Abstract
The study of non-phase modulation of different frequencies in the human electroencephalography (EEG) is revealing new mechanisms involved in information processing. In particular, it has been described that the alpha band, through a desynchronization of its non-phase component, could represent a mechanism for sensory gain in visual stimulus processing. One key question to address is whether this activity can be modulated (increased) by the recall of a previously memorized stimulus. The objective of this study is to answer this question by recording EEG activity with 58 electrodes and applying time-frequency analysis techniques (Temporal Spectral Evolution and the Hilbert Transform) in a sample of 27 human participants during a word recall task. The results of the study showed an increase in alpha phase modulation for recalled words compared to not recalled words, which included modulation of the P1 component. Additionally, alpha non-phase modulation also increased for recalled words, suggesting that the enhanced P1 component response could, in fact, be an indirect result of the attenuation of background neural noise, as proposed by the sensory gain hypothesis.
Keywords:
EEG; ERPs; Hilbert transformation; N1; non-phase alpha; P1; recalled word; Temporal Spectral Evolution 1. Introduction
The analysis of various frequencies in the human EEG is providing valuable insights into information processing in the brain [1,2,3]. Undoubtedly, one of the most studied frequency bands is the alpha band (8–12 Hz) [4,5]. Initially considered an indicator of cortical idling [6], recent research has described alternative functions, suggesting an active role in information processing [7,8]. One of the latest proposals is that the alpha band may be involved in a sensory gain mechanism to enhance the processing of stimuli presented to a subject [9].
This recent proposal stems from advancements in technology applied to the time-frequency analysis of human EEG. Initially, spectral modulations were studied using the Fourier Transform (FFT) [10]; however, this type of analysis does not allow for the millisecond-resolution study of the temporal evolution of oscillations. Since the 1970s, various time-frequency techniques have been developed, such as event-related desynchronization/synchronization (ERD/ERS) [11], wavelets [12], and Temporal Spectral Evolution (TSE) [13]. The latter presents an advantage over other techniques as it can capture non-phase modulations related to stimulus presentation (non-phase locked, time-locked) [9].
The study of non-phase modulation in the alpha band (and other frequencies) is revealing new cognitive mechanisms that are not observable with traditional analyses focused solely on phase activity (i.e., event-related potentials) [14,15]. To date, various studies have shown that these non-phase modulations occur across different frequency bands (alpha, beta, gamma, etc.); sometimes manifesting as synchronies or desynchronies, with specific latencies and topographies, and demonstrating a high level of replicability in longitudinal studies [16,17,18].
The proposal that non-phase alpha activity may represent a sensory gain mechanism comes from a study that observed a key distinction: while phase modulation of the alpha band (linked to P1 and N1 components and thus to stimulus processing) exhibited a synchronous process, non-phase activity displayed a desynchronous process with a latency and topography very similar to phase activity [9,18]. In other words, the desynchronization of non-phase alpha would represent the attenuation of background noise in the brain at that frequency and in the same region responsible for processing the stimulus, ultimately enhancing the perception of the presented stimulus.
This sensory gain mechanism appears to operate in the early stages of stimulus processing, based on its predominantly occipito-parietal topography for visual stimuli and its early latencies, around 120 ms [9,18]. However, it can also emerge at other moments, such as during the anticipation of stimulus onset [19] or in post-processing phases of the stimulus [17].
The objective of this study is to analyze simultaneously whether the recall of previously presented words can modulate both ERPs and phase and non-phase alpha band activities. In the case of ERPs, contradictory results have been reported. In particular, it seems that not always both components are modulated in visual memory tasks. In some cases, neither of the components is modulated by the recognition of the visual stimuli [20], and in other cases, modulation occurred only in one of the components [21,22]. Additionally, the simultaneous study of the P1 and N1 components along with the phase and non-phase modulations of the alpha band will help determine whether both domains (time and frequency) are interconnected, as suggested by the phase resetting hypothesis as a mechanism for the generation of ERPs [23].
Furthermore, to the best of our knowledge based on the existing scientific literature, there are no previous studies on the effect of word recall on non-phase-locked alpha activity. Since we consider non-phase-locked alpha activity to be a psychophysiological indicator of the sensory gain mechanism, the presence or absence of changes can provide highly informative insights into the events occurring during the early stages of information processing associated with word recall. In this particular study, we employed a combination of time-frequency techniques (TSE and Hilbert transform) to isolate the non-phase-locked component from the rest of the EEG signal. To investigate this, we will use a word memorization task followed by a recognition task, alongside electroencephalographic recordings with 58 scalp electrodes.
2. Materials and Methods
2.1. Ethical Statement
This study was carried out in compliance with the Helsinki Declaration. The study protocol was approved previously by the ethics committee of the Junta de Andalucía (project code: PSI2010-16825). All participants enrolled in the present study signed informed consent before their inclusion.
2.2. Participants
Twenty-seven healthy adults, who were university employees and students, were recruited from the University of Seville (16 women and 11 men). The participants’ ages ranged from 20 to 50 years (mean age: 37.7 ± 11.4). Only two participants were left-handed.
2.3. Cognitive Task
Participants were seated in a sound-attenuated room in front of a Liquid Crystal Display (LCD) monitor. Stimuli were created by E-prime 2.0 (Psychology Software Tools, Inc., Pittsburgh, PA, USA). The experiment consisted of an initial stimulation routine in which participants were instructed to memorize the presented words. The number of words to remember was 20; all were bisyllabic, frequently used in the Spanish language, and were presented three times to each participant in a random order mixed with the other words to be remembered. Each word was displayed for 1 s in a central position, with a size of 3.6 × 1.2 degrees of visual angle, while participants’ eyes were positioned 70 cm from the screen.
Next, to prevent continuous effort in recalling the words by the participant, a visual oddball task was presented in which the participant had to identify a target stimulus among other distractor stimuli (rectangles with a chessboard pattern in different colors) and indicate it by pressing a button. The task lasted 3 min.
Finally, a stimulation routine was presented in which participants had to identify the words presented in the first routine from another set of 20 new words that served as a control for the number of intrusions. The words were of the same size and position as those used in the first routine but displayed during two seconds. Each word—both those to be identified and the new ones to be ignored—was presented three times in random order and intermixed with the others. Participants were instructed to press the right mouse button with their index finger whenever they recognized the presented word as one that had appeared during the memorization task. At the end of the recording, the number of correct responses in the recognition o-f memorized words was calculated, as well as the percentage of intrusions made by the participant.
2.4. EEG Recording and Analysis
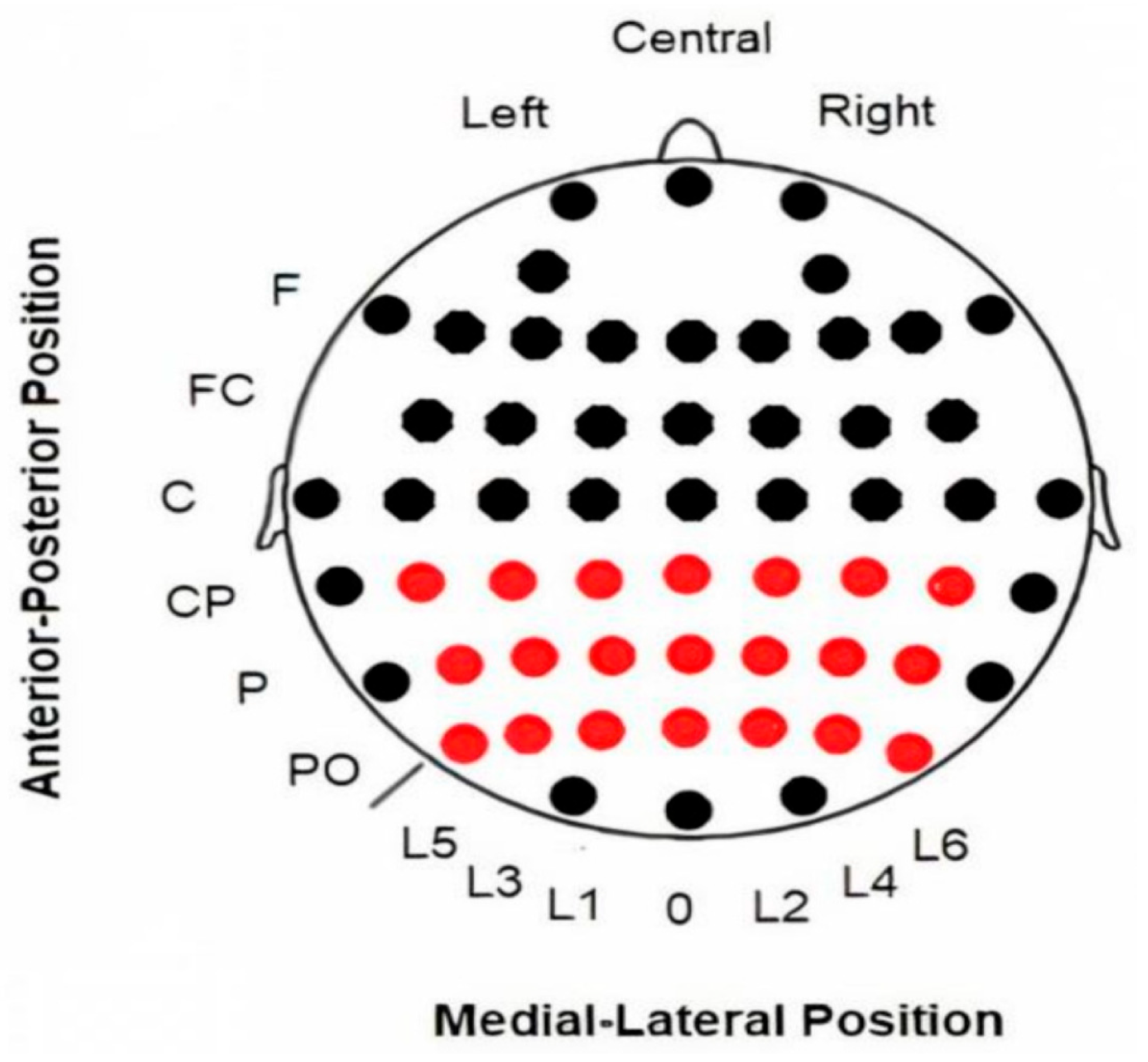
EEG data were recorded from 58 electrodes (Ag/AgCl) in standard locations of a 10-10 system (American Electroencephalographic Society, 1994) [24] (Figure 1), then amplified with BrainAmp amplifiers (BrainProducts GmbH, Gilching, Germany) and digitized at a rate of 500 Hz using Recorder software v.1.26 (BrainProducts GmbH, Germany) [25]. The electrodes used in the present study were limited to a 3 × 7 matrix located in posterior regions where the maximum activity of all analyzed activities (ERPs, evoked and induced alpha modulations) was observed: CP5, CP3, CP1, CPz, CP2, CP4, CP6, P5, P3, P1, Pz, P2, P4, P6, PO5, PO3, PO1, POz, PO2, PO4, PO6 (see Figure 1).
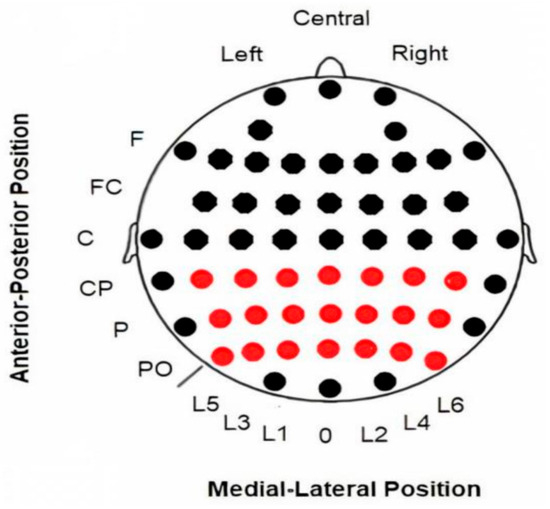
Figure 1.
Locations of electrodes on the scalp. EEG data were collected using all electrodes displayed in the figure. However, only the 3 × 7 electrode array highlighted in red was used for the subsequent analysis. Abbreviations: F (frontal), FC (fronto-central), C (central), CP (central-parietal), P (parietal), PO (parieto-occipital), L (Lines 1–6; “0” refers to the midline).
An online reference was placed at the ear lobes, while an offline reference was set to the common average. Eye movements were monitored by placing electrodes on the outer lateral eye orbits for horizontal (HEOG) and on the superior and inferior orbits of the left eye for vertical (VEOG) movements. A bandpass filter was applied within the 0.01–100 Hz range. Impedance levels were kept below 5 kΩ throughout the recording.
The following protocol was used: eye movement correction to eliminate artifacts, based on the algorithm developed by Gratton et al. [26]; segmentation of the data in a −200 to 1000 ms interval; baseline correction from −200 to 0 ms; and rejection of any artifacts exceeding ±75 μV at electrodes Pz, and HEOG. After applying the artifact rejection protocol, the average number of trials across subjects was 47.5 for the recalled words condition and 47.7 for the not recalled words condition.
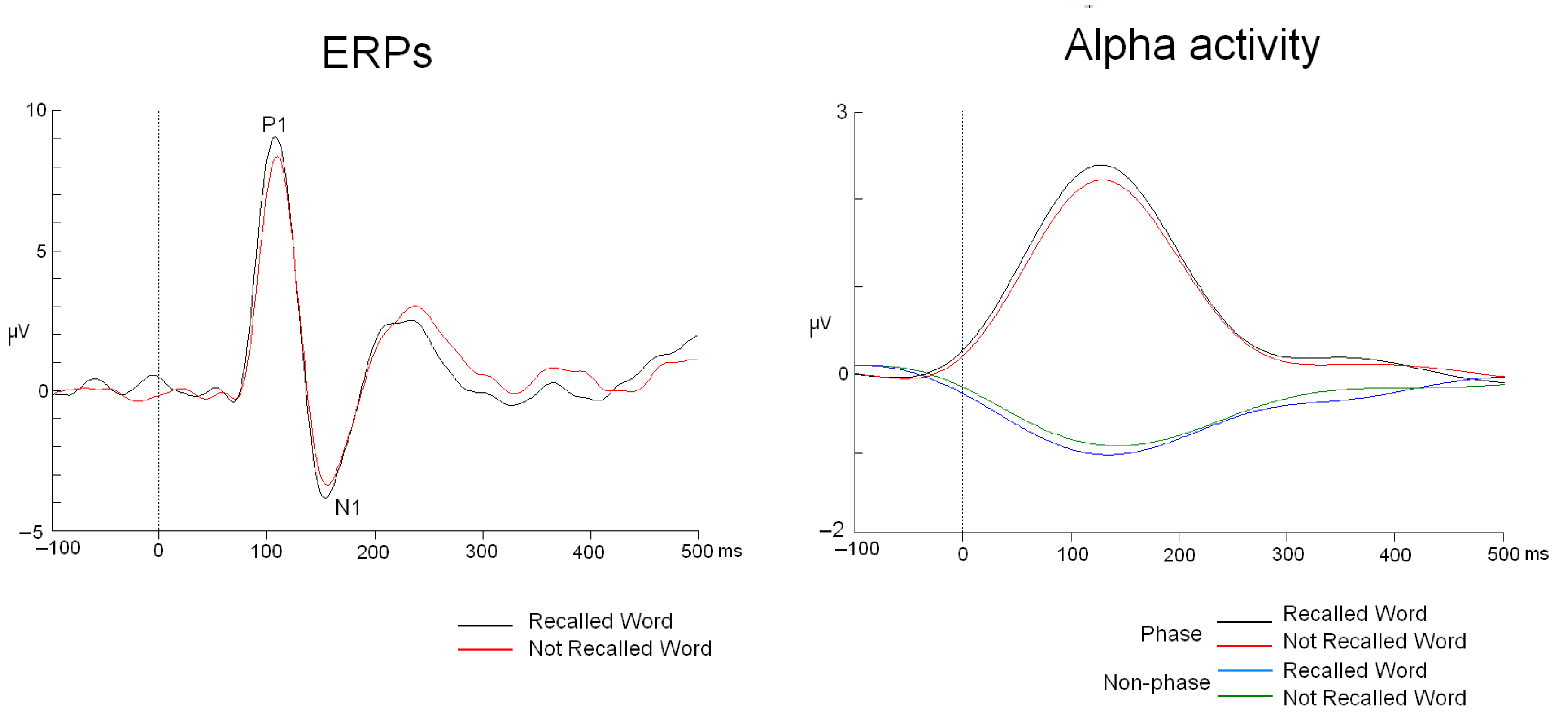
From this point, the analysis of phase and non-phase activities proceeded differently. Phase activity was analyzed by applying the following steps: averaging (ERP modulations were analyzed), bandpass filtering in the 8–12 Hz range (48 dB/octave Butterworth zero phase), rectification, low-pass filtering at 5 Hz (48 dB/octave Butterworth zero phase), and baseline correction from −200 to 0 ms. In contrast, non-phase activity was calculated using this protocol: bandpass filtering in the 8–12 Hz range (48 dB/octave Butterworth zero phase), rectifying the signal, averaging all trials for each experimental condition, subtracting phase activity from this result, applying 5 Hz low-pass filtering (48 dB/octave Butterworth zero phase), and baseline correction from −200 to 0 ms (Figure 2) [18].
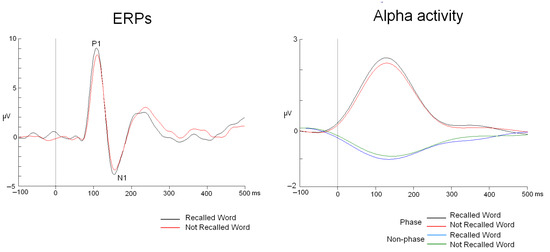
Figure 2.
ERPs and phase and non-phase alpha modulations. The waveforms correspond to the PO5 electrode.
It is important to highlight that both procedures include the same steps up to the point of obtaining phase or non-phase modulations. The main difference lies in the order of these steps. The critical step is the rectification of the signal. In the case of the non-phase section study, rectification is applied to the EEG recording segments before they are averaged. This approach allows us to observe non-phase modulations, which are usually canceled out by the averaging process. A detailed description of the procedure can be found in [27].
Latency values for ERPs and phase and non-phase modulations were calculated at the peak/valley amplitude for each participant to evaluate whether ERPs and phase/non-phase activities occur at similar latencies for recalled words compared to not recalled words [28]. The electrode showing the highest ERP amplitude and phase/non-phase activities was positioned at PO5. To calculate the possible effects on amplitude, the mean voltage value was exported for each electrode in the 3 × 7 matrix (see Figure 1) across two time intervals: (1) within the latency range of the P1 component (90–130 ms) and (2) within the latency range of the N1 component (140–180 ms).
To examine the potential voltage difference between the two conditions (recalled vs. not recalled words) for ERPs and spectral modulations (phase and non-phase), the intervals were defined based on the grand average peaks or valleys (90–130 and 140–180 ms, corresponding to the P1 and N1 windows). To ensure comparability for the topographic amplitude analyses between the two types of activity (phase and non-phase), the non-phase activity was converted to absolute values.
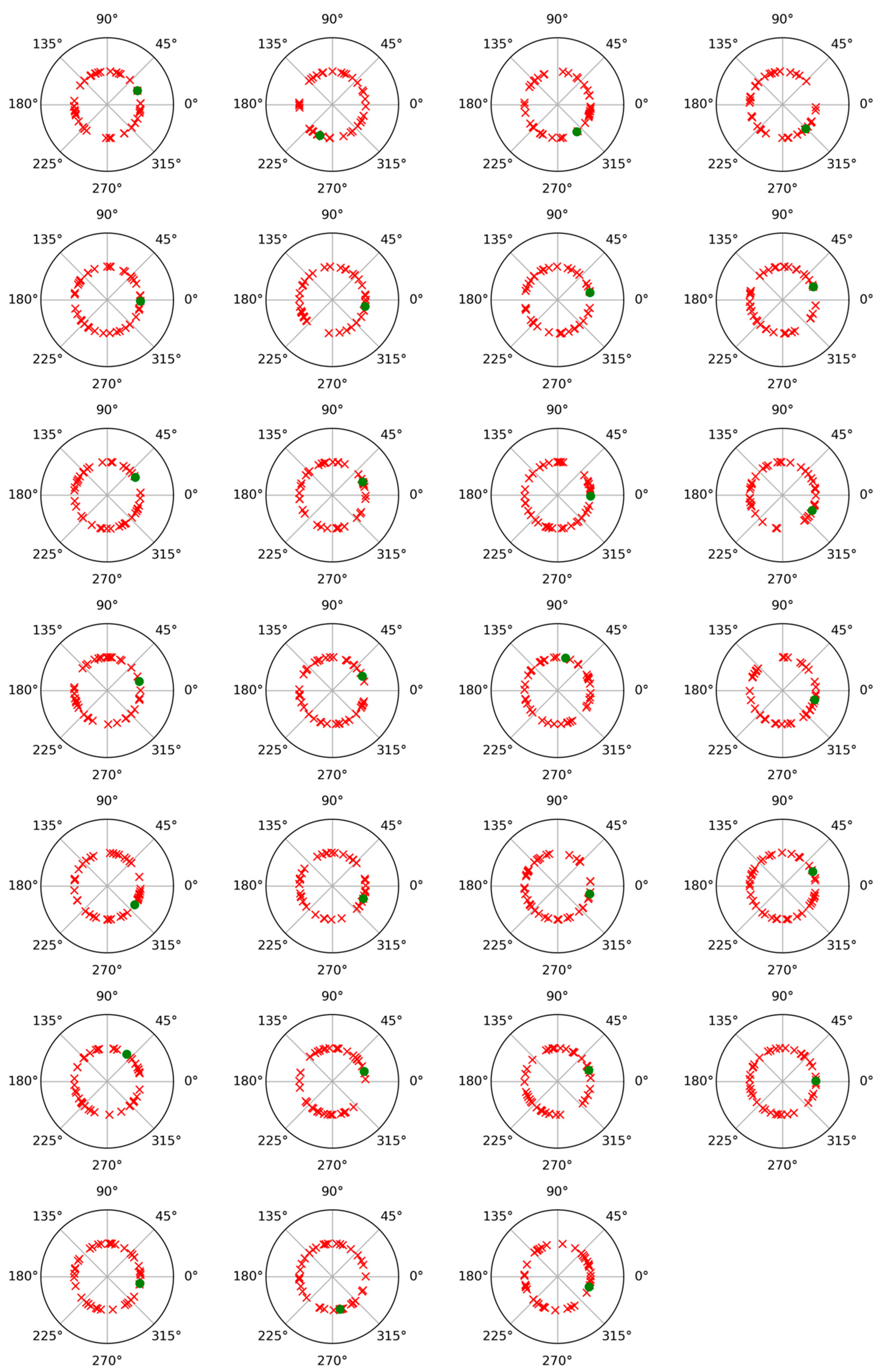
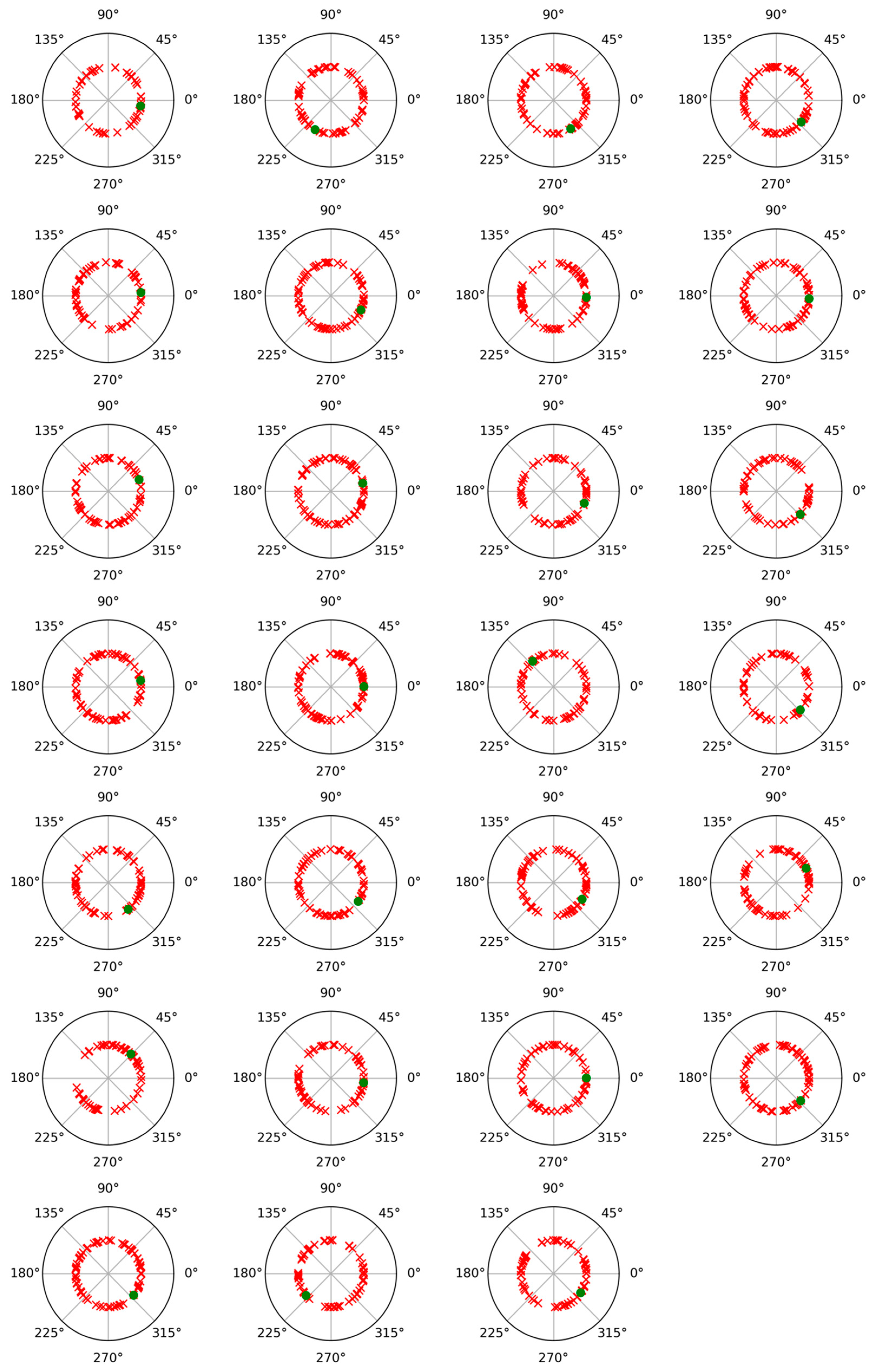
To check the possibility of cross-contribution between phase and non-phase modulations, a phase angle analysis was performed for both modulations. For this, after subtracting the average trial from each individual trial, the Hilbert transform was applied to represent the alpha band-filtered EEG signals as complex waveforms of the form xn(t) = Cn(t) exp (i wn(t)), where xn(t) denotes any of the average-subtracted trials, such as the nth, wn(t) is its instantaneous phase or angle (measured in radians), and i is the imaginary unit [29,30]. Figure 3 and Figure 4 display polar plots showing the distribution of these angles, grouped by subject.
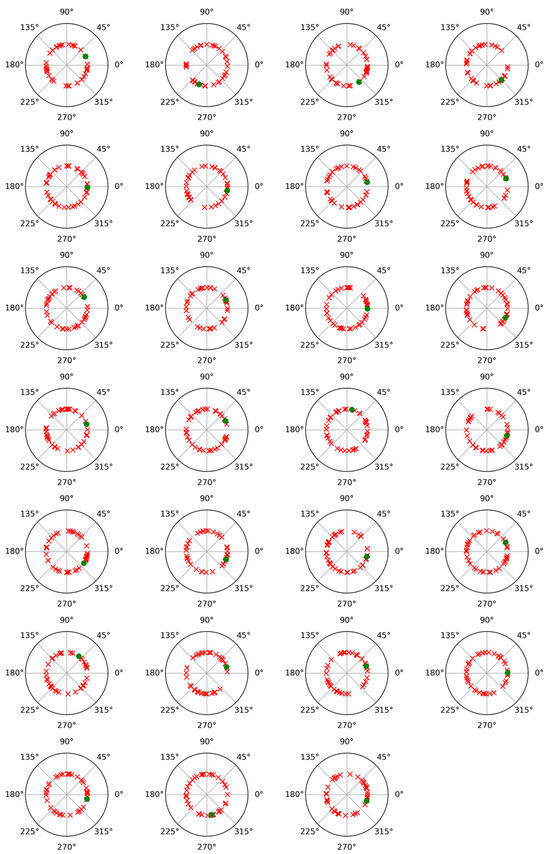
Figure 3.
Polar plot of phase values results for phase-locked and non-phase-locked activities in the alpha band (8–12 Hz) for each of the twenty-seven participants and for the recalled words. Green dots correspond to phase-locked activity, and red crosses correspond to non-phase-locked activity.
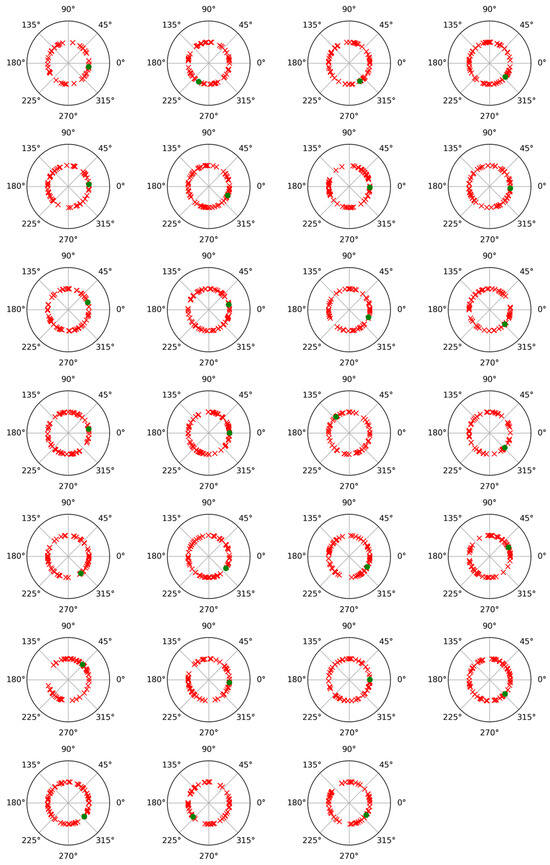
Figure 4.
Polar plot of phase values results for phase-locked and non-phase-locked activities in the alpha band (8–12 Hz) for each of the twenty-seven participants and for the not recalled words. Green dots correspond to phase-locked activity, and red crosses correspond to non-phase-locked activity.
2.5. Statistical Analysis
Statistical analyses were performed using SPSS v29 (IBM) [31]. The potential effect of the “RECALL” factor on ERP latencies (P1 and N1 components) was analyzed separately using paired t-tests. In the case of spectral modulation analysis, a repeated-measures ANOVA (RM-ANOVA) was conducted with the following factors and levels: (1) “RECALL” (levels: Recalled/Not recalled word); and (2) “TYPE OF ACTIVITY” (levels: Phase (P)/Non-phase (NP)).
For topographical differences in ERP amplitude (P1 and N1), an RM-ANOVA was applied with the following factors and levels: (1) “RECALL” (levels: Recalled/Not recalled word); (2) “ANTERO-POSTERIOR LOCATION” (levels: Central-parietal, Parietal, and Parietal-posterior); and (3) “LATERAL-MEDIAL LOCATION” (L1, L2, L3, L4, L5, L6, L7) (see Figure 1). For the analysis of phase and non-phase alpha amplitudes, the same factors and levels used in the ERP amplitude analysis were applied, with the addition of an extra factor: (4) “TYPE OF ACTIVITY” (levels: Phase (P)/Non-phase (NP)). In all cases, a Bonferroni correction was applied in the multiple comparison post hoc analysis.
Topographic map amplitude correlation analyses were conducted using Pearson’s r. In accordance with the recommendations proposed by Kileny and Kripal [32] and referenced in Vázquez-Marrufo et al. [33], the significance threshold of 0.05 was adjusted by dividing it by the total number of contrasts performed across all correlation analyses (n = 6). As a result, a revised significance level of p < 0.008 was established.
3. Results
3.1. Behavior
The analysis of the participants’ behavioral responses showed an average accuracy rate of 79.3 ± 15.9% and a percentage of 20.5 ± 13.5% for intrusions. These results suggest that the task was suitable for measuring the participants’ recall ability, without being overly difficult or too easy in terms of recalling the word list.
3.2. ERPs
The study of the P1 and N1 components showed that there were no latency differences due to the “RECALL” factor (t26 = −0.65, p = 0.518 and t26 = −0.46, p = 0.644, for P1 and N1, respectively). The mean latency values are shown in Table 1, and the ERP traces for recalled and non-recalled words are presented in Figure 2.

Table 1.
Mean latency and amplitude values at the electrode with maximum voltage (PO5) for ERPs (P1 and N1) and phase and non-phase alpha activities.
Regarding amplitude, the export of the mean value between 90 and 130 ms after stimulus onset (corresponding to the P1 component) showed an increase in the amplitude of this component for recalled words compared to new words (F(2,52) = 6.82, p = 0.002, ŋ2: 0.208). The mean value for each condition at the electrode with the maximum amplitude is presented in Table 1. A post-hoc comparison revealed that the increase was observed across all electrodes included in the analysis (3 × 7 matrix) (see Figure 1). No other interaction involving the “Recall” factor was found to be significant.
On the other hand, the analysis performed in the 140–180 ms interval for the study of the N1 component did not show, unlike what was observed with the P1 component, a statistically significant increase in amplitude (Figure 1) for recalled words compared to new words (F(1,26) = 0.79, p = 0.380, ŋ2: 0.030).
3.3. Alpha Phase and Non-Phase Activities
Concerning the latency values of the phase and non-phase alpha responses, the mean values for the different experimental conditions can be found in Table 1. The repeated measures ANOVA conducted to study the latency of these modulations revealed that there were no statistically significant differences due to the main factors “RECALL” (Recalled word/Not recalled word) F(1,26) = 2.65, p = 0.115, ŋ2: 0.093); “TYPE OF ACTIVITY” (Phase/Non-phase) F(1,26) = 1.96, p = 0.172, ŋ2: 0.070); or the interaction of both factors (F(1,26) = 1.63, p = 0.212, ŋ2: 0.059) (Figure 2).
In the case of the amplitude, ANOVA analysis of the “TYPE OF ACTIVITY” factor revealed that, in the first interval (90–130 ms), phase activity exhibited greater amplitude than non-phase activity across all electrodes analyzed in the 3 × 7 matrix (F(1,26) = 31.47, p < 0.001, ŋ2: 0.548) (see Table 1 for values at the electrode with the highest amplitude) (Figure 2).
Regarding the “RECALL” factor, the statistical analysis revealed that the four-way interaction “RECALL” × “TYPE OF ACTIVITY” × “ANTERO-POSTERIOR LOCATION” × “LATERAL-MEDIAL LOCATION” was significant (F(12,312) = 1.81, p = 0.045, ŋ2: 0.065). Post hoc analysis indicated that all electrodes in the 3 × 7 matrix exhibited greater amplitude for both phase and non-phase activities for recalled words compared to new words (Figure 2).
When the same analyses were conducted for the 140–180 ms window, an initial result showed that phase activity had greater amplitude than non-phase activity (factor “TYPE OF ACTIVITY”: F(1,26) = 22.98, p < 0.001, ŋ2: 0.469) (see Table 1 for mean values) (Figure 2).
With respect to the “RECALL” factor, the four-way interaction involving this factor along with “TYPE OF ACTIVITY”, “ANTERO-POSTERIOR LOCATION”, and “LATERAL-MEDIAL LOCATION” was also significant (F(12,312) = 3.30, p < 0.001, ŋ2: 0.113). Subsequent post hoc analysis showed that all electrodes, for phase and non-phase activities, exhibited higher amplitude when the word was recalled compared to new words.
3.4. Topographic Correlation Analysis
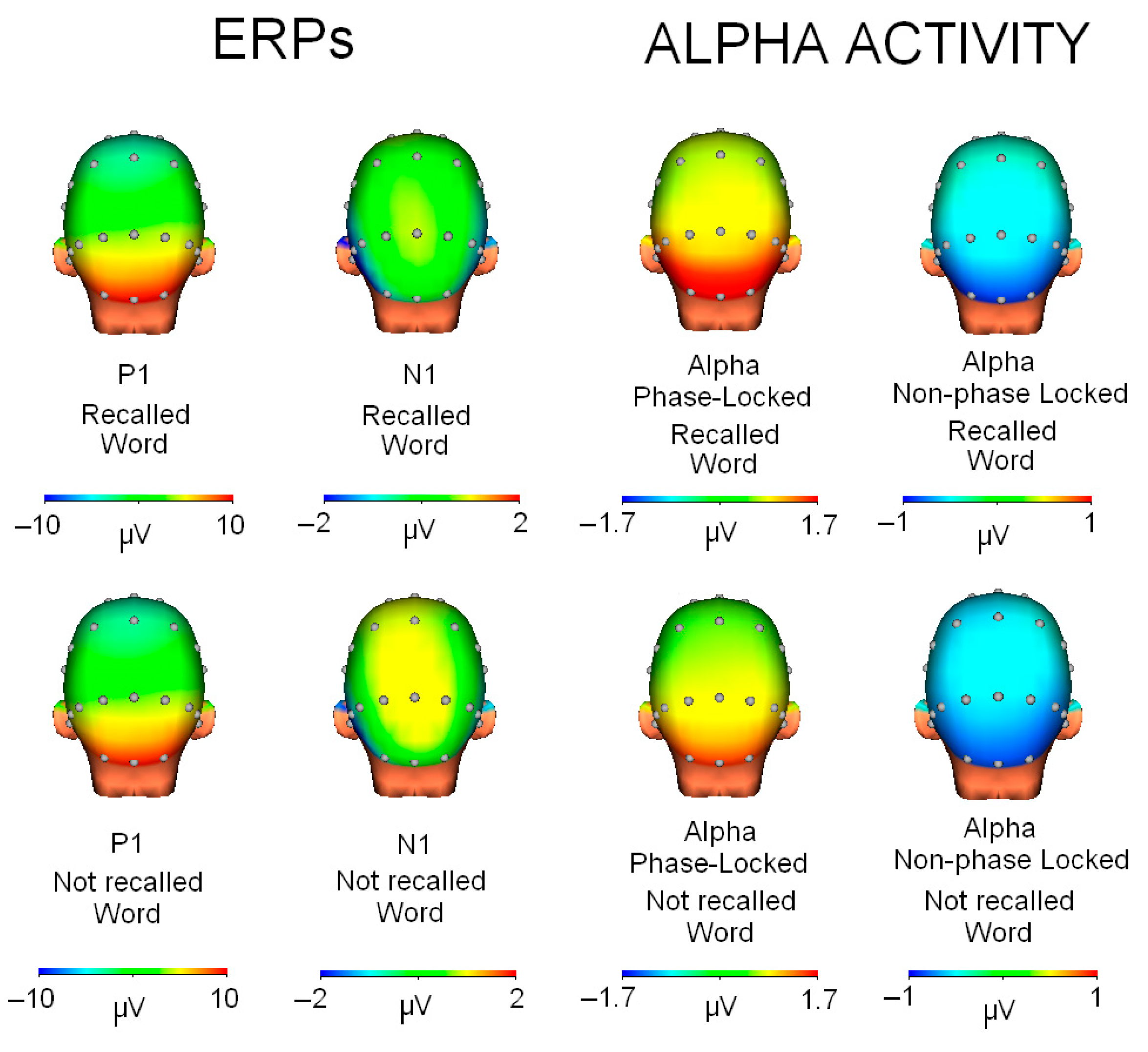
The correlation analyses first showed that the map associated with the ERPs produced when the word was recalled compared to when the word was new to the subject was virtually identical (90–130 ms interval: r = 0.999, p < 0.001; 140–180 ms interval: r = 0.974, p < 0.001). That is, the P1 component was almost identical for both types of words, and somewhat less so for the N1 component (see Figure 5).
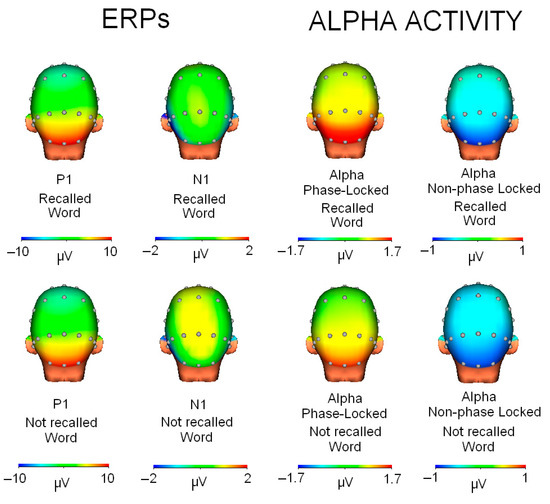
Figure 5.
Topographic maps for each condition (recalled/not recalled word) and type of activity (ERPs or alpha activity).
Regarding the comparisons between the ERP maps and the phase and non-phase alpha activities, two types of results were found. On the one hand, the comparison of the P1 map (90–130 ms interval) showed a high correlation with the phase alpha map (r = 0.895, p < 0.001) and also a very high anticorrelation with the non-phase activity map (r = −0.908, p < 0.001). However, when comparing the N1 topography with alpha activity, the results showed that the N1 component had a low correlation with the phase alpha modulation (r = −0.277, p = 0.075) and even lower with the non-phase modulation (r = 0.159, p = 0.313).
4. Discussion
Behavioral responses indicated that the task was well-adjusted for our sample, with an average accuracy of approximately 80% and a low intrusion rate of 20%. These results suggest that any potential modulations observed in the psychophysiological measures are not compromised by excessive difficulty or simplicity of the task presented to the participants.
In terms of ERP analysis, the present study revealed that no changes occurred in the latency of the analyzed components as a result of word recall. This suggests that any potential effects of recall do not manifest as either slower or faster processing speed for either type of stimulus. Previous studies have confirmed the absence of differences [21], although some research has reported their presence during face processing [22].
Regarding the amplitude study, the P1 component showed an increase when the word was recalled compared to when it was presented for the first time to the subject. In the case of the N1 component, the amplitude comparison between recalled and new words was not significant. In this regard, the literature has shown contradictory results. Some studies have not observed P1 and N1 changes related to memory [20], while others have found modulations in these components [21,22]. However, even among studies that have reported modulations, the findings are not entirely consistent. In Jha’s study, increases in P1 and N1 amplitude were observed due to memory recall, whereas other studies have reported a dissociation between these components in relation to stimulus recall—where N1 is modulated, but P1 is not [22]. In the present study, the modulation due to the recall of memorized words was observed in P1 but not in N1, although the latter showed a subtle amplitude increase for recalled words. Therefore, it is clear that further studies are needed to clarify which factors, apart from memory, may be influencing these tasks and contributing to the diversity of results.
When analyzing phase and non-phase alpha activity, neither showed latency changes caused by the “Recall” factor. This result aligns with the findings observed in ERPs and suggests that memory recall does not seem to influence the speed of these cognitive mechanisms represented by these modulations. In other words, the sensory gain mechanism does not operate by accelerating stimulus processing but rather by increasing the amplitude of brain activity associated with the stimulus, as we will discuss later.
In the case of the amplitude, the first finding is that phase and non-phase activities exhibited opposite polarities. While phase activity always manifests as synchronization, non-phase modulation generally appears as desynchronization, although not always [17]. This balance between the two alpha activities has been previously observed in other experimental paradigms (oddball, attention network test, etc.) [9,16,17,18,27], suggesting that the proposed sensory gain mechanism operates commonly during the early stages of information processing.
Additionally, a second finding revealed that the phase component of alpha, in absolute terms (without considering modulation polarity), has greater amplitude compared to the non-phase component. This result has also been reported in several previous studies [9,18]. It appears that non-phase alpha desynchronization does not require neural resource recruitment to the same extent as phase modulation. However, alternative explanations for this difference, such as the orientation of the generating dipole, cannot be ruled out.
On the other hand, regarding the possible influence of the non-phase component on the phase activity in the alpha band, the analysis of phase values revealed that the induced activity (non-phase) did not show a relationship with the phase activity (evoked). This can be inferred from the random distribution of phase values for each trial of the induced activity, which did not concentrate around the phase value of the evoked activity (phase-locked). This result has been observed in other studies conducted in our laboratory with different experimental tasks and subject samples [9,16,17,18,27]. Although both activities seem to exhibit some link, the phase value analysis suggests that they represent segregated processes in visual information processing.
Concerning the effect of the “Recall” factor, the results showed an increase in the amplitude of both phase and non-phase alpha activities when the word was remembered. This effect was observed in both the 90–130 ms interval (corresponding to the P1 component) and the 140–180 ms interval (corresponding to the N1 component). A key implication of this finding is that alpha activity and the P1 component appear to be highly correlated both topographically and functionally. However, the N1 component seems to be dissociated from alpha activity. This result supports the idea that P1 and N1 are not equally involved in the cognitive mechanisms triggered by word recall, as previously indicated.
In fact, the proposal of a dissociation of P1 and N1 related to sensory gain mechanism is not recent. Several studies have demonstrated the existence of this mechanism, particularly in experiments with attentional tasks [34,35]. In those studies, it was suggested that the P1 component was related to the suppression of activity from distractors that might be present in the visual space in regions different from the one where the relevant stimulus for the task was located, while the N1 component was more associated with the signal amplification of the target stimulus [36].
The combined study of different modulations (ERPs and time-frequency) has allowed us to observe that the phase and non-phase modulations of the alpha band are dissociable from the N1 component, at least in the context of this visual memory task. This result is significant considering that, until now, the P1 and N1 components had been associated with the resetting of the alpha band, showing a common alpha content [23].
In the case of the P1 component, it does seem to be related to both the phase and non-phase content of the alpha band, even showing a high degree of topographic similarity. In this study, based on the hypothesis that the P1 component acts as a filter attenuating the processing of irrelevant stimuli, words that did not belong to the memorized list were processed with lower amplitude as a result of this attenuation, as expected [34,35].
The striking result in this study is that the processing of non-recalled words showed a smaller decrease in non-phase alpha activity compared to that observed for recalled words. In other words, the attenuation in the processing of irrelevant stimuli for the task (indicated by P1) could be a product (perhaps partially) of a lesser attenuation of background neural noise.
In conclusion, we would like to emphasize that the simultaneous study of different modulations (ERPs and spectral content) has shown that the mechanisms operating in the early stages of information processing in a word recognition task represent a complex landscape, being represented by diverse activities to which we must assign different roles, particularly in the case of the lesser-known non-phase content of the alpha band.
Author Contributions
Conceptualization, M.V.-M.; Methodology, M.V.-M.; Software, R.M.-C.; Validation, M.V.-M. and R.M.-C.; Formal Analysis, M.V.-M., R.N.-M. and N.N.-G.; Investigation, R.M.-C., R.N.-M. and N.N.-G.; Resources, M.V.-M. and R.M.-C.; Data Curation, R.N.-M. and N.N.-G.; Writing—Original Draft Preparation, M.V.-M.; Writing—Review & Editing, R.M.-C., R.N.-M. and N.N.-G.; Visualization, R.N.-M. and N.N.-G.; Supervision, M.V.-M.; Project Administration, M.V.-M.; Funding Acquisition, M.V.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the ethics committee of the Junta de Andalucía (project code: PSI2010-16825 2014-05-27).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original data presented in the study are openly available in [Deposit in institutional repository requested and pending confirmation].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gevins, A.S.; Schaffer, R.E. A critical review of electroencephalographic (EEG) correlates of higher cortical functions. Crit. Rev. Bioeng. 1980, 4, 113–164. [Google Scholar] [PubMed]
- Lopes da Silva, F. Neural mechanisms underlying brain waves: From neural membranes to networks. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Ünsal, E.; Duygun, R.; Yemeniciler, İ.; Bingöl, E.; Ceran, Ö.; Güntekin, B. From Infancy to Childhood: A Comprehensive Review of Event- and Task-Related Brain Oscillations. Brain Sci. 2024, 14, 837. [Google Scholar] [CrossRef] [PubMed]
- Markand, O.N. Alpha rhythms. J. Clin. Neurophysiol. 1990, 7, 163–189. [Google Scholar] [CrossRef]
- Morrone, J.; Minini, L. The interlinking of alpha waves and visuospatial cognition in motor-based domains. Neurosci. Biobehav. Rev. 2023, 149, 105152. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Stancák, A.; Neuper, C. Event-related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. Int. J. Psychophysiol. 1996, 24, 39–46. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Worden, M.S.; Foxe, J.J.; Wang, N.; Simpson, C.V. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha band electroencephalography increases over occipital cortex. J. Neurosci. 2000, 20, RC63. [Google Scholar] [CrossRef]
- Vázquez-Marrufo, M.; García-Valdecasas, M.; Caballero-Diaz, R.; Martin-Clemente, R.; Galvao-Carmona, A. Multiple evoked and induced alpha modulations in a visual attention task: Latency, amplitude and topographical profiles. PLoS ONE 2019, 14, e0223055. [Google Scholar] [CrossRef]
- Reischies, F.M.; Neuhaus, A.H.; Hansen, M.L.; Mientus, S.; Mulert, C.; Gallinat, J. Electrophysiological and neuropsychological analysis of a delirious state: The role of the anterior cingulate gyrus. Psychiatry Res. 2005, 138, 171–181. [Google Scholar] [CrossRef]
- Freichel, R.; Zink, N.; Chang, F.Y.; Vera, J.D.; Truong, H.; Michelini, G.; Loo, S.K.; Lenartowicz, A. Alpha event-related decreases during encoding in adults with ADHD—An investigation of sustained attention and working memory processes. Behav. Brain Res. 2024, 469, 115003. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, V.T.; May, P.J.; Tiitinen, H. Human auditory event-related processes in the time-frequency plane. Neuroreport 2004, 15, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Hari, R.; Salmelin, R.; Mäkelä, J.P.; Salenius, S.; Helle, M. Magnetoencephalographic cortical rhythms. Int. J. Psychophysiol. 1997, 26, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Sabate, M.; Llanos, C.; Enriquez, E.; Gonzalez, B.; Rodriguez, M. Fast modulation of alpha activity during visual processing and motor control. Neuroscience 2011, 189, 236–249. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, C.; Han, J. The neural mechanism of non-phase-locked EEG activity in task switching. Neurosci. Lett. 2023, 792, 136957. [Google Scholar] [CrossRef]
- Sarrias-Arrabal, E.; Martín-Clemente, R.; Galvao-Carmona, A.; Benítez-Lugo, M.L.; Vázquez-Marrufo, M. Effect of the side of presentation in the visual field on phase-locked and nonphase-locked alpha and gamma responses. Sci. Rep. 2022, 12, 13200. [Google Scholar] [CrossRef]
- Vazquez-Marrufo, M.; Sarrias-Arrabal, E.; Martin-Clemente, R.; Galvao-Carmona, A.; Navarro, G.; Izquierdo, G. Altered phase and nonphase EEG activity expose impaired maintenance of a spatial-object attentional focus in multiple sclerosis patients. Sci. Rep. 2020, 10, 20721. [Google Scholar] [CrossRef]
- Vázquez-Marrufo, M.; Caballero-Díaz, R.; Martín-Clemente, R.; Galvao-Carmona, A.; González-Rosa, J.J. Individual test-retest reliability of evoked and induced alpha activity in human EEG data. PLoS ONE 2020, 15, e0239612. [Google Scholar] [CrossRef]
- Sarrias-Arrabal, E.; Berchicci, M.; Bianco, V.; Vázquez-Marrufo, M.; Perri, R.L.; Di Russo, F. Temporal spectral evolution of pre-stimulus brain activity in visual and visuomotor tasks. Cogn. Neurodyn. 2023, 17, 1433–1446. [Google Scholar] [CrossRef]
- Cañete, O. Potenciales evocados auditivos de corteza: Complejo P1-N1-P2 y sus aplicaciones clínicas. Rev. Otorrinolaringol. 2014, 74, 266–274. [Google Scholar] [CrossRef]
- Jha, A.P. Tracking the time-course of attentional involvement in spatial working memory: An event-related potential investigation. Brain Res. Cogn. 2002, 15, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Itier, R.J.; Taylor, M.J. Effects of repetition and configural changes on the development of face recognition processes. Dev. Sci. 2004, 7, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Schack, B.; Schabus, M.; Doppelmayr, M.; Gruber, W.; Sauseng, P. Phase-locked alpha and theta oscillations generate the P1-N1 complex and are related to memory performance. Brain Res. Cogn. Brain Res. 2004, 19, 302–316. [Google Scholar] [CrossRef]
- Anonymous. Guideline thirteen: Guidelines for standard electrode position nomenclature. American Electroencephalographic Society. J. Clin. Neurophysiol. 1994, 11, 111–113. [Google Scholar] [PubMed]
- Topor, M.; Opitz, B.; Dean, P.J.A. In search for the most optimal EEG method: A practical evaluation of a water-based electrode EEG system. Brain Neurosci. Adv. 2021, 5, 23982128211053698. [Google Scholar] [CrossRef]
- Gratton, G.; Coles, M.G.; Donchin, E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef]
- Sarrias-Arrabal, E.; Eichau, S.; Galvao-Carmona, A.; Domínguez, E.; Izquierdo, G.; Vázquez-Marrufo, M. Deficits in Early Sensory and Cognitive Processing Are Related to Phase and Nonphase EEG Activity in Multiple Sclerosis Patients. Brain Sci. 2021, 11, 629. [Google Scholar] [CrossRef]
- Keil, A.; Müller, M.M. Feature selection in the human brain: Electrophysiological correlates of sensory enhancement and feature integration. Brain Res. 2010, 1313, 172–184. [Google Scholar] [CrossRef]
- David, O.; Kilner, J.M.; Friston, K.J. Mechanisms of evoked and induced responses in MEG/EEG. NeuroImage 2006, 31, 1580–1591. [Google Scholar] [CrossRef]
- Truccolo, W.A.; Ding, M.; Knuth, K.H.; Nakamura, R.; Bressler, S.L. Trial-to-trial variability of cortical evoked responses: Implications for the analysis of functional connectivity. Clin. Neurophysiol. 2002, 113, 206–226. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 29.0; IBM Corp: Armonk, NY, USA, 2022.
- Kileny, P.R.; Kripal, J.P. Test-Retest Variability of Auditory Event-Related Potentials. Ear Hear. 1987, 8, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Marrufo, M.; González-Rosa, J.J.; Galvao-Carmona, A.; Hidalgo-Muñoz, A.R.; Borges, M.R.S.; Peña, J.L.R.; Izquierdo, G. Retest Reliability of Individual P3 Topography Assessed by High Density Electroencephalography. PLoS ONE 2013, 8, e62523. [Google Scholar] [CrossRef] [PubMed]
- Mangun, G.R.; Hillyard, S.A.; Luck, S.J. Electrocortical substrates of visual selective attention. In Attention and Performance; Meyer, D., Konblum, S., Eds.; MIT Press: Cambridge, MA, USA, 1984; Volume 14, pp. 219–243. [Google Scholar]
- Harter, M.R.; Miller, S.L.; Price, N.J.; LaLonde, M.E.; Keyes, A.L. Neural processes involved in directing attention. J. Cogn. Neurosci. 1989, 1, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Heinze, H.J.; Mangun, G.R.; Hillyard, S.A. Visual event-related potentials index focused attention within bilateral stimulus arrays. II. Functional dissociation of P1 and N1 components. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 528–542. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).