Secondary Metabolites from a New Antibiotic-Producing Endophytic Streptomyces Isolate Inhibited Pathogenic and Multidrug-Resistant Mycobacterium tuberculosis Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Ethyl Acetate Extraction of Culture Filtrate Extracts from Actinomycetes Strains
2.3. Genomic DNA Isolation and PCR Amplification of 16S rRNA Gene
2.4. Mycobacterial Cell Viability Assay
2.5. Microplate Nitrate Reductase Assay (MNRA)
2.6. Cytotoxicity Assays in Human Peripheral Blood Mononuclear Cells (hPBMCs)
2.7. Infection Assays
2.8. LC-MS Analysis
Preprocessing of Raw LC-MS Data
3. Results and Discussion
3.1. An Endophytic Strain with Anti-Mycobacterial Properties Isolated from Tectona Grandis
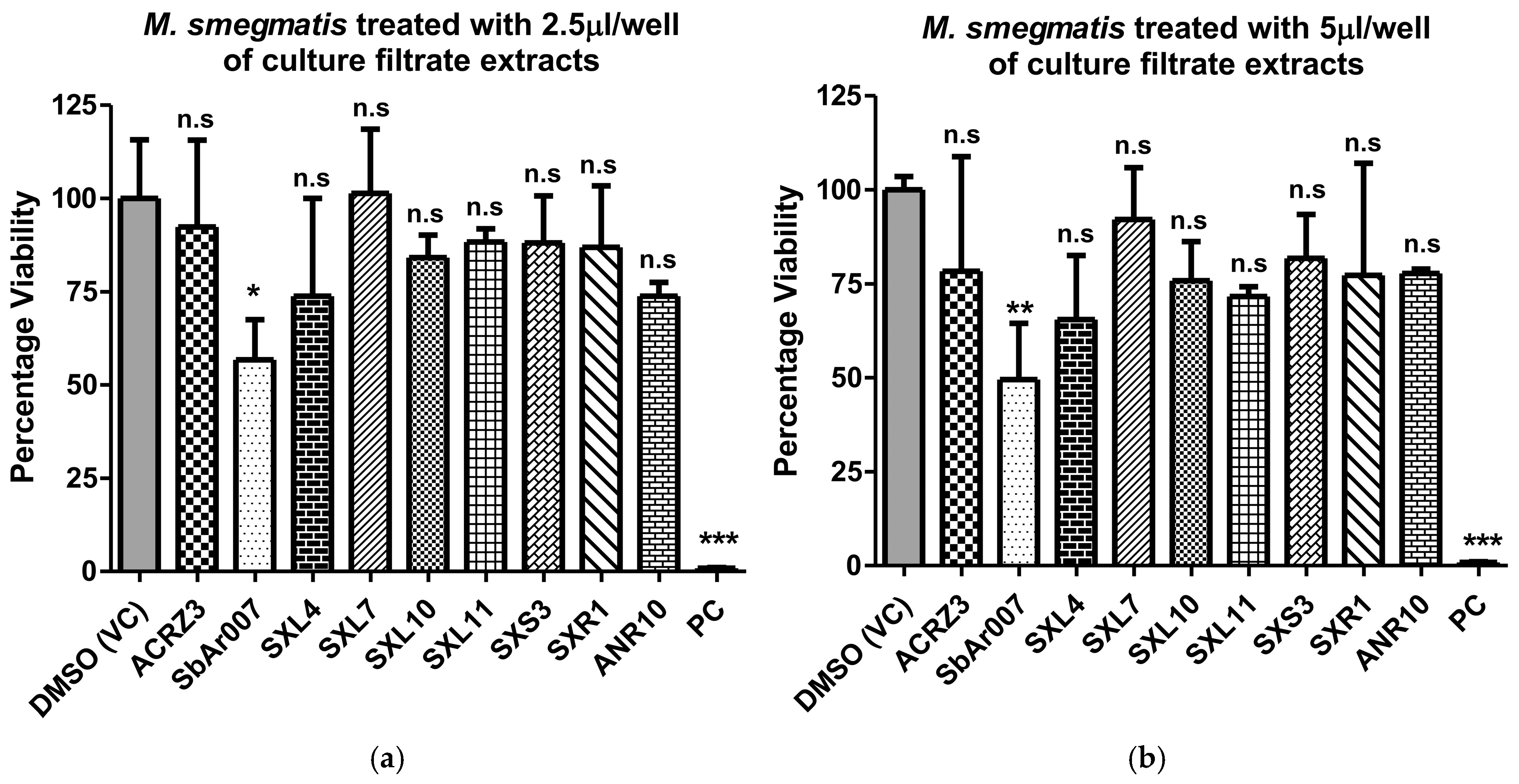
3.2. Culture Filtrate Extracts from Streptomyces sp. SbAr007 Showed Potent Anti-M.tb Activity
3.3. Culture Filtrate Extract from Streptomyces sp. SbAr007 Showed Negligible Cytotoxicity to Human Peripheral Blood Mononuclear Cells (hPBMCs)
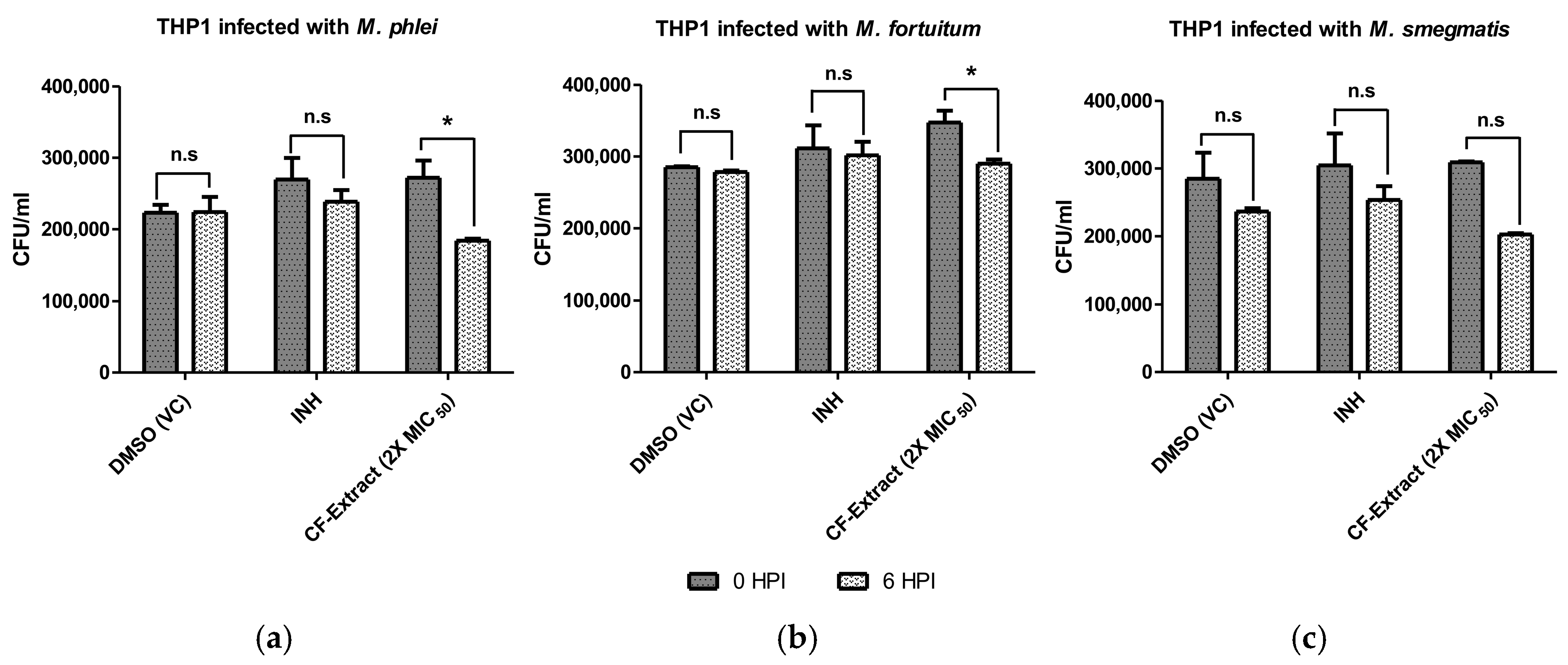
3.4. Culture Filtrate Extract of Streptomyces sp. SbAr007 Decreased Intracellular Mycobacterial Load
3.5. Characterization of Streptomyces sp. SbAr007
3.5.1. Phylogenetic Analysis Revealed Streptomyces sp. SbAr007 Clustered with Streptomyces samsunensis, Streptomyces malaysiensis, and Streptomyces solisilvae
3.5.2. Strain Comparison Between the Closely Related Subclades of Streptomyces sp. SbAr007
3.6. Identification of Potential Bioactive Molecules in the Culture Filtrate Extracts of Streptomyces sp. SbAr007
Analysis of Metabolites Obtained in the Active Ethyl Acetate Extracts of Streptomyces sp. SbAr007 Culture Filtrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. History of World TB Day. 2024. Available online: https://www.cdc.gov/tb/worldtbday/history.htm (accessed on 14 October 2024).
- World Health Organization. Licence: CC BY-NC-SA 3.0 IGO; Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- World Health Organization. Licence: CC BY-NC-SA 3.0 IGO; Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Gagneux, S. Host–Pathogen Coevolution in Human Tuberculosis. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Treatment for TB Disease|TB|CDC. Available online: https://www.cdc.gov/tb/topic/treatment/tbdisease.htm (accessed on 5 December 2024).
- Mukherjee, A.; Lodha, R.; Kabra, S.K. Current Therapies for the Treatment of Multidrug-Resistant Tuberculosis in Children in India. Expert Opin. Pharmacother. 2017, 18, 1595. [Google Scholar] [CrossRef] [PubMed]
- Provisional CDC Guidance for the Use of Pretomanid as Part of a Regimen [Bedaquiline, Pretomanid, and Linezolid (BPaL)] to Treat Drug-Resistant Tuberculosis Disease|Tuberculosis (TB)|CDC. Available online: https://www.cdc.gov/tb/hcp/treatment/bpal.html (accessed on 5 December 2024).
- Nawwar, A.; Madrid-Morales, J.; Velez-Mejia, C.; Pizarro, R.D.J.; Cepeda, V.; Reveles, K.R.; Cadena-Zuluaga, J.; Javeri, H. 1402. NTM Infections; A Rising Global Health Problem/Clinical Characteristics and Outcomes of Patients with Non-Tuberculous Mycobacterial Infections at Two Tertiary Academic Medical Centers. Open Forum Infect. Dis. 2021, 8, S785–S786. [Google Scholar] [CrossRef]
- Lin, C.; Russell, C.; Soll, B.; Chow, D.; Bamrah, S.; Brostrom, R.; Kim, W.; Scott, J.; Bankowski, M.J. Increasing Prevalence of Nontuberculous Mycobacteria in Respiratory Specimens from US-Affiliated Pacific Island Jurisdictions. Emerg. Infect. Dis. 2018, 24, 485. [Google Scholar] [CrossRef]
- Boshoff, H.I.; Malhotra, N.; Barry, C.E.; Oh, S. The Antitubercular Activities of Natural Products with Fused-Nitrogen-Containing Heterocycles. Pharmaceuticals 2024, 17, 211. [Google Scholar] [CrossRef]
- Kisil, O.V.; Efimenko, T.A.; Efremenkova, O.V. Looking Back to Amycolatopsis: History of the Antibiotic Discovery and Future Prospects. Antibiotics 2021, 10, 1254. [Google Scholar] [CrossRef]
- Michelow, I.C.; McCracken, G.H. Antibacterial Therapeutic Agents. In Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 6th ed.; Saunders: Philadelphia, PA, USA, 2009; pp. 3178–3227. [Google Scholar] [CrossRef]
- Starke, J.R. Tuberculosis. In Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 6th ed.; Saunders: Philadelphia, PA, USA, 2009; pp. 1426–1469. [Google Scholar] [CrossRef]
- Baranova, A.A.; Alferova, V.A.; Korshun, V.A.; Tyurin, A.P. Modern Trends in Natural Antibiotic Discovery. Life 2023, 13, 1073. [Google Scholar] [CrossRef]
- Quinn, G.A.; Dyson, P.J. Going to Extremes: Progress in Exploring New Environments for Novel Antibiotics. npj Antimicrob. Resist. 2024, 2, 8. [Google Scholar] [CrossRef]
- Mast, Y.; Stegmann, E. Actinomycetes: The Antibiotics Producers. Antibiotics 2019, 8, 105. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary Metabolites and Biodiversity of Actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72. [Google Scholar] [CrossRef]
- Samad, M.A. A Comprehensive Review of Antimicrobial Resistance Beginning from the Discovery of the First Antibiotic until the Present-Day Situation with One Health Approach with Special Emphasis on Bangladesh. J. Vet. Med. OH Res. 2023, 5, 1–86. [Google Scholar] [CrossRef]
- Khunjamayum, R.; Ningthoujam, D.S.; Sarita Devi, A.; Nimaichand, S.; Banerjee, S.; Sankati, S.; Rizvi, A. In-Vitro Antimycobacterial Activities of Endophytic Bacteria Associated with Medicinal Plant of Manipur. J. Bacteriol. Mycol. Open Access 2017, 4, 104–107. [Google Scholar] [CrossRef]
- Vadankula, G.R.; Nilkanth, V.V.; Rizvi, A.; Yandrapally, S.; Agarwal, A.; Chirra, H.; Biswas, R.; Arifuddin, M.; Nema, V.; Mallika, A.; et al. Confronting Tuberculosis: A Synthetic Quinoline-Isonicotinic Acid Hydrazide Hybrid Compound as a Potent Lead Molecule Against Mycobacterium Tuberculosis. ACS Infect. Dis. 2024, 10, 2288–2302. [Google Scholar] [CrossRef]
- Gomes, N.C.M.; Heuer, H.; Schönfeld, J.; Costa, R.; Mendonça-Hagler, L.; Smalla, K. Bacterial Diversity of the Rhizosphere of Maize (Zea Mays) Grown in Tropical Soil Studied by Temperature Gradient Gel Electrophoresis. Plant Soil 2001, 232, 167–180. [Google Scholar] [CrossRef]
- Asalla, S.; Mohareer, K.; Banerjee, S. Small Molecule Mediated Restoration of Mitochondrial Function Augments Anti-Mycobacterial Activity of Human Macrophages Subjected to Cholesterol Induced Asymptomatic Dyslipidemia. Front. Cell Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, S.K.; Singh, P.P.; Singh, V.K.; Rai, A.C.; Srivastava, A.K.; Shukla, L.; Kesawat, M.S.; Kumar Jaiswal, A.; Chung, S.M.; et al. Potential Anti-Mycobacterium tuberculosis Activity of Plant Secondary Metabolites: Insight with Molecular Docking Interactions. Antioxidants 2021, 10, 1990. [Google Scholar] [CrossRef]
- Hernández-García, E.; García, A.; Garza-González, E.; Avalos-Alanís, F.G.; Rivas-Galindo, V.M.; Rodríguez-Rodríguez, J.; Alcantar-Rosales, V.M.; Delgadillo-Puga, C.; del Rayo Camacho-Corona, M. Chemical Composition of Acacia farnesiana (L) Wild Fruits and Its Activity against Mycobacterium tuberculosis and Dysentery Bacteria. J. Ethnopharmacol. 2019, 230, 74–80. [Google Scholar] [CrossRef]
- Fadipe, V.O.; Mongalo, N.I.; Opoku, A.R.; Dikhoba, P.M.; Makhafola, T.J. Isolation of Anti-Mycobacterial Compounds from Curtisia dentata (Burm.f.) C.A.Sm (Curtisiaceae). BMC Complement. Altern. Med. 2017, 17, 306. [Google Scholar] [CrossRef]
- Gouveia-Figueira, S.C.; Gouveia, C.A.; Carvalho, M.J.; Rodrigues, A.I.; Nording, M.L.; Castilho, P.C. Antioxidant Capacity, Cytotoxicity and Antimycobacterial Activity of Madeira Archipelago Endemic Helichrysum Dietary and Medicinal Plants. Antioxidants 2014, 3, 713–729. [Google Scholar] [CrossRef]
- Mohamad, S.; Zin, N.M.; Wahab, H.A.; Ibrahim, P.; Sulaiman, S.F.; Zahariluddin, A.S.M.; Noor, S.S.M. Antituberculosis Potential of Some Ethnobotanically Selected Malaysian Plants. J. Ethnopharmacol. 2011, 133, 1021–1026. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Ganesh, M.; Peng, M.M.; Aziz, A.S.; Jang, H.T. Comparative Antioxidant and Antimycobacterial Activities of Opuntia ficus-indica Fruit Extracts from Summer and Rainy Seasons. Front. Life Sci. 2015, 8, 182–191. [Google Scholar] [CrossRef]
- Jiménez-Arellanes, A.; Meckes, M.; Torres, J.; Luna-Herrera, J. Antimycobacterial Triterpenoids from Lantana hispida (Verbenaceae). J. Ethnopharmacol. 2007, 111, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Sommart, U.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Sakayaroj, J.; Kirtikara, K. Hydronaphthalenones and a Dihydroramulosin from the Endophytic Fungus PSU-N24. Chem. Pharm. Bull. 2008, 56, 1687–1690. [Google Scholar] [CrossRef]
- Reheman, A.; Lu, D.; Wang, Y.; Chen, X.; Cao, G.; Wan, C. Screening of Microbial Fermentation Products for Anti-M. tuberculosis Activity. Animals 2022, 12, 1947. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Huang, Y.; Chen, H.; Li, Y.; Zhong, L.; Chen, Y.; Chen, S.; Wang, J.; Kang, J.; et al. Anti-Mycobacterial Activity of Marine Fungus-Derived 4-Deoxybostrycin and Nigrosporin. Molecules 2013, 18, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Nandhini, U.; Sreenivasan, A.; Kaari, M.; Kalyanasundaram, R.; Manikkam, R. AntiMycobacterial Activity of Endophytic Actinobacteria from Selected Medicinal Plants. Biomed. Biotechnol. Res. J. 2020, 4, 193–199. [Google Scholar] [CrossRef]
- Silva, E.M.S.; da Silva, I.R.; Ogusku, M.M.; Carvalho, C.M.; Maki, C.S.; de, L. Procópio, R.E. Metabolites from Endophytic Aspergillus fumigatus and Their in Vitro Effect against the Causal Agent of Tuberculosis. Acta Amaz. 2018, 48, 63–69. [Google Scholar] [CrossRef]
- Reingewertz, T.H.; Meyer, T.; McIntosh, F.; Sullivan, J.; Meir, M.; Chang, Y.F.; Behr, M.A.; Barkana, D. Differential Sensitivity of Mycobacteria to Isoniazid Is Related to Differences in KatG-Mediated Enzymatic Activation of the Drug. Antimicrob. Agents Chemother. 2020, 64, e01899-19. [Google Scholar] [CrossRef]
- Beye, M.; Fahsi, N.; Raoult, D.; Fournier, P.E. Careful Use of 16S RRNA Gene Sequence Similarity Values for the Identification of Mycobacterium Species. N. Microbes N. Infect. 2017, 22, 24. [Google Scholar] [CrossRef]
- Kim, M.; Chun, J. 16S RRNA Gene-Based Identification of Bacteria and Archaea Using the EzTaxon Server. Methods Microbiol. 2014, 41, 61–74. [Google Scholar] [CrossRef]
- Ezbiocloud.Net. Available online: https://www.ezbiocloud.net/identify/result?id=66f2775dcdc00f6ac0a9f52d (accessed on 2 November 2024).
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.Fr: Robust Phylogenetic Analysis for the Non-Specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.Fr: New Generation Phylogenetic Services for Non-Specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef] [PubMed]
- Sazak, A.; Şahin, N.; Güven, K.; Işik, K.; Goodfellow, M. Streptomyces samsunensis sp. Nov., a Member of the Streptomyces violaceusniger Clade Isolated from the Rhizosphere of Robinia pseudoacacia. Int. J. Syst. Evol. Microbiol. 2011, 61, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Al-Tai, A.; Bongcheol, K.; Seung Bum, K.; Manfio, G.P.; Goodfellow, M. Streptomyces malaysiensis sp. Nov., a New Streptomycete Species with Rugose, Ornamented Spores. Int. J. Syst. Bacteriol. 1999, 49, 1395–1402. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, X.; Huang, D.; Huang, X. Streptomyces solisilvae sp. Nov., Isolated from Tropical Forest Soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 3553–3558. [Google Scholar] [CrossRef]
- Chopra, S.; Matsuyama, K.; Hutson, C.; Madrid, P. Identification of Antimicrobial Activity among FDA-Approved Drugs for Combating Mycobacterium abscessus and Mycobacterium chelonae. J. Antimicrob. Chemother. 2011, 66, 1533–1536. [Google Scholar] [CrossRef]
- Kamal El-Sagheir, A.M.; Abdelmesseh Nekhala, I.; Abd El-Gaber, M.K.; Aboraia, A.S.; Persson, J.; Schäfer, A.B.; Wenzel, M.; Omar, F.A. N4-Substituted Piperazinyl Norfloxacin Derivatives with Broad-Spectrum Activity and Multiple Mechanisms on Gyrase, Topoisomerase IV, and Bacterial Cell Wall Synthesis. ACS Bio Med. Chem. Au 2023, 3, 494–506. [Google Scholar] [CrossRef]
- Kawakami, K.; Namba, K.; Tanaka, M.; Matsuhashi, N.; Sato, K.; Takemura, M. Antimycobacterial Activities of Novel Levofloxacin Analogues. Antimicrob. Agents Chemother. 2000, 44, 2126. [Google Scholar] [CrossRef]
- Rastogi, N.; Labrousse, V.; Goh, K.S.; De Sousa, J.P. Antimycobacterial Spectrum of Sparfloxacin and Its Activities Alone and in Association with Other Drugs against Mycobacterium Avium Complex Growing Extracellularly and Intracellularly in Murine and Human Macrophages. Antimicrob. Agents Chemother. 1991, 35, 2473. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Senchani, G.; Banerjee, D. Newer Tetracycline Derivatives: Synthesis, Anti-HIV, Antimycobacterial Activities and Inhibition of HIV-1 Integrase. Bioorganic Med. Chem. Lett. 2007, 17, 2372–2375. [Google Scholar] [CrossRef]
- Sanders, W.E.; Pejovic, I.; Cacciatore, R.; Valdez, H.; Dunbar, F.P. Activity of Gentamicin against Mycobacteria In Vitro and against Mycobacterium tuberculosis in Mice. J. Infect. Dis. 1971, 124, S33–S36. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Rajasekaran, P.; Haldimann, K.; Vasella, A.; Böttger, E.C.; Hobbie, S.N.; Crich, D. Synthesis of Gentamicins C1, C2, and C2a and Antiribosomal and Antibacterial Activity of Gentamicins B1, C1, C1a, C2, C2a, C2b, and X2. ACS Infect. Dis. 2023, 9, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Murohashi, T.; Yanagisawa, K. Anti-Mycobacterial Activity of Kanamycin Derivatives In Vitro and In Vivo. J. Antibiot. Ser. A 1960, 13, 177–179. [Google Scholar] [CrossRef]
- Ramos, D.F.; Matthiensen, A.; Colvara, W.; de Votto, A.P.S.; Trindade, G.S.; da Silva, P.E.A.; Yunes, J.S. Antimycobacterial and Cytotoxicity Activity of Microcystins. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 9. [Google Scholar] [CrossRef]
- Kimura, K.I. Liposidomycin, the First Reported Nucleoside Antibiotic Inhibitor of Peptidoglycan Biosynthesis Translocase I: The Discovery of Liposidomycin and Related Compounds with a Perspective on Their Application to New Antibiotics. J. Antibiot. 2019, 72, 877–889. [Google Scholar] [CrossRef]
- Gillespie, S.H.; Billington, O. Activity of Moxifloxacin against Mycobacteria. J. Antimicrob. Chemother. 1999, 44, 393–395. [Google Scholar] [CrossRef]
- Brandish, P.E.; Kimura, K.I.; Inukai, M.; Southgate, R.; Lonsdale, J.T.; Bugg, T.D.H. Modes of Action of Tunicamycin, Liposidomycin B, and Mureidomycin A: Inhibition of Phospho-N-Acetylmuramyl-Pentapeptide Translocase from Escherichia coli. Antimicrob. Agents Chemother. 1996, 40, 1640–1644. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The Bioavailability, Extraction, Biosynthesis and Distribution of Natural Dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Wen, Y.; Zhu, S.; Huang, S.; He, L.; Hou, S.; Lai, X.; Chen, S.; Dai, Z.; et al. Phloridzin Ameliorates Lipid Deposition in High-Fat-Diet-Fed Mice with Nonalcoholic Fatty Liver Disease via Inhibiting the MTORC1/SREBP-1c Pathway. J. Agric. Food Chem. 2021, 69, 8671–8683. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.S.; Kim, C.T.; Kim, I.H.; Kim, Y. Ginsenoside Rg3 Reduces Lipid Accumulation with AMP-Activated Protein Kinase (AMPK) Activation in HepG2 Cells. Int. J. Mol. Sci. 2012, 13, 5729–5739. [Google Scholar] [CrossRef]
- Muthukumaran, P.; Thiyagarajan, G.; Arun Babu, R.; Lakshmi, B.S. Raffinose from Costus Speciosus Attenuates Lipid Synthesis through Modulation of PPARs/SREBP1c and Improves Insulin Sensitivity through PI3K/AKT. Chem. Biol. Interact. 2018, 284, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tie, F.; Ding, J.; Hu, N.; Dong, Q.; Chen, Z.; Wang, H. Kaempferol and Kaempferide Attenuate Oleic Acid-Induced Lipid Accumulation and Oxidative Stress in HepG2 Cells. Int. J. Mol. Sci. 2021, 22, 8847. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Chao, T.Y.; Hsu, H.J.; Wang, C.Y.; Lin, C.Y.; Gao, W.Y.; Wu, M.J.; Yen, J.H. The Lipid-Modulating Effect of Tangeretin on the Inhibition of Angiopoietin-like 3 (Angptl3) Gene Expression through Regulation of Lxrα Activation in Hepatic Cells. Int. J. Mol. Sci. 2021, 22, 9853. [Google Scholar] [CrossRef]
- Yang, F.; Li, N.; Gaman, M.A.; Wang, N. Raloxifene Has Favorable Effects on the Lipid Profile in Women Explaining Its Beneficial Effect on Cardiovascular Risk: A Meta-Analysis of Randomized Controlled Trials. Pharmacol. Res. 2021, 166, 105512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; He, J.; Zhao, Y. Quercetin Regulates Lipid Metabolism and Fat Accumulation by Regulating Inflammatory Responses and Glycometabolism Pathways: A Review. Nutrients 2024, 16, 1102. [Google Scholar] [CrossRef]
- Jiang, Z.; Qu, H.; Lin, G.; Shi, D.; Chen, K.; Gao, Z. Lipid-Lowering Efficacy of the Capsaicin in Patients With Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 812294. [Google Scholar] [CrossRef]
- Evans, M.; Rumberger, J.A.; Azumano, I.; Napolitano, J.J.; Citrolo, D.; Kamiya, T. Pantethine, a Derivative of Vitamin B5, Favorably Alters Total, LDL and Non-HDL Cholesterol in Low to Moderate Cardiovascular Risk Subjects Eligible for Statin Therapy: A Triple-Blinded Placebo and Diet-Controlled Investigation. Vasc. Health Risk Manag. 2014, 10, 89. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Vacante, M.; Avitabile, T.; Malaguarnera, M.; Cammalleri, L.; Motta, M. L-Carnitine Supplementation Reduces Oxidized LDL Cholesterol in Patients with Diabetes. Am. J. Clin. Nutr. 2009, 89, 71–76. [Google Scholar] [CrossRef]
- Guo, X.; Ou, T.; Yang, X.; Song, Q.; Zhu, L.; Mi, S.; Zhang, J.; Zhang, Y.; Chen, W.; Guo, J. Untargeted Metabolomics Based on Ultra-High Performance Liquid Chromatography-Mass Spectrometry/MS Reveals the Lipid-Lowering Mechanism of Taurine in Hyperlipidemia Mice. Front. Nutr. 2024, 11, 1367589. [Google Scholar] [CrossRef]
- Adams, S.P.; Sekhon, S.S.; Tsang, M.; Wright, J.M. Fluvastatin for Lowering Lipids. Cochrane Database Syst. Rev. 2018, 3, CD012282. [Google Scholar] [CrossRef]
- Raffaele, M.; Barbagallo, I.; Licari, M.; Carota, G.; Sferrazzo, G.; Spampinato, M.; Sorrenti, V.; Vanella, L. N-Acetylcysteine (NAC) Ameliorates Lipid-Related Metabolic Dysfunction in Bone Marrow Stromal Cells-Derived Adipocytes. Evid. Based Complement. Altern. Med. 2018, 2018, 5310961. [Google Scholar] [CrossRef]
- Amengual, J.; Ribot, J.; Bonet, M.L.; Palou, A. Retinoic Acid Treatment Enhances Lipid Oxidation and Inhibits Lipid Biosynthesis Capacities in the Liver of Mice. Cell Physiol. Biochem. 2010, 25, 657–666. [Google Scholar] [CrossRef]
- Sogabe, N.; Maruyama, R.; Baba, O.; Hosoi, T.; Goseki-Sone, M. Effects of Long-Term Vitamin K1 (Phylloquinone) or Vitamin K2 (Menaquinone-4) Supplementation on Body Composition and Serum Parameters in Rats. Bone 2011, 48, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, Y. Revisiting the Interconnection between Lipids and Vitamin K Metabolism: Insights from Recent Research and Potential Therapeutic Implications: A Review. Nutr. Metab. 2024, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.S.; Anderson, J.W.; Bridges, S.R. Propionate Inhibits Hepatocyte Lipid Synthesis. Proc. Soc. Exp. Biol. Med. 1990, 195, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xiao, Y.; Wang, Y. Monomethylarsonous Acid Inhibited Endogenous Cholesterol Biosynthesis in Human Skin Fibroblasts. Toxicol. Appl. Pharmacol. 2014, 277, 21–29. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, T.S.; Lee, M.K.; Park, Y.B.; Choi, M.S. Lipid-Lowering Efficacy of Hesperetin Metabolites in High-Cholesterol Fed Rats. Clin. Chim. Acta 2003, 327, 129–137. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, J.Y.; Kim, S.H.; Oh, I.J.; Lee, Y.H.; Lee, K.W.; Lee, W.H.; Kim, J.H. Pharmaceutical Efficacy of Gypenoside LXXV on Non-Alcoholic Steatohepatitis (NASH). Biomolecules 2020, 10, 1426. [Google Scholar] [CrossRef]
- Merola, A.J.; Arnold, A. Estrone Inhibition of Cholesterol Biosynthesis at the Mevalonic Acid Stage. Science 1964, 144, 301–302. [Google Scholar] [CrossRef]
- Soto-Ramirez, M.D.; Aguilar-Ayala, D.A.; Garcia-Morales, L.; Rodriguez-Peredo, S.M.; Badillo-Lopez, C.; Rios-Muñiz, D.E.; Meza-Segura, M.A.; Rivera-Morales, G.Y.; Leon-Solis, L.; Cerna-Cortes, J.F.; et al. Cholesterol Plays a Larger Role during Mycobacterium tuberculosis in Vitro Dormancy and Reactivation than Previously Suspected. Tuberculosis 2017, 103, 1–9. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for Characterization of Streptomyces Species. Int. J. Syst. Evol. Microbiol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Pridham, T.G.; Gottlieb, D. The Utilization of Carbon Compounds by Some Actinomycetales as an Aid for Species Determination. J. Bacteriol. 1948, 56, 107–114. [Google Scholar] [CrossRef]
- Woods, G.L.; Brown-Elliott, B.A.; Conville, P.S.; Desmond, E.P.; Hall, G.S.; Lin, G.; Pfyffer, G.E.; Ridderhof, J.C.; Siddiqi, S.H.; Wallace, R.J.; et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. In Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes 2011; Report No.: M24-A2; CLSI: Malvern, PA, USA, 2011. [Google Scholar]
- Rudrappa, M.; Kumar, M.S.; Kumar, R.S.; Almansour, A.I.; Perumal, K.; Nayaka, S. Bioproduction, Purification and Physicochemical Characterization of Melanin from Streptomyces Sp. Strain MR28. Microbiol. Res. 2022, 263, 127130. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; El-Ewasy, S.M. Bioproduction, Characterization, Anticancer and Antioxidant Activities of Extracellular Melanin Pigment Produced by Newly Isolated Microbial Cell Factories Streptomyces Glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef]




| Streptomyces sp. SbAr007 | S. samsunensis [41] | S. malaysiensis [42] | S. solisilvae [43] | |
|---|---|---|---|---|
| Sole carbon utilization tests (% w/v) | ||||
| Cellobiose | + | − | + | − |
| Xylose | + | − | + | + |
| Sucrose | − | − | − | + |
| Fructose | + | + | + | NA |
| D-galactose | + | + | + | + |
| Mannitol | − | + | + | − |
| Maltose | + | + | + | − |
| Dextrose/Glucose | + | + | + | + |
| L-Rhamnose | + | + | + | NA |
| Sole nitrogen utilization tests (% w/v) | ||||
| L-arginine | − | + | NA | + |
| L-asparagine | + | NA | NA | + |
| Histidine | + | + | + | − |
| L-Cysteine | − | − | NA | − |
| Growth at pH 4.0 | + | − | − | − |
| Growth at pH 5 to 10 | + | + | NA | + |
| Growth in NaCl (3%, w/v) | + | NA | + | + |
| Growth in NaCl (5%, w/v) | + | NA | − | + |
| Growth at 25–30 °C | + | + | + | + |
| Growth at 37 °C | − | + | + | + |
| Antibiotic resistance (µg) | ||||
| Ampicillin (10) | − | NA | + | NA |
| Rifampicin (32 and 64) | +/− | NA | NA | + |
| Gentamicin sulphate (10) | − | + | − | − |
| Chloramphenicol (30) | − | NA | − | + |
| Kanamycin (30) | − | NA | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadankula, G.R.; Rizvi, A.; Ali, H.; Khunjamayum, R.; Eedara, V.V.R.; Nema, V.; Ningthoujam, D.S.; Suresh Babu, K.; Shetty, P.R.; Mande, S.C.; et al. Secondary Metabolites from a New Antibiotic-Producing Endophytic Streptomyces Isolate Inhibited Pathogenic and Multidrug-Resistant Mycobacterium tuberculosis Strains. Trop. Med. Infect. Dis. 2025, 10, 117. https://doi.org/10.3390/tropicalmed10050117
Vadankula GR, Rizvi A, Ali H, Khunjamayum R, Eedara VVR, Nema V, Ningthoujam DS, Suresh Babu K, Shetty PR, Mande SC, et al. Secondary Metabolites from a New Antibiotic-Producing Endophytic Streptomyces Isolate Inhibited Pathogenic and Multidrug-Resistant Mycobacterium tuberculosis Strains. Tropical Medicine and Infectious Disease. 2025; 10(5):117. https://doi.org/10.3390/tropicalmed10050117
Chicago/Turabian StyleVadankula, Govinda Raju, Arshad Rizvi, Haider Ali, Rakhi Khunjamayum, V. V. Ramprasad Eedara, Vijay Nema, Debananda Singh Ningthoujam, Katragadda Suresh Babu, Prakasham Reddy Shetty, Shekhar C. Mande, and et al. 2025. "Secondary Metabolites from a New Antibiotic-Producing Endophytic Streptomyces Isolate Inhibited Pathogenic and Multidrug-Resistant Mycobacterium tuberculosis Strains" Tropical Medicine and Infectious Disease 10, no. 5: 117. https://doi.org/10.3390/tropicalmed10050117
APA StyleVadankula, G. R., Rizvi, A., Ali, H., Khunjamayum, R., Eedara, V. V. R., Nema, V., Ningthoujam, D. S., Suresh Babu, K., Shetty, P. R., Mande, S. C., & Banerjee, S. (2025). Secondary Metabolites from a New Antibiotic-Producing Endophytic Streptomyces Isolate Inhibited Pathogenic and Multidrug-Resistant Mycobacterium tuberculosis Strains. Tropical Medicine and Infectious Disease, 10(5), 117. https://doi.org/10.3390/tropicalmed10050117







