Cost Effectiveness of a Shorter Moxifloxacin Based Regimen for Treating Drug Sensitive Tuberculosis in India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Intervention and Comparator
2.2. Time Horizon
2.3. Model Description
2.4. Decision Tree
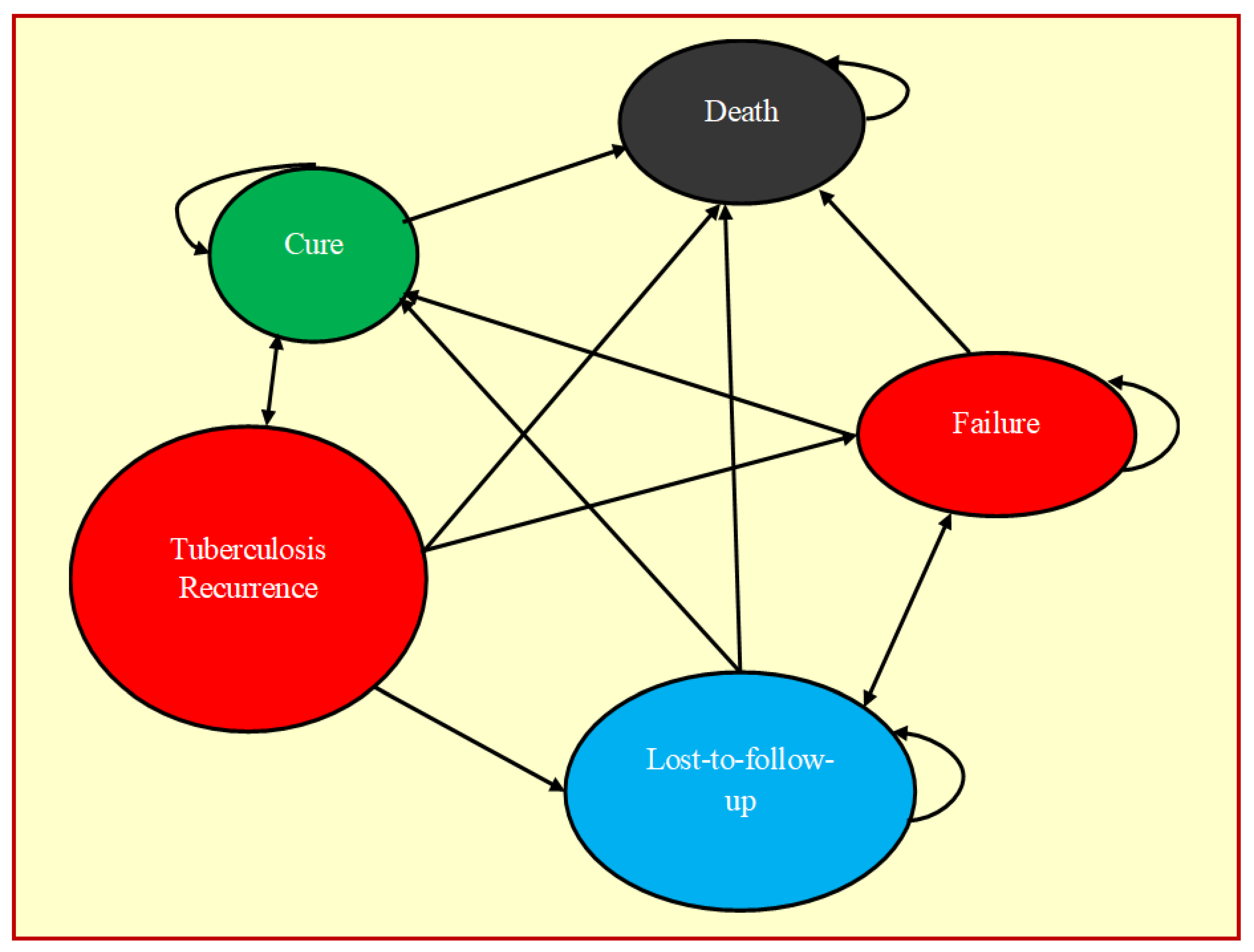
2.5. Markov Model
2.6. Model Input Parameters
2.7. Model Outcome Parameters
2.8. Willingness to Pay (WTP)
2.9. Sensitivity Analysis
2.10. Model Calibration
2.11. Study Oversight
3. Results
3.1. Base Case Analysis
3.2. Sensitivity Analysis
3.3. Model Calibration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, D.R.; Mello, F.C.D.Q.; Migliori, G.B. Shortened tuberculosis treatment regimens: What is new? J. Bras. Pneumol. 2020, 46, e20200009. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.G.; Mittal, A.; Jain, S.; Tripathy, J.P.; Satyanarayana, S.; Tharyan, P.; Kirubakaran, R. Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis. Cochrane Database Syst. Rev. 2019, 12, CD012918. [Google Scholar] [CrossRef] [PubMed]
- Conde, M.B.; Lapa, E.; Silva, J.R. New regimens for reducing the duration of the treatment of drug-susceptible pulmonary tuber-culosis. Drug Dev. Res. 2011, 72, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, E.J.; Mizrahi, V. Shortening the Short Course of Tuberculosis Treatment. N. Engl. J. Med. 2021, 384, 1764–1765. [Google Scholar] [CrossRef] [PubMed]
- Matteelli, A.; Rendon, A.; Tiberi, S.; Al-Abri, S.; Voniatis, C.; Carvalho, A.C.C.; Centis, R.; D’Ambrosio, L.; Visca, D.; Spanevello, A.; et al. Tuberculosis elimination: Where are we now? Eur. Respir. Rev. 2018, 27, 180035. [Google Scholar] [CrossRef] [Green Version]
- Gomez, G.B.; Dowdy, D.W.; Bastos, M.L.; Zwerling, A.; Sweeney, S.; Foster, N.; Trajman, A.; Islam, M.A.; Kapiga, S.; Sinanovic, E.; et al. Cost and cost-effectiveness of tuberculosis treatment shortening: A model-based analysis. BMC Infect. Dis. 2016, 16, 726. [Google Scholar] [CrossRef] [Green Version]
- Knight, G.M.; Gomez, G.B.; Dodd, P.J.; Dowdy, D.; Zwerling, A.; Wells, W.A.; Cobelens, F.; Vassall, A.; White, R.G. The Impact and Cost-Effectiveness of a Four-Month Regimen for First-Line Treatment of Active Tuberculosis in South Africa. PLoS ONE 2015, 10, e0145796. [Google Scholar] [CrossRef] [Green Version]
- Fofana, M.O.; Knight, G.M.; Gomez, G.B.; White, R.G.; Dowdy, D.W. Population-Level Impact of Shorter-Course Regimens for Tuberculosis: A Model-Based Analysis. PLoS ONE 2014, 9, e96389. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Chimeh, R.A.; Gafar, F.; Pradipta, I.S.; Akkerman, O.; Hak, E.; Alffenaar, J.-W.C.; van Boven, J. Clinical and economic impact of medication non-adherence in drug-susceptible tuberculosis: A systematic review. Int. J. Tuberc. Lung Dis. 2020, 24, 811–819. [Google Scholar] [CrossRef]
- Velayutham, B.; Jawahar, M.S.; Nair, D.; Navaneethapandian, P.; Ponnuraja, C.; Chandrasekaran, K.; Sivaramakrishnan, G.N.; Kumar, M.M.; Kumaran, P.P.; Kumar, S.R.; et al. 4-month moxifloxacin containing regimens in the treatment of patients with sputum-positive pulmonary tuberculosis in South India—A randomised clinical trial. Trop. Med. Int. Health 2020, 25, 483–495. [Google Scholar] [CrossRef]
- The Central TB Division (CTD); Ministry of Health and Family Welfare; Government of India. India TB Report: National Tu-berculosis Elimination Programme Annual Report. Ministry of Health with Family Welfare, Nirman Bhawan, New Delhi 2020 (p. 38). Available online: https://tbcindia.gov.in/showfile.php?lid=3538 (accessed on 14 June 2022).
- Imam, F.; Sharma, M.; Khayyam, K.U.; Al-Harbi, N.O.; Rashid, M.K.; Ali, M.D.; Ahmad, A.; Qamar, W. Adverse drug reaction prevalence and mechanisms of action of first-line anti-tubercular drugs. Saudi Pharm. J. 2020, 28, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Singh, A.; Gupta, N. Adverse drug reactions in tuberculosis and management. Indian J. Tuberc. 2019, 66, 520–532. [Google Scholar] [CrossRef]
- Haacker, M.; Hallett, T.; Atun, R. On discount rates for economic evaluations in global health. Health Policy Plan. 2019, 35, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Verma, M.; Bhilwar, M.; Shekhar, H.; Roy, N.; Verma, A.; Pardeshi, G. Epidemiological profile of tuberculosis patients in Delhi, India: A retrospective data analysis from the directly observed treatment short-course (DOTS) center. J. Fam. Med. Prim. Care 2019, 8, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Office of the Registrar General and Census Commissioner. SRS Based Life Table. Office of the Registrar General & Census Commissioner, India Ministry of Home Affairs, Government of India 2012–2016. Available online: https://censusindia.gov.in/Vital_Statistics/SRS_Life_Table/SRS-12-16/3.Lftb%202012-16_85.pdf (accessed on 3 February 2022).
- National Pharmaceutical Pricing Authority. Pharma Sahi Daam. National Pharmaceutical Pricing Authority. Department of Pharmaceuticals; Ministry of chemicals and fertilizers, Government of India, New Delhi 2020. Available online: https://nppaimis.nic.in/nppaprice/newmedicinepricesearch.aspx (accessed on 10 March 2022).
- Sajith, M.A.N.J.U.S.H.A.; Thomas, A.N.S.U.; Kothia, J.J.; Chandrakar, B.H.U.M.I.K.A.; Pawar, A.T.M.A.R.A.M.; Bargaje, M.D. Cost of therapy incurred for tuberculosis patients receiving directly observed therapy (DOT). Int. J. Pharm. Pharm. Sci. 2015, 7, 141–144. [Google Scholar]
- Muniyandi, M.; Rajeswari, R.; Balasubramanian, R. Estimating provider cost for treating patients with tuberculosis under revised National Tuberculosis Control Programme (RNTCP). Indian J. Tuberc. 2006, 53, 12–17. [Google Scholar]
- Patel, K.J.; Kedia, M.S.; Bajpai, D.; Mehta, S.S.; Kshirsagar, N.A.; Gogtay, N.J. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: A prospective study. BMC Clin. Pharmacol. 2007, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Kundu, D.; Katre, V.; Singh, K.; Deshpande, M.; Nayak, P.; Khaparde, K.; Moitra, A.; Nair, S.A.; Parmar, M. Innovative social protection mechanism for alleviating catastrophic expenses on multidrug-resistant tuberculosis patients in Chhattisgarh, India. WHO South-East Asia J. Public Health 2015, 4, 69–77. [Google Scholar] [CrossRef]
- Chandra, A.; Kumar, R.; Kant, S.; Parthasarathy, R.; Krishnan, A. Direct and indirect patient costs of tuberculosis care in India. Trop. Med. Int. Health 2020, 25, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Rupani, M.P.; Cattamanchi, A.; Shete, P.B.; Vollmer, W.M.; Basu, S.; Dave, J.D. Costs incurred by patients with drug-susceptible pulmonary tuberculosis in semi-urban and rural settings of Western India. Infect. Dis. Poverty 2020, 9, 144. [Google Scholar] [CrossRef]
- Poornima, M.P.; Shruthi, M.N.; Chingale, A.L.; Veena, V.; Nagaraja, S.B.; Madhukeshwar, A.K. Cost of Tuberculosis Care in Programmatic Settings from Karnataka, India: Is It Catastrophic for the Patients? Tuberc. Res. Treat. 2020, 2020, 3845694. [Google Scholar] [CrossRef] [PubMed]
- Muniyandi, M.; Rajeswari, R.; Balasubramanian, R.; Narayanan, P. A Comparison of Costs to Patients with Tuberculosis Treated in a DOTS Programme with Those in a Non-DOTS Programme in South India. J. Health Manag. 2008, 10, 9–24. [Google Scholar] [CrossRef]
- Ramkumar, S.; Vijayalakshmi, S.; Seetharaman, N.; Pajanivel, R.; Lokeshmaran, A. Health-related quality of life among tuberculosis patients under Revised National Tuberculosis Control Programme in rural and urban Puducherry. Indian J. Tuberc. 2017, 64, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ara, R.; Brazier, J. Deriving an Algorithm to Convert the Eight Mean SF-36 Dimension Scores into a Mean EQ-5D Preference-Based Score from Published Studies (Where Patient Level Data Are Not Available). Value Health 2008, 11, 1131–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikaodinaka, A.A. Health-Related Quality of Life (HRQoL) scores vary with treatment and may identify potential defaulters during treatment of tuberculosis. Malawi Med. J. 2018, 30, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Charan, J.; Tank, N.; Reljic, T.; Singh, S.; Bhardwaj, P.; Kaur, R.; Goyal, J.; Kumar, A. Prevalence of multidrug resistance tuberculosis in adult patients in India: A systematic review and meta-analysis. J. Fam. Med. Prim. Care 2019, 8, 3191–3201. [Google Scholar] [CrossRef]
- The World Bank. World Development Indicators, GDP per Capita (Current US$), India 2020. World Bank Group 2020. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?end=2020&locations=IN&start=1960 (accessed on 16 June 2022).
- Kazibwe, J.; Gheorghe, A.; Wilson, D.; Ruiz, F.; Chalkidou, K.; Chi, Y.-L. The Use of Cost-Effectiveness Thresholds for Evaluating Health Interventions in Low- and Middle-Income Countries From 2015 to 2020: A Review. Value Health 2021, 25, 385–389. [Google Scholar] [CrossRef]
- Moriña, D.; De Sanjosé, S.; Diaz, M. Impact of model calibration on cost-effectiveness analysis of cervical cancer prevention. Sci. Rep. 2017, 7, 17208. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, S.H.; Crook, A.M.; McHugh, T.D.; Mendel, C.M.; Meredith, S.K.; Murray, S.R.; Pappas, F.; Phillips, P.P.J.; Nunn, A.J. Four-Month Moxifloxacin-Based Regimens for Drug-Sensitive Tuberculosis. N. Engl. J. Med. 2014, 371, 1577–1587. [Google Scholar] [CrossRef] [Green Version]
- Floyd, K.; Glaziou, P.; Houben, R.M.G.J.; Sumner, T.; White, R.; Raviglione, M. Global tuberculosis targets and milestones set for 2016–2035: Definition and rationale. Int. J. Tuberc. Lung Dis. 2018, 22, 723–730. [Google Scholar] [CrossRef]
- Muniyandi, M.; Thomas, B.E.; Karikalan, N.; Kannan, T.; Rajendran, K.; Saravanan, B.; Vohra, V.; Okorosobo, T.; Lönnroth, K.; Tripathy, S.P. Association of Tuberculosis with Household Catastrophic Expenditure in South India. JAMA Netw. Open 2020, 3, e1920973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, B.M.; Tripathy, J.P.; Muraleedharan, V.R.; Tonsing, J. Rising Catastrophic Expenditure on Households Due to Tuberculosis: Is India Moving Away From the END-TB Goal? Front. Public Health 2021, 9, 614466. [Google Scholar] [CrossRef] [PubMed]


| Input Parameters | Base Case | Lower | Upper | Distribution | Source | |
|---|---|---|---|---|---|---|
| Demographic values | Average age of TB patient | 32 | 26 | 38 | Normal | 17 |
| Cohort population | 100,000 | 100,000 | 100,000 | NA | Assumption | |
| Life expectancy at age 32 | 44 | 44 | 44 | NA | 18 | |
| Mortality | All-cause mortality | 0.095 | 0.008 | 0.011 | Beta | 18 |
| Treatment outcome of 4-month regimen | Mortality due to TB | 0.003 | 0.002 | 0.004 | Beta | 11 |
| Failure | 0.020 | 0.016 | 0.024 | Beta | 11 | |
| Cure | 0.920 | 0.736 | 1.000 | Beta | 11 | |
| Lost-to-follow-up (LTF) | 0.040 | 0.032 | 0.048 | Beta | 11 | |
| Adverse drug reaction | 0.065 | 0.052 | 0.078 | Beta | 11 | |
| Treatment outcome of 6-month regimen | Mortality due to TB | 0.041 | 0.033 | 0.049 | Beta | 12 |
| Failure | 0.010 | 0.008 | 0.012 | Beta | 12 | |
| Cure | 0.930 | 0.744 | 1.000 | Beta | 13 | |
| Lost-to-follow-up | 0.040 | 0.032 | 0.048 | Beta | 12 | |
| Adverse drug reaction | 0.130 | 0.104 | 0.156 | Beta | 14 | |
| Recurrence | 4-month regimen | 0.041 | 0.033 | 0.049 | Beta | 11 |
| 6-month regimen | 0.069 | 0.055 | 0.083 | Beta | 12 | |
| Treatment outcome of LTF | Remain LTF | 0.200 | 0.160 | 0.240 | Beta | 14 |
| Cure | 0.630 | 0.504 | 0.756 | Beta | 14 | |
| Failure among LTF | 0.107 | 0.085 | 0.128 | Beta | 14 | |
| Treatment outcome of failures | Remain failure | 0.420 | 0.336 | 0.504 | Beta | 14 |
| LTF | 0.710 | 0.568 | 0.852 | Beta | 14 | |
| Cure | 0.420 | 0.336 | 0.504 | Beta | 14 | |
| Treatment outcome of recurrence | LTF among recurrence | 0.110 | 0.088 | 0.132 | Beta | 14 |
| Failure | 0.950 | 0.760 | 1.000 | Beta | 14 | |
| Prevalence of DR-TB | 0.290 | 0.232 | 0.348 | Beta | 31 | |
| Treatment outcome of DR-TB | Alive | 0.890 | 0.712 | 1.000 | Beta | 12 |
| Death | 0.110 | 0.088 | 0.132 | Beta | 12 | |
| Quality of life | Utility (cured TB patients) | 0.870 | 0.696 | 1.000 | Beta | 28, 29 |
| Utility (LTF, failure, recurrence, MDR-TB) | 0.623 | 0.498 | 0.747 | Beta | 30 | |
| Cost for 4-month regimen | Drugs | USD 72 | USD 57 | USD 86 | Gamma | 19 |
| Investigations | USD 5 | USD 4 | USD 6 | Gamma | 20 | |
| Hospitalization for ADR | USD 246 | USD 197 | USD 296 | Gamma | 22 | |
| Staff | USD 30 | USD 24 | USD 35 | Gamma | 21 | |
| Loss of income-patient | USD 6 | USD 5 | USD 7 | Gamma | 27 | |
| Food-patient | USD 4 | USD 3 | USD 5 | Gamma | 24 | |
| Travel-patient | USD 25 | USD 20 | USD 30 | Gamma | 25 | |
| Attendee cost | USD 2 | USD 2 | USD 3 | Gamma | 26 | |
| Cost for 6-month regimen | Drugs | USD 34 | USD 27 | USD 41 | Gamma | 19 |
| Investigation | USD 5 | USD 4 | USD 6 | Gamma | 20 | |
| Hospitalization for ADR | USD 246 | USD 197 | USD 296 | Gamma | 22 | |
| Staff | USD 44 | USD 35 | USD 53 | Gamma | 21 | |
| Loss of income-patient | USD 9 | USD 7 | USD 11 | Gamma | 27 | |
| Food-patient | USD 7 | USD 5 | USD 8 | Gamma | 24 | |
| Travel-patient | USD 38 | USD 30 | USD 45 | Gamma | 25 | |
| Attendee cost | USD 3 | USD 2 | USD 4 | Gamma | 26 | |
| Treatment for DR-TB | Drug | USD 274 | USD 219 | USD 329 | Gamma | 19 |
| Investigations | USD 12 | USD 10 | USD 15 | Gamma | 20 | |
| Staff | USD 66 | USD 53 | USD 80 | Gamma | 22 | |
| Travel-patient & attendee | USD 35 | USD 27 | USD 40 | Gamma | 25, 26 | |
| Hospital stay-patient | USD 0.490 | USD 0.390 | USD 0.580 | Gamma | 25 | |
| Food-patient | USD 7 | USD 6 | USD 7 | Gamma | 24 | |
| Travel-patient | USD 13 | USD 10 | USD 15 | Gamma | 25 | |
| Willingness to pay threshold | Willingness to pay threshold (GDP per capita) (in Indian Rupees) | USD 1900 | - | - | NA | 32 |
| Discounted | ||||||||
|---|---|---|---|---|---|---|---|---|
| Strategy | Total | Incremental | ICER | |||||
| Cost | Life Years | QALY | Cost | Life Years | QALY | Life Years | QALY | |
| 4-month regimen | USD 14,345,701 | 3,152,643 | 2,732,616 | USD −693,541 | 150,059 | 131,176 | USD −4.62 | USD −5.29 |
| 6-month regimen | USD 15,039,242 | 3,002,584 | 2,601,440 | - | - | - | - | - |
| Undiscounted | ||||||||
| 4-month regimen | USD 17,047,946 | 3,545,629 | 3,073,028 | USD −762,033 | 182,370 | 158,727 | USD −4.18 | USD −4.80 |
| 6-month regimen | USD 17,809,978 | 3,363,259 | 2,914,301 | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniyandi, M.; Karikalan, N.; Velayutham, B.; Rajsekar, K.; Padmapriyadarsini, C. Cost Effectiveness of a Shorter Moxifloxacin Based Regimen for Treating Drug Sensitive Tuberculosis in India. Trop. Med. Infect. Dis. 2022, 7, 288. https://doi.org/10.3390/tropicalmed7100288
Muniyandi M, Karikalan N, Velayutham B, Rajsekar K, Padmapriyadarsini C. Cost Effectiveness of a Shorter Moxifloxacin Based Regimen for Treating Drug Sensitive Tuberculosis in India. Tropical Medicine and Infectious Disease. 2022; 7(10):288. https://doi.org/10.3390/tropicalmed7100288
Chicago/Turabian StyleMuniyandi, Malaisamy, Nagarajan Karikalan, Banurekha Velayutham, Kavitha Rajsekar, and Chandrasekaran Padmapriyadarsini. 2022. "Cost Effectiveness of a Shorter Moxifloxacin Based Regimen for Treating Drug Sensitive Tuberculosis in India" Tropical Medicine and Infectious Disease 7, no. 10: 288. https://doi.org/10.3390/tropicalmed7100288





