Evaluation of Ground and Aerial Ultra-Low Volume Applications Using ReMoa Tri Against Deltamethrin-Resistant Aedes aegypti from Collier County, Florida
Abstract
:1. Introduction
2. Methods
2.1. Mosquito Collections and Rearing
2.2. Insecticide Susceptibility Tests
2.3. Ground-Based Field Evaluation of ReMoa Tri and DeltaGard
2.4. Aerial Evaluation of ReMoa Tri
3. Results
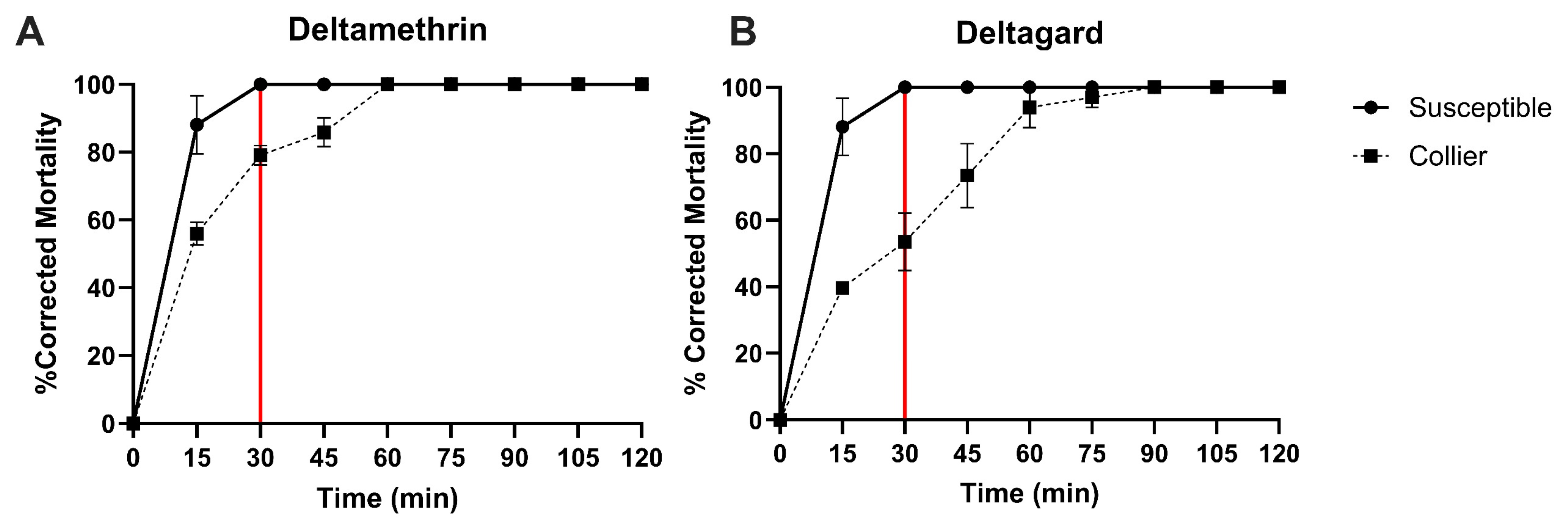
3.1. Susceptibility of Collier-Aedes aegypti to Deltamethrin and Deltagard
3.2. Ground Applications of ReMoa Tri and DeltaGard Targeting Collier-Aedes aegypti
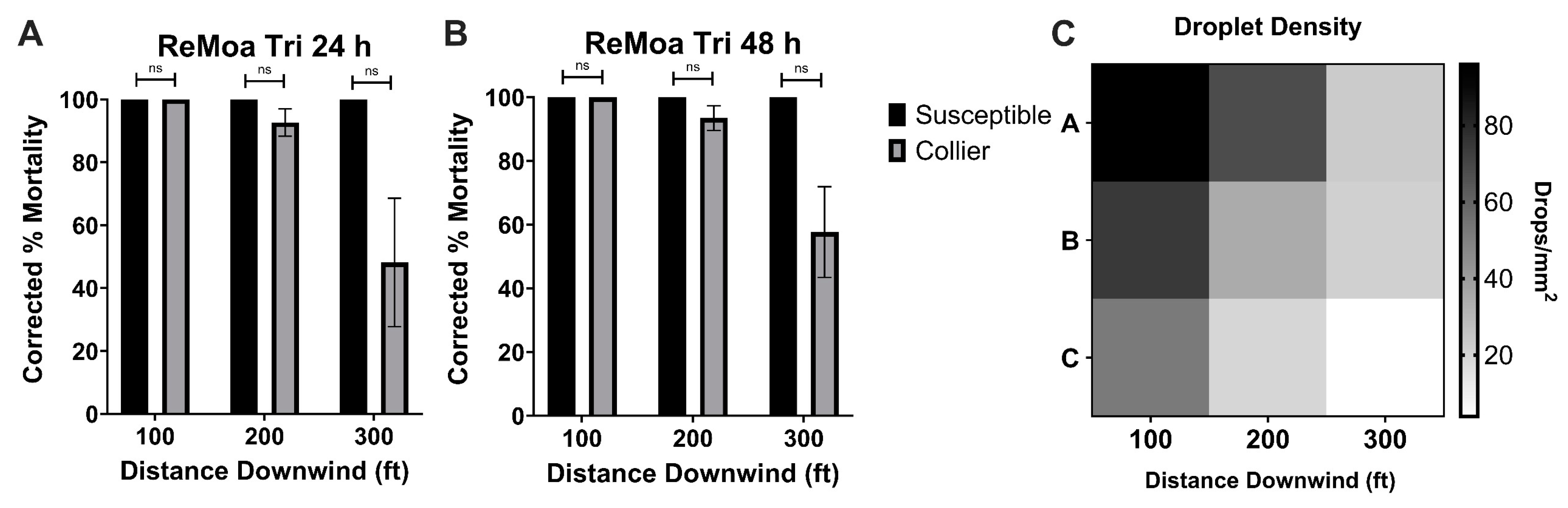
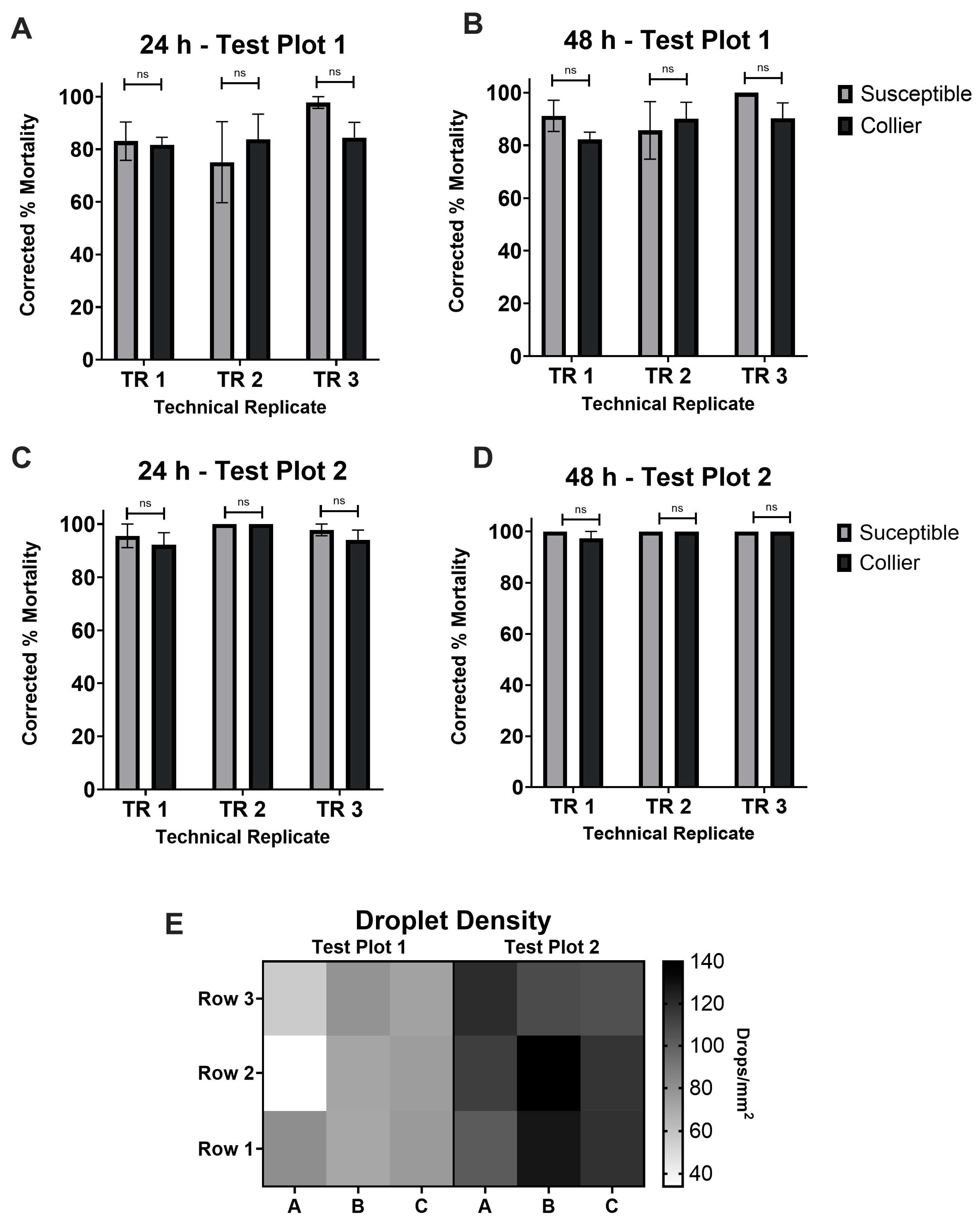
3.3. Aerial Applications of ReMoa Tri Targeting Collier-Aedes aegypti
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monaghan, A.J.; Eisen, R.J.; Eisen, L.; McAllister, J.; Savage, H.M.; Mutebi, J.P.; Johansson, M.A. Consensus and uncertainty in the geographic range of Aedes aegypti and Aedes albopictus in the contiguous United States: Multi-model assessment and synthesis. PLoS Comput. Biol. 2019, 15, e1007369. [Google Scholar] [CrossRef]
- Khan, S.U.; Ogden, N.H.; Fazil, A.A.; Gachon, P.H.; Dueymes, G.U.; Greer, A.L.; Ng, V. Current and projected distributions of Aedes aegypti and Ae. albopictus in Canada and the U.S. Environ. Health Perspect. 2020, 128, 57007. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, K.; Stanek, D.; Blackmore, C.; Centers for Disease Control and Prevention (CDC). Notes from the field: Transmission of chikungunya virus in the continental United States—Florida, 2014. MMWR Morb. Mortal Wkly Rep. 2014, 63, 1137. [Google Scholar]
- Philip, C.; Novick, C.G.; Novick, L.F. Local Transmission of Zika Virus in Miami-Dade County: The Florida Department of Health Rises to the Challenge. J. Public Health Manag. Pract. 2019, 25, 277–287. [Google Scholar] [CrossRef]
- Sharp, T.M.; Morris, S.; Morrison, A.; de Lima Corvino, D.; Santiago, G.A.; Shieh, W.J.; Rico, E.; Kopp, E.; Muñoz-Jordán, J.L.; Marttos, A.; et al. Fatal Dengue Acquired in Florida. N. Engl. J. Med. 2019, 384, 2257–2259. [Google Scholar] [CrossRef]
- Florida Department of Health. Mosquito-Borne Disease Surveillance. Tallahassee FL: Florida Department of Health. 2023. Available online: https://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/surveillance.html (accessed on 29 September 2023).
- Bowman, L.R.; Donegan, S.; McCall, P.J. Is Dengue Vector Control Deficient in Effectiveness or Evidence? Systematic Review and Meta-analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004551. [Google Scholar] [CrossRef]
- McAllister, J.C.; Porcelli, M.; Medina, J.M.; Delorey, M.J.; Connelly, C.R.; Godsey, M.S.; Panella, N.A.; Dzuris, N.; Boegler, K.A.; Kenney, J.L.; et al. Mosquito Control Activities during Local Transmission of Zika Virus, Miami-Dade County, Florida, USA, 2016. Emerg. Infect. Dis. 2020, 26, 881–890. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, Biologics and Nematicides: Updates to IRAC’s Mode of Action Classification—A Tool For Resistance Management. Pestic. Biochem. Phys. 2020, 167, 104587. [Google Scholar] [CrossRef]
- Estep, A.S.; Sanscrainte, N.D.; Waits, C.M.; Bernard, S.J.; Lloyd, A.M.; Lucas, K.J.; Buckner, E.A.; Vaidyanathan, R.; Morreale, R.; Conti, L.A.; et al. Quantification of permethrin resistance and kdr alleles in Florida strains of Aedes aegypti (L.) and Aedes albopictus (Skuse). PLoS Negl. Trop. Dis. 2018, 12, e0006544. [Google Scholar] [CrossRef]
- Parker, C.; Ramirez, D.; Thomas, C.; Connelly, R.C. Baseline Susceptibility Status of Florida Populations of Aedes aegypti (Diperta: Culicide) and Aedes albopictus. J. Med. Entomol. 2020, 57, 1550–1559. [Google Scholar] [CrossRef]
- Schluep, S.M.; Buckner, E.A. Metabolic Resistance in Permethrin-Resistant Florida Aedes aegypti (Diptera: Culicidae). Insects 2021, 12, 866. [Google Scholar] [CrossRef]
- Scott, M.L.; Hribar, L.J.; Leal, A.L.; McAllister, J.C. Characterization of Pyrethroid Resistance Mechanisms in Aedes aegypti from the Florida Keys. Am. J. Trop. Med. Hyg. 2021, 104, 1111–1122. [Google Scholar] [CrossRef]
- Lucas, K.L.; Bales, R.B. Insecticide Resistance Evaluation of Aedes aegypti from Collier County, Florida. Arthropod Manag. Tests 2022, 47, tsac120. [Google Scholar] [CrossRef]
- Murray, H.L.; Hribar, L.J. Resistance and inhibitor testing on Aedes aegypti (Linnaeus) (Culicidae: Diptera) populations in the Florida Keys. J. Vector Ecol. 2023, 49, 53–63. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Brown, D.K.; An, M.; Xue, R.D.; Liu, N. Insecticide resistance: Status and potential mechanisms in Aedes aegypti. Pestic. Biochem. Physiol. 2023, 195, 105577. [Google Scholar] [CrossRef]
- Estep, A.S.; Sanscrainte, N.D.; Lamberg, F.; McStoots, D.; Gosslin, S. Detection of the 1016Gly and 989Pro Knockdown Resistance Mutations in Florida, USA Aedes aegypti. Insects 2024, 15, 863. [Google Scholar] [CrossRef]
- Lucas, K.J.; Heinig, R.; Lake, L.; Williams, K.; Parker-Crockett, C.; Bales, R.; McDuffie, D. Evaluation of a novel triple-action adulticide containing a pyrethroid, macrocyclic lactone, and fatty acid against pyrethroid-resistant Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2024, 61, 701–709. [Google Scholar] [CrossRef]
- Brogdon, W.G.; McAllister, J.C. Insecticide resistance and vector control. Emerg. Infect. Dis. 1998, 4, 605–613. [Google Scholar] [CrossRef]
- Unlu, I.; Buckner, E.A.; Medina, J.; Vasquez, C.; Cabrera, A.; Romero-Weaver, A.L.; Ramirez, D.; Kendziorski, N.L.; Kosinski, K.J.; Fedirko, T.J.; et al. Insecticide resistance of Miami-Dade Culex quinquefasciatus populations and initial field efficacy of a new resistance-breaking adulticide formulation. PLoS ONE 2024, 19, e0296046. [Google Scholar] [CrossRef]
- Schleier III JJ and Peterson, R.K. Pyrethrins and pyrethroid insecticides. RSC Green. Chem. 2011, 11, 94–131. [Google Scholar]
- Centers for Disease Control and Prevention. CONUS Manual for Evaluating Insecticide Resistance in Mosquitoes Using the CDC Bottle Bioassay Kit. 2020. Available online: https://www.cdc.gov/mosquitoes/php/toolkit/cdc-bottle-bioassay.html (accessed on 2 May 2022).
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes; World Health Organization: Geneva, Switzerland, 2013; Available online: https://iris.who.int/bitstream/10665/250677/1/9789241511575-eng.pdf (accessed on 3 July 2017).
- Kandel, Y.; Vulcan, J.; Rodriguez, S.D.; Moore, E.; Chung, H.; Mitra, S.; Cordova, J.J.; Martinez, K.J.L.; Moon, A.S.; Lulkarni, A.; et al. Widespread insecticide resistance in Aedes aegypti L. from New Mexico, U.S.A. PLoS ONE 2019, 14, e0212693. [Google Scholar] [CrossRef]
- Cornel, A.J.; Holeman, J.; Nieman, C.C.; Lee, Y.; Smith, C.; Amorino, M.; Brisco, K.K.; Barrera, R.; Lanzaro, G.C.; Mulligan, F.S. Surveillance, insecticide resistance and control of an invasive Aedes aegypti (Diptera: Culicidae) population in California. F1000Res. 2016, 5, 194. [Google Scholar] [CrossRef]
- Bonds, J.A.S.; Greer, M.J.; Fritz, B.K.; Hoffmann, W.C. Aerosol sampling: Comparison of two rotating impactors for field droplet sizing and volumetric measurements. J. Am. Mosq. Control Assoc. 2009, 25, 474–479. [Google Scholar] [CrossRef]
- Buckner, W.; Latham, M.; Lesser, C.; Marsicano, A.; Williams, K. An evaluation of 6 ground ULV adulticides against local populations of Aedes taeniorhynchus and Ae. aegypti in Manatee County, FL. Wing Beats 2016, 27, 17–19. [Google Scholar]
- Hart, J.D.; Hare, K. Cage Match: A Semi-Field Comparison of Three Ground ULV Products. Wingbeats 2024, 35, 27–30. [Google Scholar]
- Pu, X.; Yang, Y.; Wu, S.; Wu, Y. Characterisation of abamectin resistance in a field-evolved multiresistant population of Plutella xylostella. Pest. Manag. Sci. 2010, 66, 371–378. [Google Scholar] [CrossRef]
- Ferreira, C.B.S.; Andrade, F.H.N.; Rodrigues, A.R.S.; Siqueira, H.A.A.; Gondim, M.G.C., Jr. Resistance in field populations of Tetranychus urticae to acaricides and characterization of the inheritance of abamectin resistance. Crop Prot. 2015, 67, 77–83. [Google Scholar] [CrossRef]
- Clark, J.M.; Scott, J.G.; Campos, F.; Bloomquist, J.R. Resistance to avermectins: Extent, mechanisms, and management implications. Annu. Rev. Entomol. 1995, 40, 1–30. [Google Scholar] [CrossRef]
- Georghiou, G.P. Principles of insecticide resistance management. Phytoprotection 1994, 75, 51–59. [Google Scholar] [CrossRef]

| Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Date | Time | Type | Product | 1.524 m | 9.144 m/76.2 m | Wind Speed (kph) | Wind Direction | Relative Humidity (%) |
| 16 October 2024 | 7:24 PM | Ground | DeltaGard | 22.74 | 23.67 | 10.7 | N | 65.84 |
| 16 October 2024 | 8:25 PM | Ground | ReMoa Tri | 21.33 | 23.67 | 8.87 | N | 63.12 |
| 14 August 2024 | 6:36 AM | Aerial | ReMoa Tri | 26.11 | 24 | 12.96 | SE | 82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDuffie, D.; Kacinskas, S.; Li, S.; Parker-Crockett, C.; Lucas, K.J. Evaluation of Ground and Aerial Ultra-Low Volume Applications Using ReMoa Tri Against Deltamethrin-Resistant Aedes aegypti from Collier County, Florida. Trop. Med. Infect. Dis. 2025, 10, 119. https://doi.org/10.3390/tropicalmed10050119
McDuffie D, Kacinskas S, Li S, Parker-Crockett C, Lucas KJ. Evaluation of Ground and Aerial Ultra-Low Volume Applications Using ReMoa Tri Against Deltamethrin-Resistant Aedes aegypti from Collier County, Florida. Tropical Medicine and Infectious Disease. 2025; 10(5):119. https://doi.org/10.3390/tropicalmed10050119
Chicago/Turabian StyleMcDuffie, Decyo, Sara Kacinskas, Suzanne Li, Casey Parker-Crockett, and Keira J. Lucas. 2025. "Evaluation of Ground and Aerial Ultra-Low Volume Applications Using ReMoa Tri Against Deltamethrin-Resistant Aedes aegypti from Collier County, Florida" Tropical Medicine and Infectious Disease 10, no. 5: 119. https://doi.org/10.3390/tropicalmed10050119
APA StyleMcDuffie, D., Kacinskas, S., Li, S., Parker-Crockett, C., & Lucas, K. J. (2025). Evaluation of Ground and Aerial Ultra-Low Volume Applications Using ReMoa Tri Against Deltamethrin-Resistant Aedes aegypti from Collier County, Florida. Tropical Medicine and Infectious Disease, 10(5), 119. https://doi.org/10.3390/tropicalmed10050119






