; Akowuah, G.A.; Goh, K.W.; Liew, K.B.; Ming, L.C. Cassia alata, Coriandrum sativum, Curcuma longa and Azadirachta indica: Food ingredients as complementary and alternative therapies for atopic dermatitis-a comprehensive review. Molecules 2022, 27, 5475. [Google Scholar] [CrossRef] [PubMed]
- Hennebelle, T.; Weniger, B.; Joseph, H.; Sahpaz, S.; Bailleul, F. Senna alata. Fitoterapia 2009, 80, 385–393. [Google Scholar] [CrossRef]
- Midawa, S.; Ali, B.; Mshelia, B.; Johnson, J. Cutaneous wound healing activity of the ethanolic extracts of the leaf of Senna alata L.(Fabaceae). J. Biol. Sci. Bioconserv. 2010, 2, 63–68. [Google Scholar]
- Sangkaew, S.; Wanmasae, S.; Ongtanasup, T.; Srisang, S.; Manaspon, C.; Pooprommin, P.; Eawsakul, K. Development of nano—Emulsions for wound dressing containing Cassia alata L. leaves extract. SSRN 2022. [Google Scholar] [CrossRef]
- Agampodi, V.A. Isolation, Identification and Evaluation of Bioactive Compounds in Australian and Sri Lankan Native Plants and Their Potential Implications for Wound Healing. Ph.D. Thesis, Queensland University of Technology, Brisbane City, QLD, Australia, 2020. [Google Scholar]
- Kanedi, M.; Rokiban, A.; Widodo, S.; Nopiyansah; Isbiyantoro; Fauziah, L. Healing effect of leaf extract of candlebush (Cassia alata L.) on cutaneous wound infected with Trichophyton rubrum. World J. Pharm. Life Sci. 2016, 2, 42–50. [Google Scholar]
- Nasution, S.L.R.; Putri, M.; Hulu, W.; Girsang, E.; Nasution, A.N. Healing potential of Senna alata leaves extract in rats. J. Edu. Health Sport 2019, 9, 127–136. [Google Scholar]
- Sabbagh, B.A.; Kumar, P.V.; Chew, Y.L.; Chin, J.H.; Akowuah, G.A. Determination of metformin in fixed-dose combination tablets by ATR-FTIR spectroscopy. Chem. Data Coll. 2022, 39, 100868. [Google Scholar] [CrossRef]
- Adiana, M.A.; Mazura, M.P. Study on Senna alata and its different extracts by Fourier transform infrared spectroscopy and two-dimensional correlation infrared spectroscopy. J. Mol. Struct. 2011, 991, 84–91. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Han, X.; Liu, S.; Gao, X.; Guo, C.; Wu, X. Aramid nanofibers-reinforced rhein fibrous hydrogels as antibacterial and anti-Inflammatory burn wound dressings. ACS Appl. Mater. Interfaces 2022, 14, 45167–45177. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Han, X.; Lu, Q.; Qi, X.; Guo, C.; Wu, X. Rhein incorporated silk fibroin hydrogels with antibacterial and anti-inflammatory efficacy to promote healing of bacteria-infected burn wounds. Int. J. Biol. Macromol. 2022, 201, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, H.; Zhao, J.; Chai, P.; Ma, G.; Dong, Y.; He, X.; Jiang, Y.; Wu, Q.; Hu, Z.; et al. Body temperature-induced adhesive hyaluronate/gelatin-based hybrid hydrogel dressing for promoting skin regeneration. Int. J. Biol. Macromol. 2023, 253, 126848. [Google Scholar] [CrossRef] [PubMed]
- Chew, Y.-L.; Arasi, C.; Goh, J.-K. Pyrogallol induces antimicrobial effect and cell membrane disruption on methicillin-resistant Staphylococcus aureus (MRSA). Curr. Bioact. Compd. 2022, 18, 38–46. [Google Scholar] [CrossRef]
- Angelina, M.; Mardhiyah, A.; Dewi, R.T.; Fajriah, S.; Muthiah, N.; Ekapratiwi, Y.; Dewijanti, I.D.; Sukirno, S.; Jamilah, J.; Hartati, S. Physicochemical and phytochemical standardization, and antibacterial evaluation of Cassia alata leaves from different locations in Indonesia. Pharmacia 2021, 68, 947–956. [Google Scholar] [CrossRef]
- Duong, P.Q.; Duyen, N.T.; Quyen, P.T.; Tung, N.Q.; Son, V.H.; Hung, V.D.; Quang, L.D. Isolation and identification of phenolic compounds from the leaf extract of Cassia alata L. Vietnam J. Chem. 2017, 55, 589. [Google Scholar] [CrossRef]
- Muhammad, S.L.; Wada, Y.; Mohammed, M.; Ibrahim, S.; Musa, K.Y.; Olonitola, O.S.; Ahmad, M.H.; Mustapha, S.; Abdul Rahman, Z.; Sha’aban, A. Bioassay-guided identification of bioactive compounds from Senna alata L. against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. 2021, 1, 520–536. [Google Scholar] [CrossRef]
- Abubakar, I.; Mann, A.; Mathew, J. Phytochemical composition, antioxidant and anti-nutritional properties of root-bark and leaf methanol extracts of Senna alata L. grown in Nigeria. Afr. J. Pure Appl. Chem. 2015, 9, 91–97. [Google Scholar] [CrossRef]
- Chimi Fotso, S.; Tcho Tadjong, A.; Tsopgni, W.D.T.; Lenta, B.N.; Nkenfou, C.N.; Wansi, J.D.; Toze, F.A.A. Chemical constituents and antimicrobial activities of some isolated compounds from the Cameroonian species of Senna alata (Cassia alata L. Roxb synonym, The plant list 2013). (Leguminosae). Trends Phytochem. Res. 2021, 5, 37–43. [Google Scholar] [CrossRef]
- Isah, A.; Abdullahi, M.; Tsado, M.J. Evaluation of phytochemical, anti-nutritional and antioxidant potentials of flower and seed methanol extracts of Senna alata L. grown in Nigeria. Am. J. Appl. Chem. 2015, 3, 93. [Google Scholar] [CrossRef]
- Oladeji, S.O. Thin-layer chromatographic analysis of flavonoids and total phenolics in methanolic and ethanolic extracts of Senna alata (L.) Roxb. (Fabales: Fabaceae). Braz. J. Biol. Sci. 2016, 3, 221. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Subash-Babu, P.; Nassrallah, A.; Alshatwi, A.A.; Alkaltham, M.S. Cyclotrisiloxan and β-Sitosterol rich Cassia alata (L.) flower inhibit HT-115 human colon cancer cell growth via mitochondrial dependent apoptotic stimulation. Saudi J. Biol. Sci. 2021, 28, 6009–6016. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Ali, M.Y.; Ali, M.U.; Hasan, A.J.M.M. Studies on the lipid and glyceride compositions of Cassia alata seed oil. Bangladesh J. Sci. Ind. Res. 2007, 41, 83–88. [Google Scholar] [CrossRef]
- El-Mahmood, A.; Doughari, J. Phytochemical screening and antibacterial evaluation of the leaf and root extracts of Cassia alata Linn. Afr. J. Pharm. Pharmacol. 2008, 2, 124–129. [Google Scholar]
- Fernand, V.E.; Dinh, D.T.; Washington, S.J.; Fakayode, S.O.; Losso, J.N.; van Ravenswaay, R.O.; Warner, I.M. Determination of pharmacologically active compounds in root extracts of Cassia alata L. by use of high performance liquid chromatography. Talanta 2008, 74, 896–902. [Google Scholar] [CrossRef]
- Chatsiriwej, N.; Wungsintaweekul, J.; Panichayupakaranant, P. Anthraquinone production in Senna alata. root cultures. Pharm. Biol. 2006, 44, 416–420. [Google Scholar] [CrossRef]
- Hazni, H.; Ahmad, N.; Hitotsuyanagi, Y.; Takeya, K.; Choo, C.Y. Phytochemical constituents from Cassia alata with inhibition against methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2008, 74, 1802–1805. [Google Scholar] [CrossRef]
- Rahman, M.; Ali, M.; Ali, M. in vitro screening of two flavonoid compounds isolated from Cassia alata L. leaves for fungicidal activities. J. Biosci. 2008, 16, 139–142. [Google Scholar] [CrossRef][Green Version]
- Saito, S.T.; Trentin Dda, S.; Macedo, A.J.; Pungartnik, C.; Gosmann, G.; Silveira Jde, D.; Guecheva, T.N.; Henriques, J.A.; Brendel, M. Bioguided fractionation shows Cassia alata extract to inhibit Staphylococcus epidermidis and Pseudomonas aeruginosa growth and biofilm formation. Evid. Based Complement. Alternat. Med. 2012, 2012, 867103. [Google Scholar] [CrossRef] [PubMed]
- Okpuzor, J.; Ogbunugafor, H.A.; Kareem, G.K.; Igwo-Ezikpe, M.N. in vitro investigation of antioxidant phenolic compounds in extracts of Senna alata. Res. J. Phytochem. 2009, 3, 68–76. [Google Scholar] [CrossRef]
- Das, K.R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic potential and identification of phytotoxic substances in Cassia alata Linn. leaves. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 479–488. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Flamini, G.; Cioni, P.L.; Omikorede, O.; Azeez, R.A.; Ayodele, A.A.; Kamil, Y.O. Aromatic plants growing in Nigeria: Essential oil constituents of Cassia alata (Linn.) Roxb. and Helianthus annuus L. Rec. Nat. Prod. 2010, 4, 211. [Google Scholar]
- Phansawan, B.; Pongsabangpho, S. Determination of gallic acid and rutin in extracts Cassia alata and Andrographis paniculata. Sci. Asia 2014, 40, 414–419. [Google Scholar] [CrossRef]
- Adedayo, O.; Anderson, W.A.; Moo-Young, M.; Snieckus, V.; Patil, P.A.; Kolawole, D.O. Phytochemistry and antibacterial activity of Senna alata flower. Pharm. Biol. 2001, 39, 408–412. [Google Scholar] [CrossRef]
- Ozay, Y.; Guzel, S.; Yumrutas, O.; Pehlivanoglu, B.; Erdogdu, I.H.; Yildirim, Z.; Turk, B.A.; Darcan, S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Seo, S.H.; Lee, S.H.; Cha, P.H.; Kim, M.Y.; Min do, S.; Choi, K.Y. Polygonum aviculare L. and its active compounds, quercitrin hydrate, caffeic acid, and rutin, activate the Wnt/beta-catenin pathway and induce cutaneous wound healing. Phytother. Res. 2016, 30, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Jangir, B.L.; Kumar, V.; Nigam, A.; Sharma, V. Quercetin accelerated cutaneous wound healing in rats by modulation of different cytokines and growth factors. Growth Factors 2020, 38, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin-Akyildiz, A.; Yanikoglu, R.S.; Gulec, M.; Alim-Toraman, G.O.; Kuran, E.D.; Atasoy, S.; Olgun, A.; Topcu, G. Emodin and aloe-emodin, two potential molecules in regulating cell migration of skin cells through the MAP kinase pathway and affecting Caenorhabditis elegans thermotolerance. BMC Mol. Cell Biol. 2023, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Ribeiro, D.; Freitas, M.; Carvalho, F.; Fernandes, E. A comprehensive review on the antidiabetic activity of flavonoids targeting PTP1B and DPP-4: A structure-activity relationship analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4095–4151. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.T.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.; Barreto, R.S. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Study of antioxidant activity and immune stimulating potency of the ethnomedicinal plant, Cassia alata (L.) Roxb. Med. Aromat. Plants 2012, 2, 131. [Google Scholar] [CrossRef]
- Ling, Y.Y.; Fun, P.S.; Yeop, A.; Yusoff, M.M.; Gimbun, J. Assessment of maceration, ultrasonic and microwave assisted extraction for total phenolic content, total flavonoid content and kaempferol yield from Cassia alata via microstructures analysis. Mater. Today Proc. 2019, 19, 1273–1279. [Google Scholar] [CrossRef]
- Kaewsuwan, S. Bioassay-guided isolation of the antioxidant constituent from Cassia alata L. leaves. Songklanakarin J. Sci. Technol. 2004, 26, 103–107. [Google Scholar]
- Varghese, G.K.; Bose, L.V.; Habtemariam, S. Antidiabetic components of Cassia alata leaves: Identification through α-glucosidase inhibition studies. Pharm. Biol. 2013, 51, 345–349. [Google Scholar] [CrossRef]
- Palanichamy, S.; Nagarajan, S. Anti-inflammatory activity of Cassia alata leaf extract and kaempferol 3-O-sophoroside. Fitoterapia 1990, 61, 44–47. [Google Scholar] [CrossRef]
- Park, B.K.; Lee, S.; Seo, J.N.; Rhee, J.W.; Park, J.B.; Kim, Y.S.; Choi, I.G.; Kim, Y.E.; Lee, Y.; Kwon, H.J. Protection of burn-induced skin injuries by the flavonoid kaempferol. BMB Rep. 2010, 43, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Bohova, J.; Garcia-Villalba, R.; Tomas-Barberan, F.A.; Madakova, Z.; Majtan, T.; Majtan, V.; Klaudiny, J. Fir honeydew honey flavonoids inhibit TNF-alpha-induced MMP-9 expression in human keratinocytes: A new action of honey in wound healing. Arch. Dermatol. Res. 2013, 305, 619–627. [Google Scholar] [CrossRef]
- Hu, W.H.; Wang, H.Y.; Xia, Y.T.; Dai, D.K.; Xiong, Q.P.; Dong, T.T.; Duan, R.; Chan, G.K.; Qin, Q.W.; Tsim, K.W. Kaempferol, a major flavonoid in Ginkgo folium, potentiates angiogenic functions in cultured endothelial cells by binding to vascular endothelial growth factor. Front. Pharmacol. 2020, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, L.; Zhang, Y.; Gao, Z. Kaempferol inhibits fibroblast collagen synthesis, proliferation and activation in hypertrophic scar via targeting TGF-beta receptor type I. Biomed. Pharmacother. 2016, 83, 967–974. [Google Scholar] [CrossRef]
- Petpiroon, N.; Suktap, C.; Pongsamart, S.; Chanvorachote, P.; Sukrong, S. Kaempferol-3-O-rutinoside from Afgekia mahidoliae promotes keratinocyte migration through FAK and Rac1 activation. J. Nat. Med. 2015, 69, 340–348. [Google Scholar] [CrossRef]
- Ambiga, S.; Narayanan, R.; Gowri, D.; Sukumar, D.; Madhavan, S. Evaluation of wound healing activity of flavanoids from Ipomeoa carnea Jacq. Anc. Sci. Life 2007, 26, 45–51. [Google Scholar] [PubMed]
- Ju, P.C.; Ho, Y.C.; Chen, P.N.; Lee, H.L.; Lai, S.Y.; Yang, S.F.; Yeh, C.B. Kaempferol inhibits the cell migration of human hepatocellular carcinoma cells by suppressing MMP-9 and Akt signaling. Environ. Toxicol. 2021, 36, 1981–1989. [Google Scholar] [CrossRef]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol treats UVB-induced photoaging by anti-MMP expression, through anti-inflammatory, antioxidant, and antiapoptotic properties, and treats photoaging by upregulating VEGF-B expression. Oxid. Med. Cell Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef] [PubMed]
- Hariono, M.; Yuliani, S.H.; Istyastono, E.P.; Riswanto, F.D.; Adhipandito, C.F. Matrix metalloproteinase 9 (MMP9) in wound healing of diabetic foot ulcer: Molecular target and structure-based drug design. Wound Med. 2018, 22, 1–13. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.P. Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med. 2007, 73, 1267–1274. [Google Scholar] [CrossRef]
- Kang, B.Y.; Kim, S.; Lee, K.-H.; Lee, Y.S.; Hong, I.; Lee, M.-O.; Min, D.; Chang, I.; Hwang, J.S.; Park, J.S.; et al. Transcriptional profiling in human HaCaT keratinocytes in response to kaempferol and identification of potential transcription factors for regulating differential gene expression. Exp. Mol. Med. 2008, 40, 208–219. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Silva, E.A.; Mooney, D.J. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 2010, 31, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar] [PubMed]
- Chen, X.L.; Nam, J.-O.; Jean, C.; Lawson, C.; Walsh, C.T.; Goka, E.; Lim, S.-T.; Tomar, A.; Tancioni, I.; Uryu, S.; et al. VEGF-induced vascular permeability is mediated by FAK. Dev. Cell 2012, 22, 146–157. [Google Scholar] [CrossRef]
- Wise, L.M.; Inder, M.K.; Real, N.C.; Stuart, G.S.; Fleming, S.B.; Mercer, A.A. The vascular endothelial growth factor (VEGF)-E encoded by orf virus regulates keratinocyte proliferation and migration and promotes epidermal regeneration. Cell Microbiol. 2012, 14, 1376–1390. [Google Scholar] [CrossRef]
- Qian, L.W.; Fourcaudot, A.B.; Yamane, K.; You, T.; Chan, R.K.; Leung, K.P. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair Regen. 2016, 24, 26–34. [Google Scholar] [CrossRef]
- Ogawa, R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef]
- Phan, T.-T.; Sun, L.; Bay, B.-H.; Chan, S.-Y.; Lee, S.-T. Dietary compounds inhibit proliferation and contraction of keloid and hypertrophic scar-derived fibroblasts in vitro: Therapeutic implication for excessive scarring. J. Trauma Acute Care Surg. 2003, 54, 1212–1224. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.-F.; Wang, Z.-C.; Lou, D.; Fang, Q.-Q.; Hu, Y.-Y.; Zhao, W.-Y.; Zhang, L.-Y.; Wu, L.-H.; Tan, W.-Q. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef] [PubMed]
- Suktap, C.; Lee, H.K.; Amnuaypol, S.; Suttisri, R.; Sukrong, S. Wound healing effect of flavonoid glycosides from Afgekia mahidolae BL Burtt & Chermsir. leaves. Rec. Nat. Prod. 2018, 12, 391–396. [Google Scholar] [CrossRef]
- Chin, H.K.; Horng, C.T.; Liu, Y.S.; Lu, C.C.; Su, C.Y.; Chen, P.S.; Chiu, H.Y.; Tsai, F.J.; Shieh, P.C.; Yang, J.S. Kaempferol inhibits angiogenic ability by targeting VEGF receptor-2 and downregulating the PI3K/AKT, MEK and ERK pathways in VEGF-stimulated human umbilical vein endothelial cells. Oncol. Rep. 2018, 39, 2351–2357. [Google Scholar] [CrossRef]
- Liang, F.; Han, Y.; Gao, H.; Xin, S.; Chen, S.; Wang, N.; Qin, W.; Zhong, H.; Lin, S.; Yao, X.; et al. Kaempferol identified by zebrafish assay and fine fractionations strategy from Dysosma versipellis inhibits angiogenesis through VEGF and FGF pathways. Sci. Rep. 2015, 5, 14468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017, 41, e12376. [Google Scholar] [CrossRef]
- Suntar, I.; Kupeli Akkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: Isolation of apigenin as an active component. J. Ethnopharmacol. 2013, 149, 103–110. [Google Scholar] [CrossRef]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of apigenin loaded gellan gum-chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Camacho-Alonso, F.; Gomez-Garcia, F.; Molina Minano, F.; Canas, X.; Serafin, A.; Castillo, J.; Vicente-Ortega, V. Effects of potassium apigenin and verbena extract on the wound healing process of SKH-1 mouse skin. Int. Wound J. 2014, 11, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Rajab, A.A.; Al-Wattar, W.T.; Taqa, G.A. The roles of apigenin cream on wound healing in rabbits model. J. Appl. Vet. Sci. 2022, 7, 1–5. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.; Liu, Y.; Li, W.; He, J.; Fang, M.; Lin, D. Effects of apigenin treatment on random skin flap survival in rats. Front. Pharmacol. 2021, 12, 625733. [Google Scholar] [CrossRef] [PubMed]
- Sabino, F.; Hermes, O.; Egli, F.E.; Kockmann, T.; Schlage, P.; Croizat, P.; Kizhakkedathu, J.N.; Smola, H.; auf dem Keller, U. in vivo assessment of protease dynamics in cutaneous wound healing by degradomics analysis of porcine wound exudates. Mol. Cell Proteom. 2015, 14, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Yager, D.R.; Nwomeh, B.C. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999, 7, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R.; Ambrosch, A.; Schultz, G.; Waldmann, K.; Schiweck, S.; Lehnert, H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002, 45, 1011–1016. [Google Scholar] [CrossRef]
- Ligi, D.; Mosti, G.; Croce, L.; Raffetto, J.D.; Mannello, F. Chronic venous disease–part II: Proteolytic biomarkers in wound healing. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Pang, Q.; Chen, X.; Huang, T.; Liu, M.; Zhai, Q. Angiogenic effects of apigenin on endothelial cells after hypoxia-reoxygenation via the caveolin-1 pathway. Int. J. Mol. Med. 2017, 40, 1639–1648. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Nauta, T.D.; Van Hinsbergh, V.W.; Koolwijk, P. Hypoxic signaling during tissue repair and regenerative medicine. Int. J. Mol. Sci. 2014, 15, 19791–19815. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xia, C.; Cao, Z.; Zheng, J.Z.; Reed, E.; Jiang, B.-H. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005, 19, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Frutos, M.J.; Rincón-Frutos, L.; Valero-Cases, E. Rutin. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: London, UK, 2019; pp. 111–117. [Google Scholar]
- Shehab, N.G.; Abu-Gharbieh, E.; Ihab, M.A. Chemical composition, docking simulations and burn wound healing effect of Micromeria fruticosa extract and its isolated flavonoidal compound. Pak. J. Pharm. Sci. 2022, 35, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Birdsey, G.M.; Shah, A.V.; Dufton, N.; Reynolds, L.E.; Osuna Almagro, L.; Yang, Y.; Aspalter, I.M.; Khan, S.T.; Mason, J.C.; Dejana, E.; et al. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/β-catenin signaling. Dev. Cell 2015, 32, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shu, B.; Yang, R.; Xu, Y.; Xing, B.; Liu, J.; Chen, L.; Qi, S.; Liu, X.; Wang, P.; et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res. Ther. 2015, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Sobel, K.; Tham, M.; Stark, H.J.; Stammer, H.; Prätzel-Wunder, S.; Bickenbach, J.R.; Boukamp, P. Wnt-3a-activated human fibroblasts promote human keratinocyte proliferation and matrix destruction. Int. J. Cancer 2015, 136, 2786–2798. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Huang, C.N.; Liao, C.K.; Chang, H.M.; Kuan, Y.H.; Tseng, T.J.; Yen, K.J.; Yang, K.L.; Lin, H.C. Effects of rutin on wound healing in hyperglycemic rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef]
- Almeida, J.S.; Benvegnu, D.M.; Boufleur, N.; Reckziegel, P.; Barcelos, R.C.; Coradini, K.; de Carvalho, L.M.; Burger, M.E.; Beck, R.C. Hydrogels containing rutin intended for cutaneous administration: Efficacy in wound healing in rats. Drug Dev. Ind. Pharm. 2012, 38, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.Q.; Joung, Y.K.; Lih, E.; Park, K.D. In situ forming and rutin-releasing chitosan hydrogels as injectable dressings for dermal wound healing. Biomacromolecules 2011, 12, 2872–2880. [Google Scholar] [CrossRef]
- Kamalakkannan, N.; Prince, P.S.M. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, G.; Kaliamurthi, S.; Thiruganasambandam, R. Molecular docking studies of rutin on matrix metalloproteinase. Insights Biomed. 2016, 1. Available online: https://www.primescholars.com/articles/molecular-docking-studies-of-rutin-on-matrixmetalloproteinase-95479.html (accessed on 2 August 2023).
- Taherkhani, A.; Moradkhani, S.; Orangi, A.; Jalalvand, A.; Khamverdi, Z. Molecular docking study of flavonoid compounds for possible matrix metalloproteinase-13 inhibition. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, A.; Orangi, A.; Moradkhani, S.; Khamverdi, Z. Molecular docking analysis of flavonoid compounds with matrix metalloproteinase-8 for the identification of potential effective inhibitors. Lett. Drug. Des. Discov. 2021, 18, 16–45. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Rayment, E.A.; Upton, Z.; Shooter, G. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br. J. Dermatol. 2008, 158, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef] [PubMed]
- Doersch, K.M.; Newell-Rogers, M.K. The impact of quercetin on wound healing relates to changes in αV and β1 integrin expression. Exp. Biol. Med. 2017, 242, 1424–1431. [Google Scholar] [CrossRef]
- Kant, V.; Jangir, B.L.; Sharma, M.; Kumar, V.; Joshi, V.G. Topical application of quercetin improves wound repair and regeneration in diabetic rats. Immunopharmacol. Immunotoxicol. 2021, 43, 536–553. [Google Scholar] [CrossRef]
- Yin, G.; Wang, Z.; Wang, Z.; Wang, X. Topical application of quercetin improves wound healing in pressure ulcer lesions. Exp. Dermatol. 2018, 27, 779–786. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin improves inflammation, oxidative stress, and impaired wound healing in atopic dermatitis model of human keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Azmi, L.; Shukla, I.; Goutam, A.; Allauddin; Rao, C.V.; Jawaid, T.; Kamal, M.; Awaad, A.S.; Alqasoumi, S.I.; AlKhamees, O.A. In vitro wound healing activity of 1-hydroxy-5,7-dimethoxy-2-naphthalene-carboxaldehyde (HDNC) and other isolates of Aegle marmelos L.: Enhances keratinocytes motility via Wnt/β-catenin and RAS-ERK pathways. Saudi Pharm. J. 2019, 27, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.S.; Wei, Q.; Gurung, A.; Youn, A.; Bright, T.; Poon, R.; Whetstone, H.; Guha, A.; Alman, B.A. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J. 2006, 20, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Southworth, L.K.; Sarin, K.Y.; Venteicher, A.S.; Ma, W.; Chang, W.; Cheung, P.; Jun, S.; Artandi, M.K.; Shah, N.; et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008, 4, e10. [Google Scholar] [CrossRef] [PubMed]
- Kolquist, K.A.; Ellisen, L.W.; Counter, C.M.; Meyerson, M.M.; Tan, L.K.; Weinberg, R.A.; Haber, D.A.; Gerald, W.L. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat. Genet. 1998, 19, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Osanai, M.; Tamaki, T.; Yonekawa, M.; Kawamura, A.; Sawada, N. Transient increase in telomerase activity of proliferating fibroblasts and endothelial cells in granulation tissue of the human skin. Wound Repair Regen. 2002, 10, 59–66. [Google Scholar] [CrossRef]
- Fu, J.; Huang, J.; Lin, M.; Xie, T.; You, T. Quercetin promotes diabetic wound healing via switching macrophages from M1 to M2 polarization. J. Surg. Res. 2020, 246, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, D.T.A.; Hosper, N.A.; Schipper, M.; Koerts, J.A.; de Rond, S.; Bank, R.A. Cell plasticity in wound healing: Paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun. Signal. 2013, 11, 29. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The antioxidant effect of small extracellular vesicles derived from Aloe vera peels for wound healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef]
- Kant, V.; Jangir, B.L.; Nigam, A.; Kumar, V.; Sharma, S. Dose regulated cutaneous wound healing potential of quercetin in male rats. Wound Med. 2017, 19, 82–87. [Google Scholar] [CrossRef]
- Chew, Y.-L.; Al-Nema, M.; Ong, V.W.-M. Management and treatment of atopic dermatitis with modern therapies, complementary and alternative medicines: A review. Orient. Pharm. Exp. Med. 2018, 18, 67–76. [Google Scholar] [CrossRef]
- Diaz-Munoz, G.; Miranda, I.L.; Sartori, S.K.; de Rezende, D.C.; Diaz, M.A. Anthraquinones: An overview. Stud. Nat. Prod. Chem. 2018, 58, 313–338. [Google Scholar] [CrossRef]
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013, 49, 85–99. [Google Scholar] [CrossRef]
- Lin, L.X.; Wang, P.; Wang, Y.T.; Huang, Y.; Jiang, L.; Wang, X.M. Aloe vera and Vitis vinifera improve wound healing in an in vivo rat burn wound model. Mol. Med. Rep. 2016, 13, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, H.; Meng, X.; Wang, F.; Wang, P. Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. J. Ethnopharmacol. 2014, 153, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Kwon, H.-J.; Sung, M.-K. Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci. Biotechnol. Biochem. 2009, 73, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Sharanya, C.S.; Arun, K.G.; Sabu, A.; Haridas, M. Aloe emodin shows high affinity to active site and low affinity to two other sites to result consummately reduced inhibition of lipoxygenase. Prostaglandins Other Lipid Mediat. 2020, 150, 106453. [Google Scholar] [CrossRef] [PubMed]
- Rauwald, H.W.; Maucher, R.; Dannhardt, G.; Kuchta, K. Dihydroisocoumarins, naphthalenes, and further polyketides from Aloe vera and A. plicatilis: Isolation, identification and their 5-LOX/COX-1 inhibiting potency. Molecules 2021, 26, 4223. [Google Scholar] [CrossRef] [PubMed]
- Brogliato, A.R.; Moor, A.N.; Kesl, S.L.; Guilherme, R.F.; Georgii, J.L.; Peters-Golden, M.; Canetti, C.; Gould, L.J.; Benjamim, C.F. Critical role of 5-lipoxygenase and heme oxygenase-1 in wound healing. J. Investig. Dermatol. 2014, 134, 1436–1445. [Google Scholar] [CrossRef]
- Sivamani, R.K. Eicosanoids and keratinocytes in wound healing. Adv. Wound Care 2014, 3, 476–481. [Google Scholar] [CrossRef]
- Ramalho, T.; Filgueiras, L.; Silva-Jr, I.A.; Pessoa, A.F.M.; Jancar, S. Impaired wound healing in type 1 diabetes is dependent on 5-lipoxygenase products. Sci. Rep. 2018, 8, 14164. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Protective effects of tetrahydrocurcumin (THC) on fibroblast and melanoma cell lines in vitro: It’s implication for wound healing. J. Food Sci. Technol. 2017, 54, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Chuang, S.-Y.; Huang, T.-H.; Nguyen, T.M.H.; Wang, P.-W.; Alalaiwe, A.; Fang, J.-Y. A systematic comparison of the effect of topically applied anthraquinone aglycones to relieve psoriasiform lesion: The evaluation of percutaneous absorption and anti-inflammatory potency. Biomed. Pharmacother. 2022, 145, 112482. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Miao, J.; Lau, N.; Zhang, C.; Ye, P.; Du, S.; Mei, L.; Weng, H.; Xu, Q.; Liu, X. Rhein attenuates lipopolysaccharide-primed inflammation through NF-κB inhibition in RAW264. 7 cells: Targeting the PPAR-γ signal pathway. Can. J. Physiol. Pharmacol. 2020, 98, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Wadkhien, K.; Chinpaisal, C.; Satiraphan, M.; Wetwitayaklung, P.; Pongnimitprasert, N. Anti-inflammatory effects of rhein and crude extracts from Cassia alata L. in HaCaT cells. Sci. Eng. Health Stud. 2018, 12, 19–32. [Google Scholar] [CrossRef]
- Kim, M.; Ju Lee, H.; Randy, A.; Ho Yun, J.; Oh, S.-R.; Won Nho, C. Stellera chamaejasme and its constituents induce cutaneous wound healing and anti-inflammatory activities. Sci. Rep. 2017, 7, 42490. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chen, Y.; Guo, D.; Deng, Y.; Guo, W.; Liu, X.; Wang, Y.; Lu, H.; Liu, A.; Zhu, J.; et al. Rhein promotes the proliferation of keratinocytes by targeting oestrogen receptors for skin ulcer treatment. BMC Comp. Med. Ther. 2022, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, X.; Zhang, R.; Zhang, K.; Li, Y.; Xu, F.-J. Self-assembled herbal medicine encapsulated by an oxidation-sensitive supramolecular hydrogel for chronic wound treatment. ACS Appl. Mater. Interfaces 2020, 12, 56898–56907. [Google Scholar] [CrossRef]
- Ebada, H.M.; Nasra, M.M.; Elnaggar, Y.S.; Abdallah, O.Y. Novel rhein–phospholipid complex targeting skin diseases: Development, in vitro, ex vivo, and in vivo studies. Drug Deliv. Translat. Res. 2021, 11, 1107–1118. [Google Scholar] [CrossRef]
- Ge, H.; Tang, H.; Liang, Y.; Wu, J.; Yang, Q.; Zeng, L.; Ma, Z. Rhein attenuates inflammation through inhibition of NF-κB and NALP3 inflammasome in vivo and in vitro. Drug Des. Devel. Ther. 2017, 11, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).