Drug-Resistant Tuberculosis Treatment Outcomes among Children and Adolescents in Karachi, Pakistan
Abstract
1. Background
2. Methods
2.1. Study Setting
2.2. Patient Recruitment and Enrollment at Site
2.3. Data Extraction and Analysis
- (1)
- RR/MDR-TB: resistance to Rifampicin or both Rifampicin and Isoniazid
- (2)
- Pre-XDR-TB: RR/MDR-TB plus resistance to a fluoroquinolone
- (3)
- XDR-TB: Pre-XDR-TB plus resistance to any injectable and a fluoroquinolone
- (4)
- Other: Not meeting the definition of RR/MDR-TB, Pre-XDR-TB, or XDR-TB
- (5)
- Suspected DR-TB: not having a microbiological confirmation but resistance is suspected based on clinical symptoms, and positive exposure or contact history.
2.4. Ethics Statement
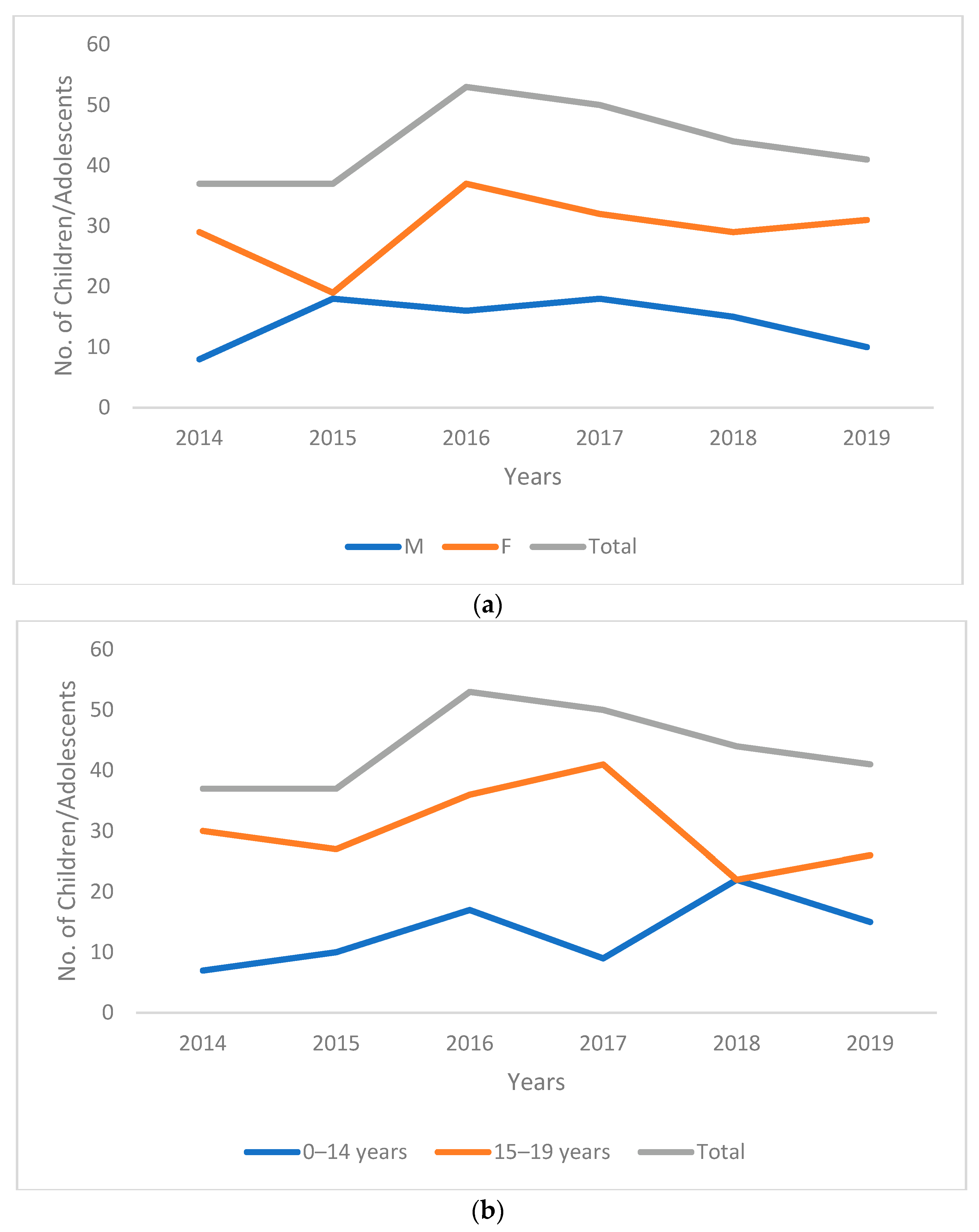
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Dodd, P.J.; Sismanidis, C.; Seddon, J.A. Global burden of drug-resistant tuberculosis in children: A mathematical modelling study. Lancet Infect. Dis. 2016, 16, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.E.; Tolman, A.W.; Yuen, C.M.; Parr, J.B.; Keshavjee, S.; Pérez-Vélez, C.M.; Pagano, M.; Becerra, M.C.; Cohen, T. Incidence of multidrug-resistant tuberculosis disease in children: Systematic review and global estimates. Lancet 2014, 383, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Song, W.M.; Li, Y.F.; Liu, Y.X.; Liu, Y.; Yu, C.B.; Liu, J.Y.; Li, H.C. Drug-Resistant Tuberculosis Among Children: A Systematic Review and Meta-Analysis. Front. Public Health 2021, 9, 721817. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.A.; Gandhi, N.R.; Marcy, O.; Walters, E.; Tejiokem, M.; Chau, G.D.; Omer, S.B.; Lash, T.L.; Becerra, M.C.; Njuguna, I.N.; et al. Development of a clinical prediction score including monocyte-to-lymphocyte ratio to inform tuberculosis treatment among children with HIV: A multi-country study. Open Forum. Infect. Dis. 2022, 9, ofac548. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.; Liang, H.; Wu, H.; Middelkoop, K.; Oni, T.; Rangaka, M.X.; Wilkinson, R.J.; Bekker, L.G.; Lawn, S.D. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int. J. Tuberc. Lung Dis. 2010, 14, 406–412. [Google Scholar]
- Seddon, J.A.; Chiang, S.S.; Esmail, H.; Coussens, A.K. The Wonder Years: What Can Primary School Children Teach Us About Immunity to Mycobacterium tuberculosis? Front. Immunol. 2018, 9, 2946. [Google Scholar] [CrossRef]
- Snow, K.J.; Cruz, A.T.; Seddon, J.A.; Ferrand, R.A.; Chiang, S.S.; Hughes, J.A.; Kampmann, B.; Graham, S.M.; Dodd, P.J.; Houben, R.M.; et al. Adolescent tuberculosis. Lancet Child Adolesc. Health 2020, 4, 68–79. [Google Scholar] [CrossRef]
- Nahid, P.; Mase, S.R.; Migliori, G.B.; Sotgiu, G.; Bothamley, G.H.; Brozek, J.L.; Cattamanchi, A.; Cegielski, J.P.; Chen, L.; Daley, C.L.; et al. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019, 200, e93–e142. [Google Scholar] [CrossRef]
- National TB Control Program. Annual Report 2019; Government of Pakistan: Islamabad, Pakistan, 2019.
- Migliori, G.B.; Network, G.T. Evolution of Programmatic Definitions Used in Tuberculosis Prevention and Care. Clin. Infect. Dis. 2018, 68, 1787–1789. [Google Scholar] [CrossRef]
- Harausz, E.P.; Garcia-Prats, A.J.; Law, S.; Schaaf, H.S.; Kredo, T.; Seddon, J.A.; Menzies, D.; Turkova, A.; Achar, J.; Amanullah, F.; et al. Treatment and outcomes in children with multidrug-resistant tuberculosis: A systematic review and individual patient data meta-analysis. PLoS Med. 2018, 15, e1002591. [Google Scholar] [CrossRef]
- Tola, H.H.; Khadoura, K.J.; Jimma, W.; Nedjat, S.; Majdzadeh, R. Multidrug resistant tuberculosis treatment outcome in children in developing and developed countries: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 12–18. [Google Scholar] [CrossRef]
- Dhakulkar, S.; Das, M.; Sutar, N.; Oswal, V.; Shah, D.; Ravi, S.; Vengurlekar, D.; Chavan, V.; Rebello, L.; Meneguim, A.C.; et al. Treatment outcomes of children and adolescents receiving drug-resistant TB treatment in a routine TB programme, Mumbai, India. PLoS ONE 2021, 16, e0246639. [Google Scholar] [CrossRef]
- Abubakar, M.; Ahmad, N.; Atif, M.; Hayat Khan, A.; Ghafoor, A. Treatment outcomes among childhood extensively drug-resistant tuberculosis patients in Pakistan. ERJ Open Res. 2022, 8. [Google Scholar] [CrossRef]
- Naz, F.; Ahmad, N.; Wahid, A.; Ahmad, I.; Khan, A.; Abubakar, M.; Khan, S.A.; Khan, A.; Latif, A.; Ghafoor, A. High rate of successful treatment outcomes among childhood rifampicin/multidrug-resistant tuberculosis in Pakistan: A multicentre retrospective observational analysis. BMC Infect. Dis. 2021, 21, 1209. [Google Scholar] [CrossRef]
- Isaakidis, P.; Paryani, R.; Khan, S.; Mansoor, H.; Manglani, M.; Valiyakath, A.; Saranchuk, P.; Furin, J. Poor outcomes in a cohort of HIV-infected adolescents undergoing treatment for multidrug-resistant tuberculosis in Mumbai, India. PLoS ONE 2013, 8, e68869. [Google Scholar] [CrossRef]
- Moyo, S.; Furin, J.J.; Hughes, J.; Daniels, J.; Snyman, L.; Muller, O.; Cox, V.; Shroufi, A.; Cox, H. Outcomes in Adolescents Undergoing Treatment for Drug-Resistant Tuberculosis in Cape Town, South Africa, 2008–2013. Arch. Pediatr. Infect. Dis. 2015, 3, e17934. [Google Scholar] [CrossRef]
- Hamid, M.; Brooks, M.B.; Madhani, F.; Ali, H.; Naseer, M.J.; The Childhood Tuberculosis Karachi, G.; Becerra, M.; Amanullah, F. Risk factors for unsuccessful tuberculosis treatment outcomes in children. PLoS ONE 2019, 14, e0222776. [Google Scholar] [CrossRef]
- Codlin, A.J.; Khowaja, S.; Chen, Z.; Rahbar, M.H.; Qadeer, E.; Ara, I.; McCormick, J.B.; Fisher-Hoch, S.P.; Khan, A.J. Short report: Gender differences in tuberculosis notification in Pakistan. Am. J. Trop. Med. Hyg. 2011, 85, 514–517. [Google Scholar] [CrossRef]
- Chiang, S.S.; Dolynska, M.; Rybak, N.R.; Cruz, A.T.; Aibana, O.; Sheremeta, Y.; Petrenko, V.; Mamotenko, A.; Terleieva, I.; Horsburgh, C.R., Jr.; et al. Clinical manifestations and epidemiology of adolescent tuberculosis in Ukraine. ERJ Open Res. 2020, 6, 00308-2020. [Google Scholar] [CrossRef]
- Laycock, K.M.; Enane, L.A.; Steenhoff, A.P. Tuberculosis in Adolescents and Young Adults: Emerging Data on TB Transmission and Prevention among Vulnerable Young People. Trop. Med. Infect. Dis. 2021, 6, 148. [Google Scholar] [CrossRef]
- Osman, M.; du Preez, K.; Seddon, J.A.; Claassens, M.M.; Dunbar, R.; Dlamini, S.S.; Welte, A.; Naidoo, P.; Hesseling, A.C. Mortality in South African Children and Adolescents Routinely Treated for Tuberculosis. Pediatrics 2021, 147, e2020032490. [Google Scholar] [CrossRef] [PubMed]
- Tierney, D.B.; Milstein, M.B.; Manjourides, J.; Furin, J.J.; Mitnick, C.D. Treatment Outcomes for Adolescents With Multidrug-Resistant Tuberculosis in Lima, Peru. Glob. Pediatr. Health 2016, 3, 2333794X16674382. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y.; Bai, K.J.; Lee, C.N.; Enarson, D.A.; Suo, J.; Luh, K.T. Inconsistent dosing of anti-tuberculosis drugs in Taipei, Taiwan. Int. J. Tuberc. Lung Dis. 2010, 14, 878–883. [Google Scholar] [PubMed]
- Enane, L.A.; Lowenthal, E.D.; Arscott-Mills, T.; Matlhare, M.; Smallcomb, L.S.; Kgwaadira, B.; Coffin, S.E.; Steenhoff, A.P. Loss to follow-up among adolescents with tuberculosis in Gaborone, Botswana. Int. J. Tuberc. Lung Dis. 2016, 20, 1320–1325. [Google Scholar] [CrossRef]
- Stevens, H.; Ximenes, R.A.; Dantas, O.M.; Rodrigues, L.C. Risk factors for tuberculosis in older children and adolescents: A matched case-control study in Recife, Brazil. Emerg. Themes Epidemiol. 2014, 11, 20. [Google Scholar] [CrossRef]
- Moore, B.K.; Anyalechi, E.; van der Walt, M.; Smith, S.; Erasmus, L.; Lancaster, J.; Morris, S.; Ndjeka, N.; Ershova, J.; Ismail, N.; et al. Epidemiology of drug-resistant tuberculosis among children and adolescents in South Africa, 2005 –2010. Int. J. Tuberc. Lung Dis. 2015, 19, 663–669. [Google Scholar] [CrossRef]
- Das, M.; Mathur, T.; Ravi, S.; Meneguim, A.C.; Iyer, A.; Mansoor, H.; Kalon, S.; Hossain, F.N.; Acharya, S.; Ferlazzo, G.; et al. Challenging drug-resistant TB treatment journey for children, adolescents and their care-givers: A qualitative study. PLoS ONE 2021, 16, e0248408. [Google Scholar] [CrossRef]
- Malik, A.A.; Gandhi, N.R.; Lash, T.L.; Cranmer, L.M.; Omer, S.B.; Ahmed, J.F.; Siddiqui, S.; Amanullah, F.; Khan, A.J.; Keshavjee, S.; et al. Effectiveness of Preventive Therapy for Persons Exposed at Home to Drug-Resistant Tuberculosis, Karachi, Pakistan. Emerg. Infect. Dis. 2021, 27, 805–812. [Google Scholar] [CrossRef]
- Chiang, S.S.; Brooks, M.B.; Jenkins, H.E.; Rubenstein, D.; Seddon, J.A.; van de Water, B.J.; Lindeborg, M.M.; Becerra, M.C.; Yuen, C.M. Concordance of Drug-resistance Profiles Between Persons With Drug-resistant Tuberculosis and Their Household Contacts: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2021, 73, 250–263. [Google Scholar] [CrossRef]

| Resistance Type | n (col%) (N = 262) | Favourable Outcome n (row%) |
|---|---|---|
| RR/MDR-TB | 198 (75.6) | 150 (75.8) |
| PreXDR-TB | 43 (16.4) | 30 (69.8) |
| XDR-TB | 3 (1.2) | 2 (66.7) |
| Other | 4 (1.5) | 3 (75.0) |
| Suspected DR-TB | 14 (5.3) | 9 (64.3) |
| Age | n (col%) (N = 262) | Favourable Outcomes n (row%) |
|---|---|---|
| 0–4 y | 16 (6.1) | 11 (68.8) |
| 5–9 y | 10 (3.8) | 8 (80.0) |
| 10–14 y | 54 (20.6) | 40 (74.1) |
| 15–19 y | 182 (69.5) | 135 (74.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, A.A.; Khan, U.; Khan, P.; Anwar, A.; Salahuddin, N.; Khowaja, S.; Khan, A.J.; Khan, S.; Hussain, H.; Amanullah, F. Drug-Resistant Tuberculosis Treatment Outcomes among Children and Adolescents in Karachi, Pakistan. Trop. Med. Infect. Dis. 2022, 7, 418. https://doi.org/10.3390/tropicalmed7120418
Malik AA, Khan U, Khan P, Anwar A, Salahuddin N, Khowaja S, Khan AJ, Khan S, Hussain H, Amanullah F. Drug-Resistant Tuberculosis Treatment Outcomes among Children and Adolescents in Karachi, Pakistan. Tropical Medicine and Infectious Disease. 2022; 7(12):418. https://doi.org/10.3390/tropicalmed7120418
Chicago/Turabian StyleMalik, Amyn A., Uzma Khan, Palwasha Khan, Aliya Anwar, Naseem Salahuddin, Saira Khowaja, Aamir J. Khan, Salman Khan, Hamidah Hussain, and Farhana Amanullah. 2022. "Drug-Resistant Tuberculosis Treatment Outcomes among Children and Adolescents in Karachi, Pakistan" Tropical Medicine and Infectious Disease 7, no. 12: 418. https://doi.org/10.3390/tropicalmed7120418
APA StyleMalik, A. A., Khan, U., Khan, P., Anwar, A., Salahuddin, N., Khowaja, S., Khan, A. J., Khan, S., Hussain, H., & Amanullah, F. (2022). Drug-Resistant Tuberculosis Treatment Outcomes among Children and Adolescents in Karachi, Pakistan. Tropical Medicine and Infectious Disease, 7(12), 418. https://doi.org/10.3390/tropicalmed7120418








