Improved Utilisation and Quality of Blood Culture Services Following Operational Research in a Tertiary Hospital in Ghana
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. General Setting
2.3. Specific Setting
2.3.1. Blood Culture Processing Workflow in HTH
2.3.2. Laboratory Processing of Blood Cultures
2.4. Study Population
2.5. Data Variables and Data Collection Process
2.6. Data Analysis
3. Results
3.1. Demographic, Clinical Characteristics, and Factors Associated with Blood Culture Request
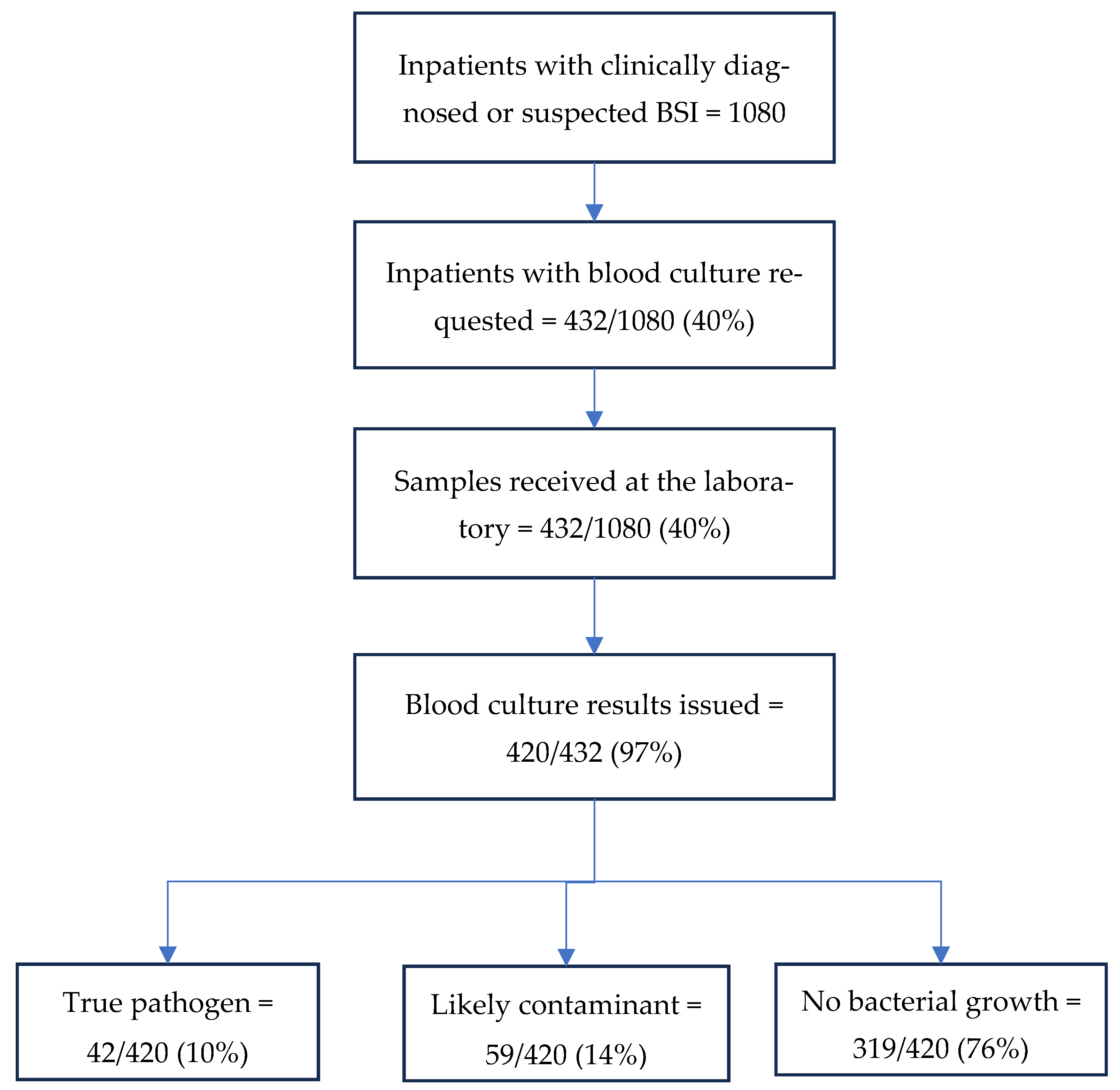
3.2. Utilisation and Quality of Blood Cultures
3.3. Organisms Isolated and Their Resistance Patterns
3.4. Factors Associated with Blood Culture Request
3.5. Factors Associated with Hospital Exit Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seni, J.; Mwakyoma, A.A.; Mashuda, F.; Marando, R.; Ahmed, M.; DeVinney, R.; Pitout, J.D.D.; Mshana, S.E. Deciphering risk factors for blood stream infections, bacteria species and antimicrobial resistance profiles among children under five years of age in North-Western Tanzania: A multicentre study in a cascade of referral health care system. BMC Pediatr. 2019, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Wolk, D.M.; Hayden, R.T.; Carroll, K.C.; Tang, Y.-W. Bloodstream Infections. Microbiol. Spectr. 2016, 4, 653–689. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- de Kraker, M.E.A. Understanding the impact of antimicrobial resistance on outcomes of bloodstream infections in low- and middle-income countries. PLoS Med. 2023, 20, e1004262. [Google Scholar] [CrossRef]
- Deku, J.G.; Dakorah, M.P.; Lokpo, S.Y.; Orish, V.N.; Ussher, F.A.; Kpene, G.E.; Eshun, V.A.; Agyei, E.; Attivor, W.; Osei-Yeboah, J. The Epidemiology of Bloodstream Infections and Antimicrobial Susceptibility Patterns: A Nine-Year Retrospective Study at St. Dominic Hospital, Akwatia, Ghana. J. Trop. Med. 2019, 2019, 6750864. [Google Scholar] [CrossRef]
- Obeng-Nkrumah, N.; Labi, A.-K.; Addison, N.O.; Labi, J.E.M.; Awuah-Mensah, G. Trends in paediatric and adult bloodstream infections at a Ghanaian referral hospital: A retrospective study. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 49. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Bassetti, M.; Blasi, F.; Goossens, H.; Rello, J.; Sotgiu, G.; Tavoschi, L.; Arber, M.R.; McCool, R.; Patterson, J.V.; et al. A Systematic Review of the Effect of Delayed Appropriate Antibiotic Treatment on the Outcomes of Patients with Severe Bacterial Infections. Chest 2020, 158, 929–938. [Google Scholar] [CrossRef]
- World Health Organization. Implementing the Global Action Plan on Antimicrobial Resistance: First Quadripartite Biennial Report; World Health Organization, Food and Agriculture Organization of the United Nations, United Nations Environment Programme and World Organization for Animal Health: Geneva, Switzerland, 2023; Available online: https://creativecommons.org/licenses/by-nc-sa/3.0/igo/ (accessed on 1 August 2025).
- Maki, G.; Smith, I.; Paulin, S.; Kaljee, L.; Kasambara, W.; Mlotha, J.; Chuki, P.; Rupali, P.; Singh, D.R.; Bajracharya, D.C.; et al. Feasibility study of the world health organization health care facility-based antimicrobial stewardship toolkit for low- and Middle-Income countries. Antibiotics 2020, 9, 556. [Google Scholar] [CrossRef]
- Krapp, F.; Rondon, C.; Amaro, C.; Barco-Yaipén, E.; Valera-Krumdieck, M.; Vásquez, R.; Briones, A.; Casapia, M.; Burgos, A.; López, F.S.; et al. Underutilization and Quality Gaps in Blood Culture Processing in Public Hospitals of Peru. Am. J. Trop. Med. Hyg. 2022, 106, 432–440. [Google Scholar] [CrossRef]
- Droz, N.; Hsia, Y.; Ellis, S.; Dramowski, A.; Sharland, M.; Basmaci, R. Bacterial pathogens and resistance causing community acquired paediatric bloodstream infections in low- and middle-income countries: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2019, 8, 207. [Google Scholar] [CrossRef]
- Pan, H.-W.; Li, W.; Li, R.-G.; Li, Y.; Zhang, Y.; Sun, E.-H. Simple sample preparation method for direct microbial identification and susceptibility testing from positive blood cultures. Front. Microbiol. 2018, 9, 481. [Google Scholar] [CrossRef]
- Opintan, J.A.; Newman, M.J. Prevalence of antimicrobial resistant pathogens from blood cultures: Results from a laboratory based nationwide surveillance in Ghana. Antimicrob. Resist. Infect. Control 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Minton, J.; Clayton, J.; Sandoe, J.; Gann, H.M.; Wilcox, M. Quality Improvement Report: Improving early management of bloodstream infection: A quality improvement project. BMJ Br. Med. J. 2008, 336, 440. [Google Scholar] [CrossRef]
- Idelevich, E.; Seifert, H.; Sundqvist, M.; Scudeller, L.; Amit, S.; Balode, A.; Bilozor, A.; Drevinek, P.; Tufan, Z.K.; Koraqi, A.; et al. Microbiological diagnostics of bloodstream infections in Europe—An ESGBIES survey. Clin. Microbiol. Infect. 2019, 25, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Boakye-Yiadom, E.; Najjemba, R.; Thekkur, P.; Labi, A.-K.; Gil-Cuesta, J.; Asafo-Adjei, K.; Mensah, P.; van Boetzelaer, E.; Jessani, N.S.; Orish, V.N. Use and Quality of Blood Cultures for the Diagnosis of Bloodstream Infections: A Cross-Sectional Study in the Ho Teaching Hospital, Ghana, 2019–2021. Int. J. Environ. Res. Public Health 2023, 20, 6631. [Google Scholar] [CrossRef] [PubMed]
- Ghana 2024 Statistical Year Overview Ghana Statistical Service. 2024. Available online: https://statsghana.gov.gh/gssmain/fileUpload/pressrelease/Ghana%20Statistical%20Service%202024%20Releases%20-%20online.pdf (accessed on 22 July 2025).
- Lewis, J.S. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2024; 382p. [Google Scholar]
- Halstead, D.C.; Sautter, R.L.; Snyder, J.W.; Crist, A.E.; Nachamkin, I. Reducing Blood Culture Contamination Rates: Experiences of Four Hospital Systems. Infect. Dis. Ther. 2020, 9, 389–401. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Blood Culture Contamination: An Overview for Infection Control and Antibiotic Stewardship Programs Working with the Clinical Laboratory. 2022. Available online: https://www.cap.org/laboratory-improvement/accreditation/ (accessed on 23 July 2025).
- Fraser, J.; Skone-James, R.; Foster, J.; Rosado, H.; Brandish, C.; Nabiryo, M. Commonwealth Partnerships for Antimicrobial Stewardship (CWPAMS) Impact Report. 2024. Available online: https://commonwealthpharmacy.org/ (accessed on 1 August 2025).
- McErlean, M.; Kpokiri, E.; Panesar, P.; Cooper, E.E.; Jato, J.; Orman, E.; Odoi, H.; Hutton-Nyameaye, A.; Somuah, S.O.; Folitse, I.; et al. Evaluation of a Hub-and-Spoke Model to Enhance Healthcare Professionals’ Practice of Antimicrobial Stewardship (AMS) Programmes in the Volta Region of Ghana. Antibiotics 2025, 14, 672. [Google Scholar] [CrossRef]
- Ashiru-Oredope, D.; Nabiryo, M.; Zengeni, L.; Kamere, N.; Makotose, A.; Olaoye, O.; Townsend, W.; Waddingham, B.; Matuluko, A.; Nambatya, W.; et al. Tackling antimicrobial resistance: Developing and implementing antimicrobial stewardship interventions in four African commonwealth countries through a health partnership model. J. Public Health Afr. 2023, 14, 7. [Google Scholar] [CrossRef]
- Ombelet, S.; Kpossou, G.; Kotchare, C.; Agbobli, E.; Sogbo, F.; Massou, F.; Lagrou, K.; Barbé, B.; Affolabi, D.; Jacobs, J. Blood culture surveillance in a secondary care hospital in Benin: Epidemiology of bloodstream infection pathogens and antimicrobial resistance. BMC Infect. Dis. 2022, 22, 119. [Google Scholar] [CrossRef]
- Standard Treatment Guidelines. 2017. Available online: www.ghndp.org (accessed on 22 July 2025).
- Rahden, P.; Barrow, E.; Bah, H.; Bittaye, S.O.; Nygren, D.; Badjan, A. Bloodstream infections at a tertiary hospital in the Gambia—A one-year retrospective study. BMC Infect. Dis. 2025, 25, 170. [Google Scholar] [CrossRef]
- Hsu, P.-H.; Chang, R.; Yin, C.-H.; Chen, Y.-S.; Chen, J.-S. Association between blood culture turnaround time and clinical prognosis in emergency department patients with community acquired bloodstream infection: A retrospective study based on electronic medical records. Heliyon 2024, 10, e27957. [Google Scholar] [CrossRef]
- Dempsey, C.; Skoglund, E.; Muldrew, K.L.; Garey, K.W. Economic health care costs of blood culture contamination: A systematic review. Am. J. Infect. Control 2019, 47, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Dräger, S.; Giehl, C.; Søgaard, K.K.; Egli, A.; de Roche, M.; Huber, L.C.; Osthoff, M. Do we need blood culture stewardship programs? A quality control study and survey to assess the appropriateness of blood culture collection and the knowledge and attitudes among physicians in Swiss hospitals. Eur. J. Intern. Med. 2022, 103, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S.; Muhsen, K.; Johnson, S.A.M.; Kotey, F.C.N.; Dayie, N.T.K.D.; Tetteh-Quarcoo, P.B.; Tette, E.M.A.; Osei, M.-M.; Egyir, B.; Nii-Trebi, N.I.; et al. Multicenter Surveillance of Antimicrobial Resistance among Gram-Negative Bacteria Isolated from Bloodstream Infections in Ghana. Antibiotics 2023, 12, 255. [Google Scholar] [CrossRef]
- Labi, A.-K.; Obeng-Nkrumah, N.; Bjerrum, S.; Enweronu-Laryea, C.; Newman, M.J. Neonatal bloodstream infections in a Ghanaian Tertiary Hospital: Are the current antibiotic recommendations adequate? BMC Infect. Dis. 2016, 16, 598. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024-Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; pp. 1–72. Available online: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf (accessed on 23 July 2025).
- Seboxa, T.; Amogne, W.; Abebe, W.; Tsegaye, T.; Azazh, A.; Hailu, W.; Fufa, K.; Grude, N.; Henriksen, T.-H.; Quach, C. High mortality from blood stream infection in Addis Ababa, Ethiopia, is due to antimicrobial resistance. PLoS ONE 2015, 10, e0144944. [Google Scholar] [CrossRef]
- Barman, P.; Chopra, S.; Thukral, T. Direct testing by VITEK® 2: A dependable method to reduce turnaround time in Gram-negative bloodstream infections. J. Lab. Physicians 2018, 10, 260–264. [Google Scholar] [CrossRef]
| Total N(%) ^ | CDST Request N(%) * | PR (95% CI) | aPR β (95% CI) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Total | 1080 | (100) | 432 | (40.0) | |||
| Age | |||||||
| Neonate (0–28 days) | 249 | (23.1) | 155 | (62.3) | 2.5 (1.9–3.3) | ||
| 29 days to <1 year | 39 | (3.6) | 24 | (61.5) | 2.5 (1.8–3.5) | ||
| 1–4 years | 77 | (7.1) | 48 | (62.3) | 2.5 (1.9–3.4) | ||
| 5–11 years | 54 | (5.0) | 37 | (68.5) | 2.8 (2.0–3.7) | ||
| 12–24 years | 114 | (10.6) | 29 | (25.4) | 1.0 (0.7–1.5) | ||
| 25–44 years | 172 | (15.9) | 37 | (21.5) | 0.9 (0.6–1.3) | ||
| 45–64 years | 173 | (16.0) | 52 | (30.1) | 1.2 (0.9–1.7) | ||
| ≥65 years | 202 | (18.7) | 50 | (24.8) | 1 | ||
| Sex | |||||||
| Male | 524 | (48.5) | 207 | (39.5) | 1 | 1 | |
| Female | 556 | (51.5) | 225 | (40.5) | 1.0 (0.9–1.2) | 1.0 (0.9–1.1) | 0.843 |
| Condition *** | |||||||
| Neonatal sepsis | 242 | (22.4) | 147 | (60.7) | 2.4 (2.0–2.9) | 1.4 (1.1–1.7) | 0.003 |
| Pneumonia | 368 | (34.1) | 93 | (25.3) | 1 | 1 | |
| Other sepsis 1 | 227 | (21.0) | 119 | (52.4) | 2.1 (1.7–2.6) | 2.0 (1.6–2.4) | <0.001 |
| Other conditions 2 | 243 | (22.5) | 73 | (30.0) | 1.2 (0.9–1.5) | 1.4 (1.1–1.7) | 0.011 |
| Department | |||||||
| Intensive Care Unit | 30 | (2.8) | 16 | (53.3) | 3.5 (2.1–5.8) | 3.5 (2.2–5.7) | <0.001 |
| Accident and Emergency | 168 | (15.6) | 41 | (24.4) | 1.6 (1.0–2.6) | 1.8 (1.1–2.8) | 0.013 |
| Child Health | 422 | (39.1) | 264 | (62.6) | 4.1 (2.8–6.1) | 4.2 (2.9–6.2) | <0.001 |
| Internal Medicine | 261 | (24.2) | 81 | (31.0) | 2.1 (1.4–3.1) | 2.4 (1.6–3.7) | <0.001 |
| Obstetrics and Gynaecology | 47 | (4.4) | 7 | (14.9) | 1.0 (0.5–2.1) | 0.9 (0.4–2.0) | 0.867 |
| Surgery | 152 | (14.1) | 23 | (15.1) | 1 | 1 | |
| Duration Between | n * | Median Days | (IQR) |
|---|---|---|---|
| Admission and receipt of blood sample at laboratory for CDST | 394 | 1 | (0–2) |
| Receipt of blood sample at laboratory and issuance of CDST report # | 390 | 5 | (5–6) |
| Admission to issuance of final CDST report | 373 | 6 | (6–8) |
| Organism | WHO Priority Category | Number of Isolates | Number Tested | Number Resistant |
|---|---|---|---|---|
| Macrolide-resistant Streptococcus pyogenes | Medium | 2 | 1 | 0 |
| MRSA (methicillin-resistant Staphylococcus aureus) | High | 10 | 7 | 5 |
| Carbapenem-resistant Pseudomonas aeruginosa | High | 4 | 4 | 0 |
| Carbapenem-resistant Acinetobacter baumanii | Critical | 3 | 1 | 0 |
| Carbapenem-resistant Enterobacterales | Critical | 19 | 10 | 1 |
| Ceftriaxone-resistant Enterobacterales | Critical | 19 | 8 | 6 |
| Ceftazidime-resistant Enterobacterales | Critical | 19 | 8 | 4 |
| Total N | Unfavourable Outcome n(%) * | RR (95% CI) | aRR β (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|
| Total | 1080 | 243 | (22.5) | |||
| Age | ||||||
| Neonate (0–28 days) | 249 | 24 | (9.6) | 1 | ||
| 29 days to <1 year | 39 | 2 | (5.1) | 0.5 (0.1–2.2) | ||
| 1–4 years | 77 | 2 | (2.6) | 0.3 (0.1–1.1) | ||
| 5–11 years | 54 | 2 | (3.7) | 0.4 (0.1–1.6) | ||
| 12–24 years | 114 | 22 | (19.3) | 2.0 (1.2–3.4) | ||
| 25–44 years | 172 | 42 | (24.4) | 2.5 (1.6–4.0) | ||
| 45–64 years | 173 | 68 | (39.3) | 4.1 (2.7–6.2) | ||
| >=65 years | 202 | 81 | (40.1) | 4.1 (2.7–6.3) | ||
| Sex | ||||||
| Male | 524 | 114 | (21.7) | 1 | 1 | |
| Female | 556 | 129 | (23.2) | 1.1 (0.9–1.3) | 1.0 (0.8–1.2) | 0.700 |
| Condition *** | ||||||
| Neonatal sepsis | 242 | 18 | (7.4) | 1 | 1 | |
| Pneumonia | 368 | 94 | (25.5) | 3.4 (2.1–5.5) | 0.7 (0.3–1.6) | 0.427 |
| Other sepsis 1 | 227 | 107 | (47.1) | 6.3 (4.0–10.1) | 1.3 (0.6–2.9) | 0.496 |
| Other conditions 2 | 243 | 24 | (9.9) | 1.3 (0.7–2.4) | 0.4 (0.2–0.9) | 0.024 |
| Department ** | ||||||
| Intensive Care Unit | 30 | 29 | (96.7) | 15.1 (10.4–21.9) | 14.3 (7.5–27.6) | <0.001 |
| Accident and Emergency | 168 | 72 | (42.9) | 6.7 (4.5–10.3) | 7.1 (3.7–13.6) | <0.001 |
| Child Health | 422 | 27 | (6.4) | 1 | 1 | |
| Internal Medicine | 261 | 83 | (31.8) | 5.0 (3.3–7.5) | 5.4 (2.8–10.3) | <0.001 |
| Obstetrics and Gynaecology | 47 | 9 | (19.2) | 3.0 (1.5–6.0) | 2.8 (1.2–6.8) | 0.019 |
| Surgery | 152 | 23 | (15.1) | 2.4 (1.4–4.0) | 3.1 (1.5–6.4) | 0.002 |
| Culture Status | ||||||
| No culture/no results | 660 | 148 | (22.4) | 1.6 (0.7–3.2) | 1.7 (1.0–3.2) | 0.071 |
| No bacterial growth | 319 | 74 | (23.2) | 1.6 (0.8–3.5) | 2.0 (1.1–3.6) | 0.024 |
| Contaminant | 59 | 15 | (25.4) | 1.8 (0.8–4.2) | 2.1 (1.1–4.0) | 0.037 |
| True bacterial pathogen | 42 | 6 | (14.3) | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sewornu, R.; Boakye-Yiadom, E.; Ativi, E.; Kwadzokpui, P.K.; Senahey, B.; Owusu, H.; Thekkur, P.; Kumar, A.M.V.; Dodoo, C.C. Improved Utilisation and Quality of Blood Culture Services Following Operational Research in a Tertiary Hospital in Ghana. Trop. Med. Infect. Dis. 2025, 10, 270. https://doi.org/10.3390/tropicalmed10090270
Sewornu R, Boakye-Yiadom E, Ativi E, Kwadzokpui PK, Senahey B, Owusu H, Thekkur P, Kumar AMV, Dodoo CC. Improved Utilisation and Quality of Blood Culture Services Following Operational Research in a Tertiary Hospital in Ghana. Tropical Medicine and Infectious Disease. 2025; 10(9):270. https://doi.org/10.3390/tropicalmed10090270
Chicago/Turabian StyleSewornu, Rita, Emily Boakye-Yiadom, Emmanuel Ativi, Precious Kwablah Kwadzokpui, Bismark Senahey, Helena Owusu, Pruthu Thekkur, Ajay M. V. Kumar, and Cornelius C. Dodoo. 2025. "Improved Utilisation and Quality of Blood Culture Services Following Operational Research in a Tertiary Hospital in Ghana" Tropical Medicine and Infectious Disease 10, no. 9: 270. https://doi.org/10.3390/tropicalmed10090270
APA StyleSewornu, R., Boakye-Yiadom, E., Ativi, E., Kwadzokpui, P. K., Senahey, B., Owusu, H., Thekkur, P., Kumar, A. M. V., & Dodoo, C. C. (2025). Improved Utilisation and Quality of Blood Culture Services Following Operational Research in a Tertiary Hospital in Ghana. Tropical Medicine and Infectious Disease, 10(9), 270. https://doi.org/10.3390/tropicalmed10090270






