The State of Antimicrobial Resistance of Gram-Negative Bacilli in Canada
Abstract
1. Introduction
2. ESBL-Producing Enterobacterales in Canada
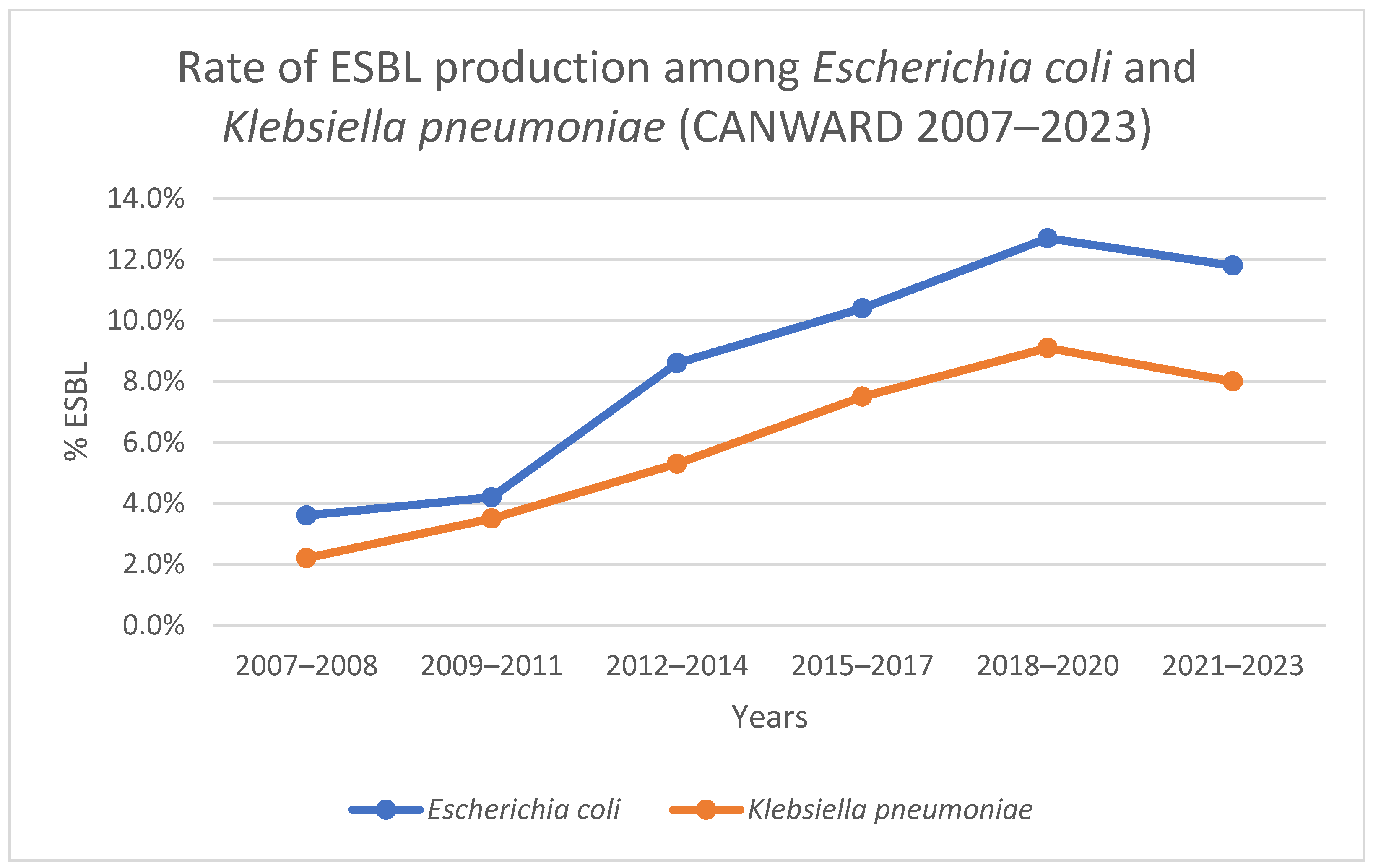
2.1. Prevalence of ESBL-Producing Enterobacterales in Canada
2.2. Outcomes of ESBL-Producing Enterobacterales Infections
2.3. ESBL-Producing Enterobacterales Are MDR
2.4. Treatment of ESBL-Producing Enterobacterales
3. Carbapenem-Resistant Enterobacterales-CRE
3.1. Definitions of CRE
3.2. Outcomes of CRE and CPE Infections
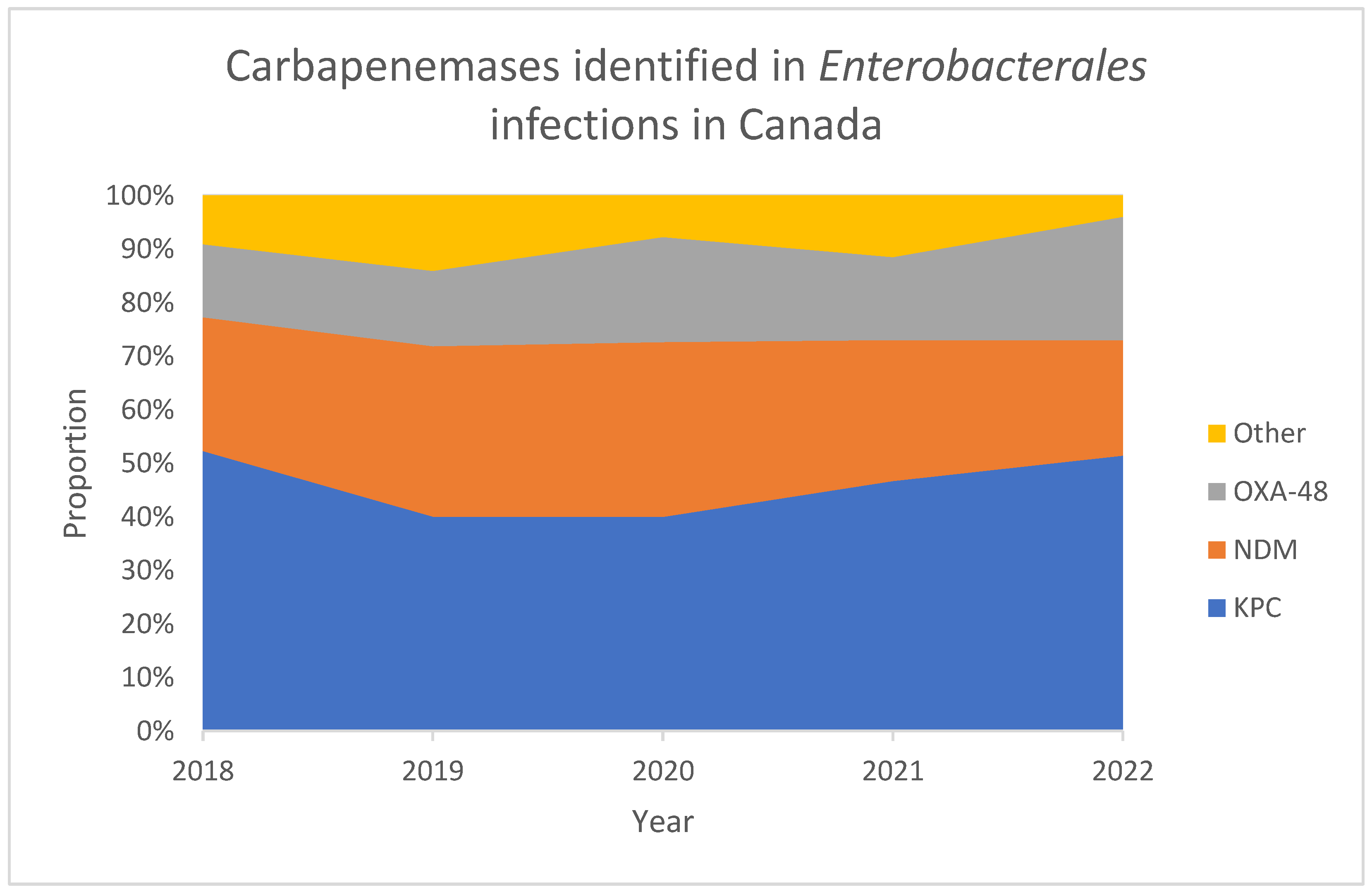
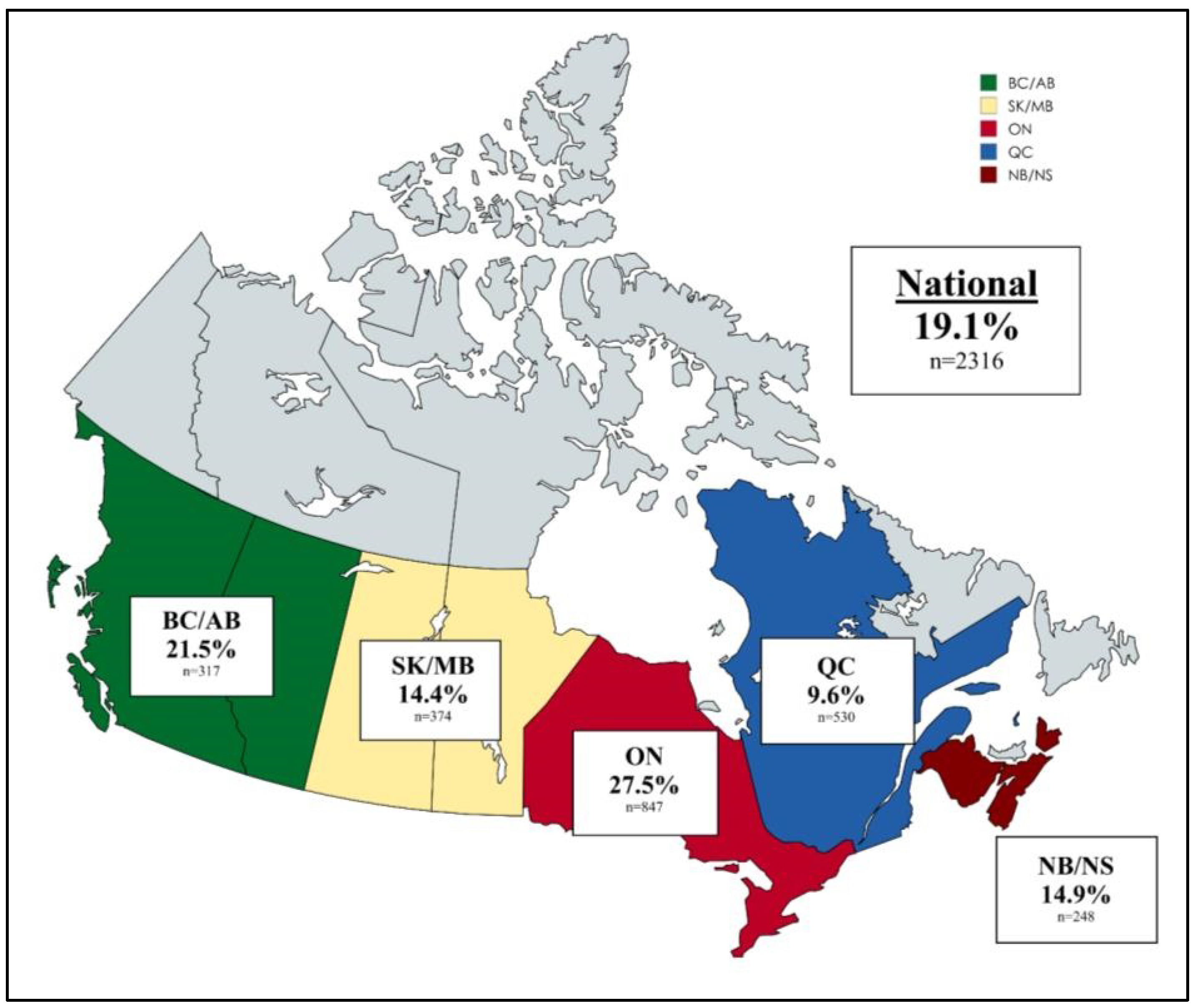
3.3. Prevalence of CRE and CPE in Canada
3.4. CRE and CPE Are MDR
3.5. Treatment of CRE and CPE
4. Multi-Drug Resistant (MDR) Pseudomonas aeruginosa
4.1. Definition of MDR P. aeruginosa
4.2. Prevalence of MDR P. aeruginosa in Canada
4.3. Resistance Mechanisms of MDR P. aeruginosa in Canada
4.4. Treatment of MDR P. aeruginosa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wei, J.; Zhou, W.; Yan, X.; Cao, X.; Zuo, L.; Chen, S.; Yao, K.; Huang, R.; Chen, Y.; et al. Risk factors and mortality for patients with Bloodstream infections of Klebsiella pneumoniae during 2014–2018: Clinical impact of carbapenem resistance in a large tertiary hospital of China. J. Infect. Public Health. 2020, 13, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Trecarichi, E.M.; Tumbarello, M.; Di Blasi, R.; Fianchi, L.; Sica, S.; Martino, B.; Candoni, A.; Nadali, G.; Pastore, D.; Perriello, V.; et al. Bloodstream Infections Caused By Klebsiella Pneumoniae in Onco-Hematological Patients: Incidence and Clinical Impact of Carbapenem Resistance in a Multicentre Prospective Survey. Blood 2015, 126, 3757. [Google Scholar] [CrossRef]
- Cosgrove, S.E. The Relationship between Antimicrobial Resistance and Patient Outcomes: Mortality, Length of Hospital Stay, and Health Care Costs. Clin. Infect. Dis. 2006, 42, S82–S89. [Google Scholar] [CrossRef]
- Council of Canadian Academies. When Antibiotics Fail; Council of Canadian Academies: Ottawa, ON, Canada, 2019. [Google Scholar]
- Luepke, K.H.; Suda, K.J.; Boucher, H.; Russo, R.L.; Bonney, M.W.; Hunt, T.D.; Mohr, J.F., III. Past, Present, and Future of Antibacterial Economics: Increasing Bacterial Resistance, Limited Antibiotic Pipeline, and Societal Implications. Pharmacotherapy 2017, 37, 71–84. [Google Scholar] [CrossRef]
- Denisuik, A.J.; Karlowsky, J.A.; Adam, H.J.; Baxter, M.R.; Lagacé-Wiens, P.R.S.; Mulvey, M.R.; Hoban, D.J.; Zhanel, G.G. Dramatic rise in the proportion of ESBL-producing Escherichia coli and Klebsiella pneumoniae among clinical isolates identified in Canadian hospital laboratories from 2007 to 2016. J. Antimicrob. Chemother. 2019, 74, iv64–iv71. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Canton, R.; Gniadkowski, M.; Nordmann, P.; Rossolini, G.M.; Arlet, G.; Ayala, J.; Coque, T.M.; Kern-Zdanowicz, I.; Luzzaro, F.; et al. CTX-M: Changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 2006, 59, 165–174. [Google Scholar] [CrossRef]
- Denisuik, A.J.; Lagacé-Wiens, P.R.S.; Pitout, J.D.; Mulvey, M.R.; Simner, P.J.; Tailor, F.; Karlowsky, J.A.; Hoban, D.J.; Adam, H.J.; Zhanel, G.G.; et al. Molecular epidemiology of extended-spectrum β-lactamase-, AmpC β-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007-11. J. Antimicrob. Chemother. 2013, 68, i57–i65. [Google Scholar] [CrossRef]
- Mathers, A.J.; Peirano, G.; Pitout, J.D.D. Chapter Four—Escherichia coli ST131: The Quintessential Example of an International Multiresistant High-Risk Clone. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 90, pp. 109–154. [Google Scholar]
- Lagacé-Wiens, P.R.S.; Mataseje, L.F.; McCracken, M.; Walkty, A.; Karlowsky, J.A.; Adam, H.J.; Baxter, M.R.; Denisuik, A.J.; Zhanel, G.G. Increasing Rates of ESBL-producing Escherichia coli and Klebsiella pneumoniae in Canadian Hospitals: 17 Years of the CANWARD Study (2007–2023). J. Antimicrob. Chemother. 2025; (unpublished manuscript). [Google Scholar]
- Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System Report 2022. Available online: https://www.canada.ca/en/public-health/services/publications/drugs-health-products/canadian-antimicrobial-resistance-surveillance-system-report-2022.html (accessed on 22 January 2025).
- McGill University Health Centre Antimicrobial Stewardship Program. Empiric Management of Urinary Tract Infections (UTI). Available online: https://www.muhcasp.com/_files/ugd/dea1a3_251daff4bac340f68b9f364170a79ebe.pdf (accessed on 3 February 2025).
- Nova Scotia Health Authority. Antimicrobial Handbook, Pyelonephritis in Adults. Available online: https://library.nshealth.ca/ld.php?content_id=36338897 (accessed on 22 January 2025).
- SH+UHN Antimicrobial Stewardship Program. Guidelines for Empiric Treatment of Urinary Tract Infection in Adults. Available online: https://www.antimicrobialstewardship.com/uti (accessed on 22 January 2025).
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Solomkin, J.S.; Mazuski, J.E.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.C.; Baron, E.J.; O’Neill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and Management of Complicated Intra-abdominal Infection in Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 133–164. [Google Scholar] [CrossRef]
- SH+UHN Antimicrobial Stewardship Program. MSH ED Sepsis Recognition and Management Algorithm. Available online: https://www.antimicrobialstewardship.com/edsepsis (accessed on 22 January 2025).
- McGill University Health Centre Antimicrobial Stewardship Program. Empiric Management of Sepsis. Available online: https://www.muhcasp.com/_files/ugd/7b3751_6e8190a0a5de4a4dbc1041ee0584d7d7.pdf (accessed on 3 February 2025).
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.; et al. Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of Piperacillin-Tazobactam vs. Meropenem on 30-Day Mortality for Patients with E coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef]
- Paterson, D.L.; Ko, W.-C.; Von Gottberg, A.; Mohapatra, S.; Casellas, J.M.; Goossens, H.; Mulazimoglu, L.; Trenholme, G.; Klugman, K.P.; Bonomo, R.A.; et al. Antibiotic Therapy for Klebsiella pneumoniae Bacteremia: Implications of Production of Extended-Spectrum β-Lactamases. Clin. Infect. Dis. 2004, 39, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Navarro, M.D.; Romero, L.; Muniain, M.A.; Cueto, M.d.; Ríos, M.J.; Hernández, J.R.; Pascual, A. Bacteremia Due to Extended-Spectrum β-Lactamase–Producing Escherichia coli in the CTX-M Era: A New Clinical Challenge. Clin. Infect. Dis. 2006, 43, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Lagacé-Wiens, P.R.S.; Adam, H.J.; Poutanen, S.; Baxter, M.R.; Denisuik, A.J.; Golden, A.R.; Nichol, K.A.; Walkty, A.; Karlowsky, J.A.; Mulvey, M.R.; et al. Trends in antimicrobial resistance over 10 years among key bacterial pathogens from Canadian hospitals: Results of the CANWARD study 2007–16. J. Antimicrob. Chemother. 2019, 74, iv22–iv31. [Google Scholar] [CrossRef]
- Janssen, J.; Kinkade, A.; Man, D. CARBapenem utilizatiON evaluation in a large community hospital (CARBON): A Quality Improvement Study. Can. J. Hosp. Pharm. 2015, 68, 327–331. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System Report 2021. Available online: https://www.canada.ca/en/public-health/services/publications/drugs-health-products/canadian-antimicrobial-resistance-surveillance-system-report-2021.html (accessed on 22 January 2025).
- Mladenovic-Antic, S.; Kocic, B.; Velickovic-Radovanovic, R.; Dinic, M.; Petrovic, J.; Randjelovic, G.; Mitic, R. Correlation between antimicrobial consumption and antimicrobial resistance of Pseudomonas aeruginosa in a hospital setting: A 10-year study. J. Clin. Pharm. Ther. 2016, 41, 532–537. [Google Scholar] [CrossRef]
- Palacios-Baena, Z.R.; Giannella, M.; Manissero, D.; Rodríguez-Baño, J.; Viale, P.; Lopes, S.; Wilson, K.; McCool, R.; Longshaw, C. Risk factors for carbapenem-resistant Gram-negative bacterial infections: A systematic review. Clin. Microbiol. Infect. 2021, 27, 228–235. [Google Scholar] [CrossRef]
- Lynch, J.P.; Clark, N.M.; Zhanel, G.G. Escalating antimicrobial resistance among Enterobacteriaceae: Focus on carbapenemases. Expert Opin. Pharmacother. 2021, 22, 1455–1474. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and Other Enterobacteriaceae: An Evolving Crisis of Global Dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef]
- Tumbarello, M.; Viale, P.; Viscoli, C.; Trecarichi, E.M.; Tumietto, F.; Marchese, A.; Spanu, T.; Ambretti, S.; Ginocchio, F.; Cristini, F.; et al. Predictors of Mortality in Bloodstream Infections Caused by Klebsiella pneumoniae Carbapenemase—Producing K. pneumoniae: Importance of Combination Therapy. Clin. Infect. Dis. 2012, 55, 943–950. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Canadian Nosocomial Infection Surveillance Program. Healthcare-associated infections and antimicrobial resistance in Canadian acute care hospitals, 2018–2022. Can. Commun. Dis. Rep. 2024, 50, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Walkty, A.; Lagace-Wiens, P.; Adam, H.; Baxter, M.; Karlowsky, J.; Mulvey, M.R.; McCracken, M.; Zhanel, G.G. Antimicrobial susceptibility of 2906 Pseudomonas aeruginosa clinical isolates obtained from patients in Canadian hospitals over a period of 8 years: Results of the Canadian Ward surveillance study (CANWARD), 2008–2015. Diagn. Microbiol. Infect. Dis. 2017, 87, 60–63. [Google Scholar] [CrossRef]
- Walkty, A.; Karlowsky, J.A.; Lagacé-Wiens, P.R.S.; Baxter, M.R.; Adam, H.J.; Bay, D.C.; Zhanel, G.G. Moving towards a standardized definition of antimicrobial resistance: A comparison of the antimicrobial susceptibility profile of difficult-to-treat resistance (DTR) versus multidrug-resistant (MDR) Pseudomonas aeruginosa clinical isolates (CANWARD, 2016–2021). Diagn. Microbiol. Infect. Dis. 2024, 108, 116130. [Google Scholar] [CrossRef]
- Ernst, P.; Lix, L.M.; Carney, G.; Daneman, N.; Dahl, M.; Scory, T.D.; Dutton, D.; Feng, X.; Janzen, D.; Ling, V.; et al. Use of Oral Fluoroquinolones in Canada: A Drug Utilization Study Update; Canadian Network for Observational Drug Effect Studies (CNODES): Ottawa, ON, Canada, 2024. [Google Scholar]
- Marketed Health Products Directorate (Health Canada). Summary Safety Review—Fluoroquinolones—Assessing the Potential Risk of Persistent and Disabling Side Effects. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/summary-safety-review-fluoroquinolones-assessing-potential-risk-persistent-disabling-effects.html (accessed on 22 January 2025).
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef]
- CLSI M100; Performance Standards for Antimicrobial Susceptiblity Testing, 33rd ed. Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2023.
- Mataseje, L.F.; Bryce, E.; Roscoe, D.; Boyd, D.A.; Embree, J.; Gravel, D.; Katz, K.; Kibsey, P.; Kuhn, M.; Mounchili, A.; et al. Carbapenem-resistant Gram-negative bacilli in Canada 2009-10: Results from the Canadian Nosocomial Infection Surveillance Program (CNISP). J. Antimicrob. Chemother. 2012, 67, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, J.-M.; Poirel, L.; Nordmann, P. Molecular Epidemiology and Mechanisms of Carbapenem Resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2009, 53, 4783–4788. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Reserve List for Antimicrobial Drugs. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/reserve-list-antimicrobial.html (accessed on 22 January 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Walkty, A.; Lagacé-Wiens, P.; Karlowsky, J.; Zhanel, G. The State of Antimicrobial Resistance of Gram-Negative Bacilli in Canada. Trop. Med. Infect. Dis. 2025, 10, 115. https://doi.org/10.3390/tropicalmed10040115
Li J, Walkty A, Lagacé-Wiens P, Karlowsky J, Zhanel G. The State of Antimicrobial Resistance of Gram-Negative Bacilli in Canada. Tropical Medicine and Infectious Disease. 2025; 10(4):115. https://doi.org/10.3390/tropicalmed10040115
Chicago/Turabian StyleLi, Jeremy, Andrew Walkty, Philippe Lagacé-Wiens, James Karlowsky, and George Zhanel. 2025. "The State of Antimicrobial Resistance of Gram-Negative Bacilli in Canada" Tropical Medicine and Infectious Disease 10, no. 4: 115. https://doi.org/10.3390/tropicalmed10040115
APA StyleLi, J., Walkty, A., Lagacé-Wiens, P., Karlowsky, J., & Zhanel, G. (2025). The State of Antimicrobial Resistance of Gram-Negative Bacilli in Canada. Tropical Medicine and Infectious Disease, 10(4), 115. https://doi.org/10.3390/tropicalmed10040115





