Yellow Fever Virus (YFV) Detection in Different Species of Culicids Collected During an Outbreak in Southeastern Brazil, 2016–2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Epizootic Events and Mosquito Collection
2.3. YFV RNA Detection and Statistical Analysis
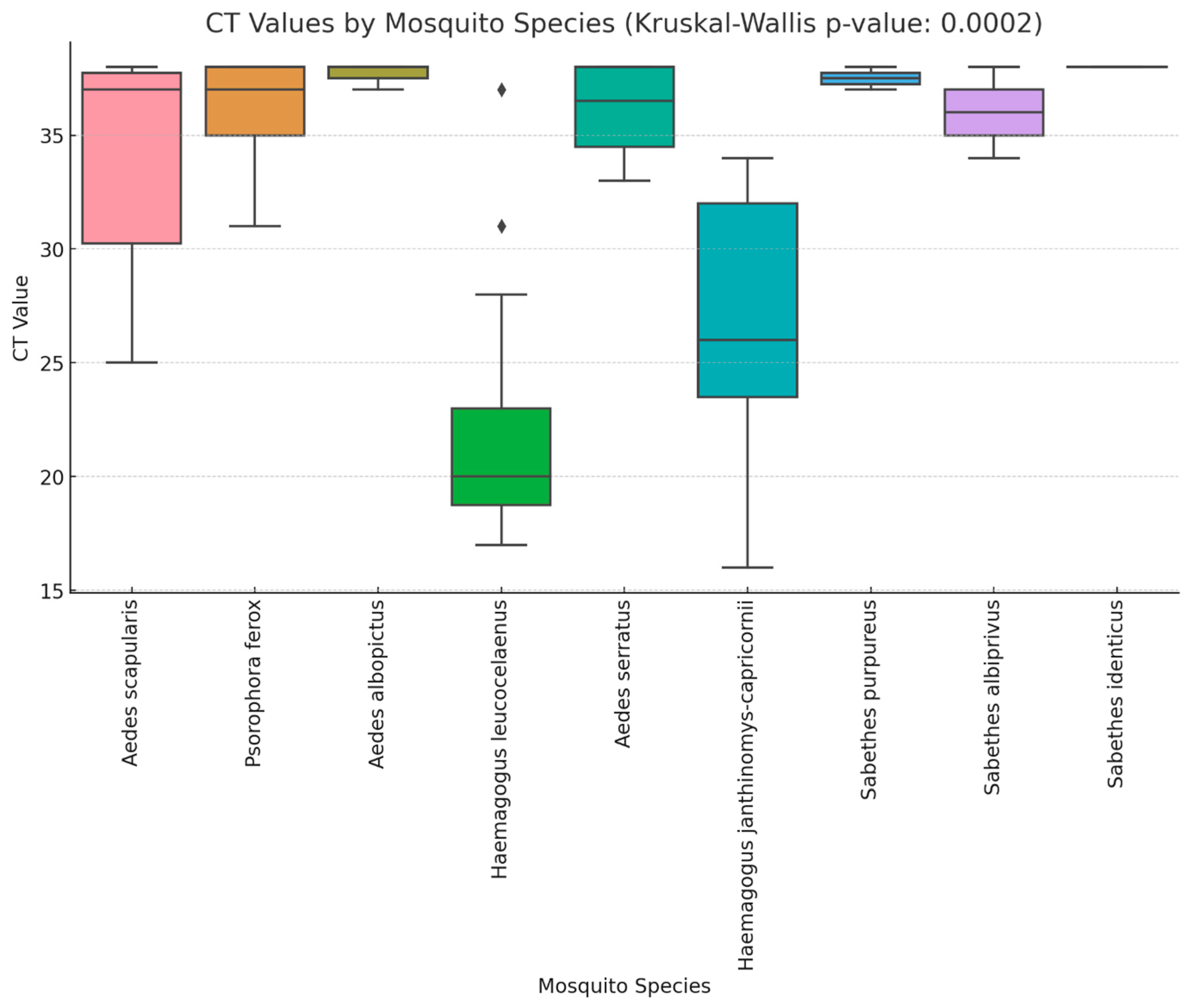
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasconcelos, P.F.C.; Monath, T.P. Yellow Fever Remains a Potential Threat to Public Health. Vector Borne Zoonotic Dis. 2016, 16, 566–567. [Google Scholar] [CrossRef]
- de Azevedo Fernandes, N.C.C.; Guerra, J.M.; Díaz-Delgado, J.; Cunha, M.S.; Saad, L.D.; Iglezias, S.D.; Ressio, R.A.; Cirqueira, C.d.S.; Kanamura, C.T.; Jesus, I.P.; et al. Differential Yellow Fever Susceptibility in New World Nonhuman Primates, Comparison with Humans, and Implications for Surveillance. Emerg. Infect. Dis. 2021, 27, 47–56. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.; Costa, Z.G.; Travassos, D.R.E.S.; Luna, E.; Rodrigues, S.G.; Barros, V.L.; Dias, J.P.; Monteiro, H.A.; Oliva, O.F.; Vasconcelos, H.B.; et al. Epidemic of jungle yellow fever in Brazil, 2000: Implications of climatic alterations in disease spread. J. Med. Virol. 2001, 65, 598–604. [Google Scholar] [CrossRef]
- Ministerio da Saúde. Brasília-Df 2017 Guia de Vigilância de Epizootias em Primatas não Humanos e Entomologia Aplicada à Vigilância da Febre Amarela Ministério da Saúde 2 a Edição Atualizada. 2017. Available online: https://www.gov.br/saude/pt-br (accessed on 11 December 2024).
- Li, S.L.; Acosta, A.L.; Hill, S.C.; Brady, O.J.; de Almeida, M.A.B.; Cardoso, J.d.C.; Hamlet, A.; Mucci, L.F.; de Deus, J.T.; Iani, F.C.M.; et al. Mapping environmental suitability of Haemagogus and Sabethes spp. mosquitoes to understand sylvatic transmission risk of yellow fever virus in Brazil. PLoS Neglected Trop. Dis. 2022, 16, e0010019. [Google Scholar] [CrossRef] [PubMed]
- Silva-Inacio, C.L.; Ximenes, M.d.F.F.d.M. Haemagogus spegazzinii Brèthes, 1912 (Diptera: Culicidae) in Brazilian semiarid: Resistance in eggs and scale color variation in adults. Rev. Bras. Entomol. 2021; 65, Epub ahead of print. [Google Scholar] [CrossRef]
- Cunha, M.S.; da Costa, A.C.; de Azevedo Fernandes, N.C.C.; Santos, F.C.P.D.; Nogueira, J.S.; D’Agostino, L.G.; Komninakis, S.V.; Witkin, S.S.; Ressio, R.A.; Maeda, A.Y.; et al. Epizootics due to Yellow Fever Virus in São Paulo State, Brazil: Viral dissemination to new areas (2016–2017). Sci. Rep. 2019, 9, 5474. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.C.C.D.A.; Cunha, M.S.; Guerra, J.M.; Réssio, R.A.; Cirqueira, C.D.S.; Iglezias, S.D.; Albergaria, R.R.; Araujo, E.L.; Catão-Dias, J.L.; Díaz-Delgado, J. Outbreak of Yellow Fever among Nonhuman Primates, Espirito Santo, Brazil, 2017. Emerg. Infect. Dis. 2017, 23, 2038–2041. [Google Scholar] [CrossRef]
- Rezende, I.M.; Sacchetto, L.; Munhoz de Mello, É.; Alves, P.A.; Iani, F.C.M.; Adelino, T.É.R.; Duarte, M.M.; Cury, A.L.F.; Bernardes, A.F.L.; Santos, T.A.; et al. Persistence of Yellow fever virus outside the Amazon Basin, causing epidemics in Southeast Brazil, from 2016 to 2018. PLoS Neglected Trop. Dis. 2018, 12, e0006538. [Google Scholar] [CrossRef]
- Moreira-Soto, A.; Torres, M.; de Mendonça, M.L.; Mares-Guia, M.; Rodrigues, C.d.S.; Fabri, A.; dos Santos, C.; Araújo, E.M.; Fischer, C.; Nogueira, R.R.; et al. Evidence for multiple sylvatic transmission cycles during the 2016–2017 yellow fever virus outbreak, Brazil. Clin. Microbiol. Infect. 2018, 24, e1–e1019. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Sao Paulo. Available online: https://www.ibge.gov.br/cidades-e-estados/sp/sao-paulo.html (accessed on 5 March 2025).
- Faria, N.R.; Kraemer, M.U.G.; Hill, S.C.; De Jesus, J.G.; Aguiar, R.S.; Iani, F.C.M.; Xavier, J.; Quick, J.; Du Plessis, L.; Dellicour, S.; et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 2018, 361, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.C.; de Souza, R.; Thézé, J.; Claro, I.; Aguiar, R.S.; Abade, L.; Santos, F.C.P.; Cunha, M.S.; Nogueira, J.S.; Salles, F.C.S.; et al. Genomic Surveillance of Yellow Fever Virus Epizootic in São Paulo, Brazil, 2016–2018. PLoS Pathog. 2020, 16, e1008699. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.S.; Tubaki, R.M.; de Menezes, R.M.T.; Pereira, M.; Caleiro, G.S.; Coelho, E.; Saad, L.D.C.; Fernandes, N.C.C.A.; Guerra, J.M.; Nogueira, J.S.; et al. Possible non-sylvatic transmission of yellow fever between non-human primates in São Paulo city, Brazil, 2017–2018. Sci. Rep. 2020, 10, 15751. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- de Oliveira, F.P.; Stoffella-Dutra, A.G.; Barbosa, C.G.; Silva, d.O.J.; Dourado, A.C.; Duarte, S.J.; Soares, R.K.L.; Araújo, J.J.P.; Lacerda, N.M.; Zazá, B.M.A.; et al. Re-Emergence of Yellow Fever in Brazil during 2016–2019: Challenges, Lessons Learned, and Perspectives. Viruses 2020, 12, 1233. [Google Scholar] [CrossRef]
- Abreu, F.V.S.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Santos, A.A.C.D.; Miranda, R.M.; Bonelly, I.S.; Neves, M.S.A.S.; Bersot, M.I.; Santos, T.P.D.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef]
- Souza, R.P.; Petrella, S.; Coimbra, T.L.; Maeda, A.Y.; Rocco, I.M.; Bisordi, I.; Silveira, V.R.; Pereira, L.E.; Suzuki, A.; Silva, S.J.; et al. Isolation of yellow fever virus (YFV) from naturally infectied Haemagogus (Conopostegus) leucocelaenus (diptera, cukicudae) in São Paulo State, Brazil, 2009. Rev. Inst. Med. Trop. São Paulo 2011, 53, 133–139. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.; Sperb, A.F.; Monteiro, H.A.; Torres, M.A.; Sousa, M.R.; Vasconcelos, H.B.; Mardini, L.B.; Rodrigues, S.G. Isolations of yellow fever virus from Haemagogus leucocelaenus in Rio Grande do Sul State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 60–62. [Google Scholar] [CrossRef]
- de Carvalho, G.C.; Malafronte, R.S.; Miti, I.C.; Souza, T.R.; Natal, L.; Marrelli, M.T. Blood meal sources of mosquitoes captured in municipal parks in São Paulo, Brazil. J. Vector Ecol. 2014, 39, 146–152. [Google Scholar] [CrossRef]
- Forattini, O.P.; de Castro, G.A.; Natal, D.; Kakitani, I.; Marucci, D. Preferências alimentares e domiciliação de mosquitos Culicidae no Vale do Ribeira, São Paulo, Brasil, com especial referência a Aedes scapularis e a Culex (Melanoconion). Rev. Saude Publica 1989, 23, 9–19. [Google Scholar] [CrossRef]
- Goenaga, S.; Fabbri, C.; Dueñas, J.C.R.; Gardenal, C.N.; Rossi, G.C.; Calderon, G.; Morales, M.A.; Garcia, J.B.; Enria, D.A.; Levis, S. Isolation of Yellow Fever Virus from Mosquitoes in Misiones Province, Argentina. Vector Borne Zoonotic Dis. 2012, 12, 986–993. [Google Scholar] [CrossRef]
- de Oliveira, C.H.; Andrade, M.S.; Campos, F.S.; Cardoso, J.d.C.; Gonçalves-Dos-Santos, M.E.; Oliveira, R.S.; Aquino-Teixeira, S.M.; Campos, A.A.; AB Almeida, M.; Simonini-Teixeira, D.; et al. Yellow Fever Virus Maintained by Sabethes Mosquitoes during the Dry Season in Cerrado, a Semiarid Region of Brazil, in 2021. Viruses 2023, 15, 757. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, L.M.d.A.; Motta, M.d.A.; Erbisti, R.S.; Abreu, F.V.S.d.; Nascimento-Pereira, A.C.; Ferreira-de-Brito, A.; Neves, M.S.A.S.; Pereira, G.R.; Pereira, G.R.; Santos, C.B.d.; et al. Back to Where It Was First Described: Vectors of Sylvatic Yellow Fever Transmission in the 2017 Outbreak in Espírito Santo, Brazil. Viruses 2022, 14, 2805. [Google Scholar] [CrossRef] [PubMed]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; Motta, M.d.A.; dos Santos, F.B.; Vazeille, M.; Vasconcelos, P.F.d.C.; Lourenço-De-Oliveira, R.; Failloux, A.-B. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Environ. Sci. Med. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Fernandes, N.C.C.A.; Cunha, M.S.; Suarez, P.E.N.; Machado, E.F.; Garcia, J.M.; De Carvalho, A.C.S.R.; Figueiredo, K.B.; Ressio, R.A.; Matsumoto, P.S.S.; Saad, L.D.C.; et al. Phylogenetic analysis reveals a new introduction of Yellow Fever virus in São Paulo State, Brazil, 2023. Acta Trop. 2024, 251, 107110. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-de-Oliveira, R.; Vazeille, M.; Filippis, A.M.B.d.; Failloux, A.B. Oral Susceptibility to Yellow Fever Virus of Aedes aegypti from Brazil. Memórias Inst. Oswaldo Cruz 2002, 97, 437–439. [Google Scholar] [CrossRef]
- Barbosa, G.; Sterlino, B.E.; Pereira, M.; Botti, M.V.; Perpétuo, S.M. Presença de Aedes aegypti e Aedes albopictus em ambientes urbanos adjacentes às áreas silvestres que apresentam potencial para a circulação do vírus da febre amarela no estado de São Paulo. BEPA Bol. Epidemiológico Paul. 2022, 16, 25–30. [Google Scholar] [CrossRef]
- Damasceno-Caldeira, R.; Nunes-Neto, J.P.; Aragão, C.F.; Freitas, M.N.O.; Ferreira, M.S.; de Castro, P.H.G.; Dias, D.D.; Araújo, P.A.d.S.; Brandão, R.C.F.; Nunes, B.T.D.; et al. Vector Competence of Aedes albopictus for Yellow Fever Virus: Risk of Reemergence of Urban Yellow Fever in Brazil. Viruses 2023, 15, 1019. [Google Scholar] [CrossRef]
- Amraoui, F.; Pain, A.; Piorkowski, G.; Vazeille, M.; Couto-Lima, D.; de Lamballerie, X.; Lourenço-De-Oliveira, R.; Failloux, A.-B. Experimental Adaptation of the Yellow Fever Virus to the Mosquito Aedes albopictus and Potential risk of urban epidemics in Brazil, South America. Sci. Rep. 2018, 8, 14337. [Google Scholar] [CrossRef]
- Hamlet, A.; Jean, K.K.; Perea, W.; Yactayo, S.; Biey, J.; Van Kerkhove, M.; Ferguson, N.; Garske, T. The seasonal influence of climate and environment on yellow fever transmission across Africa. PLoS Neglected Trop. Dis. 2018, 12, e0006284. [Google Scholar] [CrossRef]
- Sacchetto, L.; Silva, N.I.; De Rezende, I.M.; Arruda, M.S.; Costa, T.A.; De Mello, É.M.; Oliveira, G.F.G.; Alves, P.A.; De Mendonça, V.E.; Stumpp, R.G.A.V.; et al. Neighbor danger: Yellow fever virus epizootics in urban and urban-rural transition areas of Minas Gerais state, during 2017–2018 yellow fever outbreaks in Brazil. PLoS Neglected Trop. Dis. 2020, 14, e0008658. [Google Scholar] [CrossRef]
| Species | N | % | Positive | %Pos |
|---|---|---|---|---|
| Aedes scapularis | 893 | 26.46 | 6 | 0.67 |
| Aedes albopictus | 731 | 21.66 | 3 | 0.41 |
| Psorophora ferox | 378 | 11.20 | 5 | 1.32 |
| Haemagogus leucocelaenus | 274 | 8.09 | 16 | 5.83 |
| Aedes serratus | 193 | 5.72 | 4 | 2.07 |
| Aedes aegypti | 148 | 4.39 | 0 | 0 |
| Haemagogus janthinomys/capricornii | 127 | 3.4 | 7 | 5.51 |
| Sabethes purpureus | 96 | 2.84 | 2 | 2.08 |
| Sabethes glaucodaemon | 94 | 2.79 | 0 | 0 |
| Aedes terrens | 72 | 2.13 | 0 | 0 |
| Sabethes identicus | 57 | 1.69 | 1 | 1.75 |
| Sabethes albiprivus | 47 | 1.39 | 2 | 4.26 |
| Sabethes chloropterus | 46 | 1.23 | 0 | 0 |
| Psorophora albigenu | 31 | 0.83 | 0 | 0 |
| Sabethes intermedius | 28 | 0.83 | 0 | 0 |
| Psorophora albipes | 27 | 0.80 | 0 | 0 |
| Psorophora (Jan.) sp. | 17 | 0.50 | 0 | 0 |
| Sabethes belisarioi | 16 | 0.47 | 0 | 0 |
| Aedes argyrothorax | 13 | 0.39 | 0 | 0 |
| Psorophora sp. | 11 | 0.33 | 0 | 0 |
| Sabethes sp. | 11 | 0.30 | 0 | 0 |
| Aedes sp. | 9 | 0.27 | 0 | 0 |
| Sabethes undosus | 9 | 0.27 | 0 | 0 |
| Sabethes tridentatus | 8 | 0.24 | 0 | 0 |
| Psorophora lutzii | 6 | 0.18 | 0 | 0 |
| Sabethes soperi | 6 | 0.18 | 0 | 0 |
| Sabethes whitmani | 6 | 0.18 | 0 | 0 |
| Howardina fulvithorax | 6 | 0.18 | 0 | 0 |
| Culex sp. | 4 | 0.12 | 0 | 0 |
| Sabethes undosus aff. | 3 | 0.09 | 0 | 0 |
| Aedes fluviatilis | 2 | 0.06 | 0 | 0 |
| Culex quinquefaciatus | 2 | 0.06 | 0 | 0 |
| Limatus sp. | 1 | 0.03 | 0 | 0 |
| Psorophora lanei | 1 | 0.03 | 0 | 0 |
| Sabethes belisarioi aff. | 1 | 0.03 | 0 | 0 |
| Sabethes petrochiae | 1 | 0.03 | 0 | 0 |
| Sabethes shannoni | 1 | 0.03 | 0 | 0 |
| Pool Number | Species (n° per pool) | Local | Ct Value | Date | Season |
|---|---|---|---|---|---|
| 732 | Aedes albopictus (2) | Jundiaí | 38 | 28 August 2018 | Dry |
| 3777 | Aedes albopictus (2) | Itariri | 38 | 16 January 2019 | Rainy |
| 4231 | Aedes albopictus (6) | Pereira Barreto | 37 | 16 January 2019 | Rainy |
| 443 | Aedes scapularis (8) | Urupes | 25 | 26 November 2016 | Rainy |
| 1415 | Aedes scapularis (1) | Araçatuba | 28 | 25 November 2016 | Rainy |
| 2348 | Aedes scapularis (3) | Sao Paulo | 37 | 19 February 2018 | Rainy |
| 4232 | Aedes scapularis (1) | Pereira Barreto | 37 | 16 January 2019 | Rainy |
| 4234 | Aedes scapularis (1) | Pereira Barreto | 38 | 16 January 2019 | Rainy |
| 4238 | Aedes scapularis (3) | Sao Paulo | 38 | 16 January 2019 | Rainy |
| 2198 | Aedes serratus (1) | Jarinu | 33 | 12 February 2019 | Rainy |
| 4233 | Aedes serratus (1) | Pereira Barreto | 38 | 16 January 2019 | Rainy |
| 4276 | Aedes serratus (1) | Monteiro Lobato | 35 | 16 January 2019 | Rainy |
| 4449 | Aedes serratus (1) | Iguape | 38 | 25 March 2019 | Rainy |
| 2438 | Haemagogus janthinomys-capricornii (1) | Mairipora | 33 | 23 January 2018 | Rainy |
| 2572 | Haemagogus janthinomys-capricornii (1) | Valinhos | 31 | 4 September 2018 | Dry |
| 2577 | Haemagogus janthinomys-capricornii (12) | Valinhos | 34 | 17 September 2018 | Dry |
| 3551 | Haemagogus janthinomys-capricornii (7) | Igarata | 16 | 23 May 2018 | Dry |
| 3689 | Haemagogus janthinomys-capricornii (1) | Sao José dos Campos | 25 | 4 July 2018 | Dry |
| 3766 | Haemagogus janthinomys-capricornii (4) | Caçapava | 22 | 25 June 2018 | Dry |
| 4298 | Haemagogus janthinomys-capricornii (2) | Monteiro Lobato | 26 | 25 March 2019 | Rainy |
| 2152 | Haemagogus leucocelaenus (3) | Caieras | 23 | 16 April 2019 | Dry |
| 2163 | Haemagogus leucocelaenus (11) | Guarulhos | 21 | 14 December 2018 | Rainy |
| 2322 | Haemagogus leucocelaenus (1) | Jarinu | 37 | 3 May 2018 | Dry |
| 2377 | Haemagogus leucocelaenus (2) | Jarinu | 20 | 30 January 2018 | Rainy |
| 3268 | Haemagogus leucocelaenus (15) | Sao Paulo | 19 | 20 December 2017 | Rainy |
| 3270 | Haemagogus leucocelaenus (24) | Sao Paulo | 18 | 20 December 2017 | Rainy |
| 3318 | Haemagogus leucocelaenus (8) | Sao José dos Campos | 28 | 10 October 2018 | Dry |
| 3514 | Haemagogus leucocelaenus (11) | Piedade | 31 | 10 October 2018 | Dry |
| 3521 | Haemagogus leucocelaenus (7) | Jacarei | 20 | 4 September 2018 | Dry |
| 3530 | Haemagogus leucocelaenus (7) | Jacarei | 18 | 28 May 2018 | Dry |
| 3541 | Haemagogus leucocelaenus (5) | Igarata | 19 | 28 May 2018 | Dry |
| 3552 | Haemagogus leucocelaenus (9) | Igarata | 20 | 23 May 2018 | Dry |
| 3687 | Haemagogus leucocelaenus (6) | Sao José dos Campos | 23 | 4 July 2018 | Dry |
| 4272 | Haemagogus leucocelaenus (7) | Monteiro Lobato | 17 | 16 January 2019 | Rainy |
| 4275 | Haemagogus leucocelaenus (15) | Monteiro Lobato | 17 | 16 January 2019 | Rainy |
| 4297 | Haemagogus leucocelaenus (2) | Monteiro Lobato | 21 | 25 March 2019 | Rainy |
| 465 | Psorophora ferox (14) | Pontalinda | 38 | 21 August 2018 | Dry |
| 4188 | Psorophora ferox (2) | Jacupiranga | 31 | 10 December 2018 | Rainy |
| 4273 | Psorophora ferox (1) | Monteiro Lobato | 37 | 16 January 2019 | Rainy |
| 4568 | Psorophora ferox (1) | Sarapui | 38 | 21 May 2018 | Dry |
| 5077 | Psorophora ferox (2) | Iporanga | 35 | 25 April 2019 | Dry |
| 3542 | Sabethes albiprivus (1) | Igarata | 38 | 28 May 2018 | Dry |
| 3769 | Sabethes albiprivus (3) | Caçapava | 34 | 25 June 2018 | Dry |
| 3543 | Sabethes identicus (6) | Igarata | 38 | 28 May 2018 | Dry |
| 3491 | Sabethes purpureus (2) | Sao Miguel Arcanjo | 37 | 4 September 2018 | Dry |
| 4279 | Sabethes purpureus (1) | Monteiro Lobato | 38 | 16 January 2019 | Rainy |
| Species | Estimate (β) | SE | 95% CI | p-Value |
|---|---|---|---|---|
| Intercept (Haemagogus leucocelaenus) | 22 | 1.26 | (19.53, 24.47) | <0.001 |
| Aedes scapularis | 11.83 | 2.41 | (7.11, 16.56) | <0.001 |
| Psorophora ferox | 13.8 | 2.58 | (8.74, 18.86) | <0.001 |
| Aedes albopictus | 15.67 | 3.17 | (9.46, 21.88) | <0.001 |
| Aedes serratus | 14 | 2.81 | (8.48, 19.52) | <0.001 |
| Haemagogus janthinomys/capricornii | 4.71 | 2.28 | (0.24, 9.19) | 0.039 |
| Sabethes purpureus | 15.5 | 3.78 | (8.10, 22.90) | <0.001 |
| Sabethes albiprivus | 14 | 3.78 | (6.60, 21.40) | <0.001 |
| Sabethes identicus | 16 | 5.19 | (5.83, 26.17) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caleiro, G.S.; Vilela, L.O.; Nuevo, K.M.B.; Tubaki, R.M.; de Menezes, R.M.T.; Mucci, L.F.; Telles-de-Deus, J.; Bergo, E.S.; Araújo, E.L.L.; Cunha, M.S. Yellow Fever Virus (YFV) Detection in Different Species of Culicids Collected During an Outbreak in Southeastern Brazil, 2016–2019. Trop. Med. Infect. Dis. 2025, 10, 118. https://doi.org/10.3390/tropicalmed10050118
Caleiro GS, Vilela LO, Nuevo KMB, Tubaki RM, de Menezes RMT, Mucci LF, Telles-de-Deus J, Bergo ES, Araújo ELL, Cunha MS. Yellow Fever Virus (YFV) Detection in Different Species of Culicids Collected During an Outbreak in Southeastern Brazil, 2016–2019. Tropical Medicine and Infectious Disease. 2025; 10(5):118. https://doi.org/10.3390/tropicalmed10050118
Chicago/Turabian StyleCaleiro, Giovana Santos, Lucila Oliveira Vilela, Karolina Morales Barrio Nuevo, Rosa Maria Tubaki, Regiane Maria Tironi de Menezes, Luis Filipe Mucci, Juliana Telles-de-Deus, Eduardo Sterlino Bergo, Emerson Luiz Lima Araújo, and Mariana Sequetin Cunha. 2025. "Yellow Fever Virus (YFV) Detection in Different Species of Culicids Collected During an Outbreak in Southeastern Brazil, 2016–2019" Tropical Medicine and Infectious Disease 10, no. 5: 118. https://doi.org/10.3390/tropicalmed10050118
APA StyleCaleiro, G. S., Vilela, L. O., Nuevo, K. M. B., Tubaki, R. M., de Menezes, R. M. T., Mucci, L. F., Telles-de-Deus, J., Bergo, E. S., Araújo, E. L. L., & Cunha, M. S. (2025). Yellow Fever Virus (YFV) Detection in Different Species of Culicids Collected During an Outbreak in Southeastern Brazil, 2016–2019. Tropical Medicine and Infectious Disease, 10(5), 118. https://doi.org/10.3390/tropicalmed10050118







