Inhibiting Human and Leishmania Arginases Using Cannabis sativa as a Potential Therapy for Cutaneous Leishmaniasis: A Molecular Docking Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of C. sativa Ligands
2.2. Retrieval of Arginases’ 3D Structures
2.3. Molecular Docking
3. Results
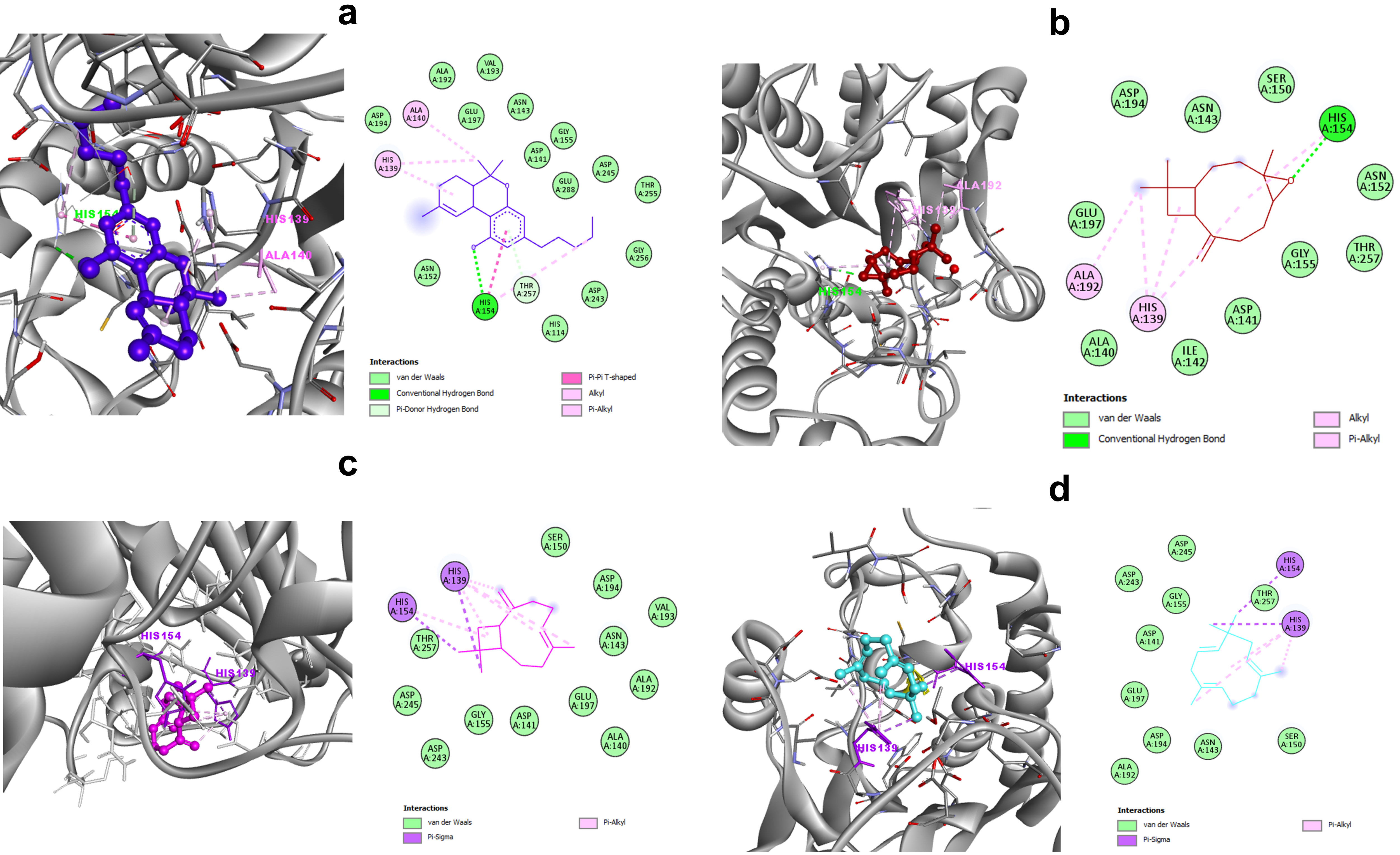
3.1. C. sativa Components and Leishmania Arginase: Molecular-Interaction Analysis
3.2. C. sativa Components and Human Arginase: Molecular-Interaction Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Leishmaniasis Fact-Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 26 September 2022).
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical Syndromes and Treatment. QJM Int. J. Med. 2014, 107, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daoui, O.; Bennaid, H.; Kbaich, M.A.; Mhaidi, I.; Aderdour, N.; Rhinane, H.; Bouhout, S.; Akarid, K.; Lemrani, M. Environmental, Climatic, and Parasite Molecular Factors Impacting the Incidence of Cutaneous Leishmaniasis Due to Leishmania Tropica in Three Moroccan Foci. Microorganisms 2022, 10, 1712. [Google Scholar] [CrossRef] [PubMed]
- Laboudi, M.; Sahibi, H.; Elabandouni, M.; Nhammi, H.; Ait Hamou, S.; Sadak, A. A Review of Cutaneous Leishmaniasis in Morocco: A Vertical Analysisto Determine Appropriate Interventions for Control and Prevention. Acta Trop. 2018, 187, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A. Plant-Derived Compounds in Treatment of Leishmaniasis. Iran. J. Vet. Res. 2015, 16, 1–19. [Google Scholar] [PubMed]
- Pearson, R.D.; Sousa, A.Q. Clinical Spectrum of Leishmaniasis. Clin. Infect. Dis. 1996, 22, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bogdan, C. Macrophages as Host, Effector and Immunoregulatory Cells in Leishmaniasis: Impact of Tissue Micro-Environment and Metabolism. Cytokine X 2020, 2, 100041. [Google Scholar] [CrossRef]
- Saha, S.; Shalova, I.N.; Biswas, S.K. Metabolic Regulation of Macrophage Phenotype and Function. Immunol. Rev. 2017, 280, 102–111. [Google Scholar] [CrossRef]
- Tomiotto-Pellissier, F.; da Silva Bortoleti, B.T.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef] [Green Version]
- Carter, N.S.; Stamper, B.D.; Elbarbry, F.; Nguyen, V.; Lopez, S.; Kawasaki, Y.; Poormohamadian, R.; Roberts, S.C. Natural Products That Target the Arginase in Leishmania Parasites Hold Therapeutic Promise. Microorganisms 2021, 9, 267. [Google Scholar] [CrossRef]
- Maksouri, H.; Dang, P.M.-C.; Rodrigues, V.; Estaquier, J.; Riyad, M.; Akarid, K. Moroccan Strains of Leishmania Major and Leishmania Tropica Differentially Impact on Nitric Oxide Production by Macrophages. Parasit Vectors 2017, 10, 506. [Google Scholar] [CrossRef]
- Liew, F.Y.; Li, Y.; Millott, S. Tumour Necrosis Factor (TNF-Alpha) in Leishmaniasis. II. TNF-Alpha-Induced Macrophage Leishmanicidal Activity Is Mediated by Nitric Oxide from L-Arginine. Immunology 1990, 71, 556–559. [Google Scholar] [PubMed]
- Wanasen, N.; Soong, L. L-Arginine Metabolism and Its Impact on Host Immunity against Leishmania Infection. Immunol. Res. 2008, 41, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Reczkowski, R.S.; Ash, D.E. EPR Evidence for Binuclear Manganese (II) Centers in Rat Liver Arginase. J. Am. Chem. Soc. 1992, 114, 10992–10994. [Google Scholar] [CrossRef]
- Kanyo, Z.F.; Scolnick, L.R.; Ash, D.E.; Christianson, D.W. Structure of a Unique Binuclear Manganese Cluster in Arginase. Nature 1996, 383, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, V.; Carlos Gómez-Nieto, L.; Molano, I.; Mohedano, A.; Carcelén, J.; Mirón, C.; Alonso, C.; Corraliza, I. Arginase I Induction in Macrophages, Triggered by Th2-Type Cytokines, Supports the Growth of Intracellular Leishmania Parasites. Parasite Immunol. 2002, 24, 113–118. [Google Scholar] [CrossRef]
- Kropf, P.; Fuentes, J.M.; Fähnrich, E.; Arpa, L.; Herath, S.; Weber, V.; Soler, G.; Celada, A.; Modolell, M.; Müller, I. Arginase and Polyamine Synthesis Are Key Factors in the Regulation of Experimental Leishmaniasis in Vivo. FASEB J. 2005, 19, 1000–1002. [Google Scholar] [CrossRef]
- Roberts, S.C.; Tancer, M.J.; Polinsky, M.R.; Gibson, K.M.; Heby, O.; Ullman, B. Arginase Plays a Pivotal Role in Polyamine Precursor Metabolism in Leishmania: Characterization of Gene Deletion Mutants*. J. Biol. Chem. 2004, 279, 23668–23678. [Google Scholar] [CrossRef] [Green Version]
- Muxel, S.M.; Aoki, J.I.; Fernandes, J.C.R.; Laranjeira-Silva, M.F.; Zampieri, R.A.; Acuña, S.M.; Müller, K.E.; Vanderlinde, R.H.; Floeter-Winter, L.M. Arginine and Polyamines Fate in Leishmania Infection. Front. Microbiol. 2018, 8, 2682. [Google Scholar] [CrossRef] [Green Version]
- Munder, M. Arginase: An Emerging Key Player in the Mammalian Immune System. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef] [Green Version]
- Abebe, T.; Hailu, A.; Woldeyes, M.; Mekonen, W.; Bilcha, K.; Cloke, T.; Fry, L.; Seich al Basatena, N.-K.; Corware, K.; Modolell, M.; et al. Local Increase of Arginase Activity in Lesions of Patients with Cutaneous Leishmaniasis in Ethiopia. PLOS Negl. Trop. Dis. 2012, 6, e1684. [Google Scholar] [CrossRef]
- Mortazavi, H.; Sadeghipour, P.; Taslimi, Y.; Habibzadeh, S.; Zali, F.; Zahedifard, F.; Rahmati, J.; Kamyab, K.; Ghandi, N.; Zamanian, A.; et al. Comparing Acute and Chronic Human Cutaneous Leishmaniasis Caused by Leishmania Major and Leishmania Tropica Focusing on Arginase Activity. J. Eur. Acad. Derm. Venereol. 2016, 30, 2118–2121. [Google Scholar] [CrossRef] [PubMed]
- França-Costa, J.; Van Weyenbergh, J.; Boaventura, V.S.; Luz, N.F.; Malta-Santos, H.; Oliveira, M.C.S.; Santos de Campos, D.C.; Saldanha, A.C.; dos-Santos, W.L.C.; Bozza, P.T.; et al. Arginase I, Polyamine, and Prostaglandin E2 Pathways Suppress the Inflammatory Response and Contribute to Diffuse Cutaneous Leishmaniasis. J. Infect. Dis. 2015, 211, 426–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iniesta, V.; Carcelén, J.; Molano, I.; Peixoto, P.M.V.; Redondo, E.; Parra, P.; Mangas, M.; Monroy, I.; Campo, M.L.; Nieto, C.G.; et al. Arginase I Induction during Leishmania Major Infection Mediates the Development of Disease. Infect. Immun. 2005, 73, 6085–6090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahidi, S.; Gholami, E.; Taslimi, Y.; Habibzadeh, S.; Seyed, N.; Davarpanah, E.; Ghanadan, A.; Rafati, S.; Taheri, T. The Outcome of Arginase Activity Inhibition in BALB/c Mice Hosting Leishmania Tropica. Parasite Immunol. 2020, 42, e12691. [Google Scholar] [CrossRef]
- Iniesta, V.; Gómez-Nieto, L.C.; Corraliza, I. The Inhibition of Arginase by N(Omega)-Hydroxy-l-Arginine Controls the Growth of Leishmania inside Macrophages. J. Exp. Med. 2001, 193, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Charmoy, M.; Romano, A.; Paun, A.; Chaves, M.M.; Cope, F.O.; Ralph, D.A.; Sacks, D.L. Mannose Receptor High, M2 Dermal Macrophages Mediate Nonhealing Leishmania Major Infection in a Th1 Immune Environment. J. Exp. Med. 2017, 215, 357–375. [Google Scholar] [CrossRef]
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Chapter 53—Cannabis sativa and Hemp. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 735–754. [Google Scholar]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A Comprehensive Ethnopharmacological Review of a Medicinal Plant with a Long History. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stambouli, H.; Bouri, A.E.; Bellemam, M.A.; Bouayoun, T.; Karni, N.E. Concentrations du Δ9—THC dans les cultures de Cannabis sativa L. du nord du Maroc. Ann. Toxicol. Anal. 2005, 17, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Freeman, A.M.; Petrilli, K.; Lees, R.; Hindocha, C.; Mokrysz, C.; Curran, H.V.; Saunders, R.; Freeman, T.P. How Does Cannabidiol (CBD) Influence the Acute Effects of Delta-9-Tetrahydrocannabinol (THC) in Humans? A Systematic Review. Neurosci. Biobehav. Rev. 2019, 107, 696–712. [Google Scholar] [CrossRef]
- El Bakali, I.; Sakar, E.H.; Boutahar, A.; Kadiri, M.; Merzouki, A. A Comparative Phytochemical Profiling of Essential Oils Isolated from Three Hemp (Cannabis sativa L.) Cultivars Grown in Central-Northern Morocco. Biocatal. Agric. Biotechnol. 2022, 42, 102327. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant Activity and Evidence for Synergism of Cannabis sativa (L.) Essential Oil with Antimicrobial Standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Schofs, L.; Sparo, M.D.; Sánchez Bruni, S.F. The Antimicrobial Effect behind Cannabis sativa. Pharmacology Research & Perspectives 2021, 9, e00761. [Google Scholar]
- Radwan, M.M.; ElSohly, M.A.; Slade, D.; Ahmed, S.A.; Khan, I.A.; Ross, S.A. Biologically Active Cannabinoids from High-Potency Cannabis sativa. J. Nat. Prod. 2009, 72, 906–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robledo, S.M.; Restrepo, A.; Yepes, L.M.; Fernandez, M.; Vélez, I.D. Studies In Vitro and In Vivo of Antileishmanial Activity and Differential Cytotoxicity of Cannabis Spp. Planta Med. Int. Open 2017, 4 (Suppl. 1), S1–S202. [Google Scholar]
- D’Antonio, E.L.; Ullman, B.; Roberts, S.C.; Dixit, U.G.; Wilson, M.E.; Hai, Y.; Christianson, D.W. Crystal Structure of Arginase from Leishmania Mexicana and Implications for the Inhibition of Polyamine Biosynthesis in Parasitic Infections. Arch. Biochem. Biophys. 2013, 535, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Di Costanzo, L.; Ilies, M.; Thorn, K.J.; Christianson, D.W. Inhibition of Human Arginase I by Substrate and Product Analogues. Arch. Biochem. Biophys. 2010, 496, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Reithinger, R.; Dujardin, J.-C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous Leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef] [Green Version]
- Mehwish, S.; Islam, A.; Ullah, I.; Wakeel, A.; Qasim, M.; Khan, M.A.; Ahmad, A.; Ullah, N. In Vitro Antileishmanial and Antioxidant Potential, Cytotoxicity Evaluation and Phytochemical Analysis of Extracts from Selected Medicinally Important Plants. Biocatal. Agric. Biotechnol. 2019, 19, 101117. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Carradori, S.; D’Antonio, M.; Orlando, G.; Cairone, F.; Cesa, S.; Filippi, A.; Fraschetti, C.; Zengin, G.; et al. Chemical and Bioinformatics Analyses of the Anti-Leishmanial and Anti-Oxidant Activities of Hemp Essential Oil. Biomolecules 2021, 11, 272. [Google Scholar] [CrossRef]
- Riley, E.; Roberts, S.C.; Ullman, B. Inhibition Profile of Leishmania Mexicana Arginase Reveals Differences with Human Arginase I. Int. J. Parasitol. 2011, 41, 545–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reguera, R.M.; Balaña-Fouce, R.; Showalter, M.; Hickerson, S.; Beverley, S.M. Leishmania Major Lacking Arginase (ARG) Are Auxotrophic for Polyamines but Retain Infectivity to Susceptible BALB/c Mice. Mol. Biochem. Parasitol. 2009, 165, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Maquiaveli, C.d.C.; Rochetti, A.L.; Fukumasu, H.; Vieira, P.C.; da Silva, E.R. Antileishmanial Activity of Verbascoside: Selective Arginase Inhibition of Intracellular Amastigotes of Leishmania (Leishmania) Amazonensis with Resistance Induced by LPS plus IFN-γ. Biochem. Pharmacol. 2017, 127, 28–33. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.R.; Maquiaveli, C.d.C.; Magalhães, P.P. The Leishmanicidal Flavonols Quercetin and Quercitrin Target Leishmania (Leishmania) Amazonensis Arginase. Exp. Parasitol. 2012, 130, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Maquiaveli, C.C.; Lucon-Júnior, J.F.; Brogi, S.; Campiani, G.; Gemma, S.; Vieira, P.C.; Silva, E.R. Verbascoside Inhibits Promastigote Growth and Arginase Activity of Leishmania Amazonensis. J. Nat. Prod. 2016, 79, 1459–1463. [Google Scholar] [CrossRef]
- da Silva, E.R.; Castilho, T.M.; Pioker, F.C.; Tomich de Paula Silva, C.H.; Floeter-Winter, L.M. Genomic Organisation and Transcription Characterisation of the Gene Encoding Leishmania (Leishmania) Amazonensis Arginase and Its Protein Structure Prediction. Int. J. Parasitol. 2002, 32, 727–737. [Google Scholar] [CrossRef]
- Crizanto de Lima, E.; Castelo-Branco, F.S.; Maquiaveli, C.C.; Farias, A.B.; Rennó, M.N.; Boechat, N.; Silva, E.R. Phenylhydrazides as Inhibitors of Leishmania Amazonensis Arginase and Antileishmanial Activity. Bioorganic Med. Chem. 2019, 27, 3853–3859. [Google Scholar] [CrossRef]
- Custot, J.; Moali, C.; Brollo, M.; Boucher, J.L.; Delaforge, M.; Mansuy, D.; Tenu, J.P.; Zimmermann, J.L. The New α-Amino Acid Nω-Hydroxy-nor-l-Arginine: A High-Affinity Inhibitor of Arginase Well Adapted to Bind to Its Manganese Cluster. J. Am. Chem. Soc. 1997, 119, 4086–4087. [Google Scholar] [CrossRef]
- Méndez-Cuesta, C.A.; Méndez-Lucio, O.; Castillo, R. Homology Modeling, Docking and Molecular Dynamics of the Leishmania Mexicana Arginase: A Description of the Catalytic Site Useful for Drug Design. J. Mol. Graph. Model. 2012, 38, 50–59. [Google Scholar] [CrossRef]
- Shishova, E.Y.; Di Costanzo, L.; Emig, F.A.; Ash, D.E.; Christianson, D.W. Probing the Specificity Determinants of Amino Acid Recognition by Arginase. Biochemistry 2009, 48, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Grobben, Y.; Uitdehaag, J.C.M.; Willemsen-Seegers, N.; Tabak, W.W.A.; de Man, J.; Buijsman, R.C.; Zaman, G.J.R. Structural Insights into Human Arginase-1 PH Dependence and Its Inhibition by the Small Molecule Inhibitor CB-1158. J. Struct. Biol. X 2020, 4, 100014. [Google Scholar] [CrossRef]
- Katchan, V.; David, P.; Shoenfeld, Y. Cannabinoids and Autoimmune Diseases: A Systematic Review. Autoimmun. Rev. 2016, 15, 513–528. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; Lima, D.; de Costa, F.; Deshmukh, K.; Li, N.; Chow, A.M.; Marques, J.A.; Pereira, R.P.; Kerman, K. Probing the Antioxidant Activity of Δ9-Tetrahydrocannabinol and Cannabidiol in Cannabis sativa Extracts. Analyst 2019, 144, 4952–4961. [Google Scholar] [CrossRef]
- Perera, P.K.; Diddeniya, J.I.D. In-Vitro and in-Vivo Supportive Research on Medicinal Properties of Cannabis sativa: A Comprehensive Review. J. Ayurvedic Herb. Med. 2022, 8, 40–47. [Google Scholar] [CrossRef]
- Pham, T.-N.; Bordage, S.; Pudlo, M.; Demougeot, C.; Thai, K.-M.; Girard-Thernier, C. Cinnamide Derivatives as Mammalian Arginase Inhibitors: Synthesis, Biological Evaluation and Molecular Docking. Int. J. Mol. Sci. 2016, 17, 1656. [Google Scholar] [CrossRef]
- Bourjot, M.; Zedet, A.; Demange, B.; Pudlo, M.; Girard, C. In Vitro Mammalian Arginase Inhibitory and Antioxidant Effects of Amide Derivatives Isolated from the Hempseed Cakes (Cannabis sativa). Planta Med. Int. Open 2017, 3, e64–e67. [Google Scholar] [CrossRef] [Green Version]
- Gaur, U.; Roberts, S.C.; Dalvi, R.P.; Corraliza, I.; Ullman, B.; Wilson, M.E. An Effect of Parasite-Encoded Arginase on the Outcome of Murine Cutaneous Leishmaniasis. J. Immunol. 2007, 179, 8446–8453. [Google Scholar] [CrossRef]

| Ligands | Characteristics | Structures |
|---|---|---|
| Delta-9-Tetrahydrocannabinol | MW:314.5 g/mol MF: C21H30O2 PubChem ID: CID 16078 |  |
| Cannabidiol (CBD) | MW:314.5 g/mol MF: C21H30O2 PubChem ID: CID 644019 |  |
| Caryophyllene Oxide | MW:220.35 g/mol MF: C15H24O PubChem ID: CID 1742210 |  |
| Beta-Caryophyllene | MW:204.35 g/mol MF: C15H24 PubChem ID: CID 5281515 |  |
| α-Pinene | MW:136.23 g/mol MF: C10H16 PubChem ID: CID 6654 |  |
| α-humulene | MW:204.35 g/mol MF: C15H24 PubChem ID: CID 5281520 |  |
| Myrcene | MW:136.23 g/mol MF: C10H16 PubChem ID: CID 31253 |  |
| Limonene | MW: 136.23 g/mol MF: C10H16 PubChem ID: CID 22311 |  |
| Glucantime® (meglumine antimoniate) | MW:365.98 g/mol MF: C7H18NO8Sb PubChem ID: CID 64953 |  |
| Ligand | Arginase | |||
|---|---|---|---|---|
| Leishmania (4ITY) | Human (3kv2) | |||
| Binding Energy (kcal/mol) | Ki (µM) | Binding Energy (kcal/mol) | Ki (µM) | |
| Delta-9-Tetrahydrocannabinol | −6.02 | 38.63 | −6.35 | 21.97 |
| Cannabidiol (CBD) | −5.41 | 108.33 | −5.60 | 78.86 |
| Caryophyllene Oxide | −5.88 | 49.07 | −5.37 | 115.51 |
| Beta-Caryophyllene | −5.79 | 56.56 | −5.47 | 97.47 |
| α-Pinene | −4.58 | 441.46 | −4.90 | 254.71 |
| α-humulene | −5.55 | 85.30 | −5.25 | 140.93 |
| Myrcene | −4.79 | 307.93 | −4.88 | 266.94 |
| Limonene | −4.49 | 507.25 | −4.61 | 416.05 |
| Glucantime® | −4.30 | 702.95 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assouab, A.; El Filaly, H.; Akarid, K. Inhibiting Human and Leishmania Arginases Using Cannabis sativa as a Potential Therapy for Cutaneous Leishmaniasis: A Molecular Docking Study. Trop. Med. Infect. Dis. 2022, 7, 400. https://doi.org/10.3390/tropicalmed7120400
Assouab A, El Filaly H, Akarid K. Inhibiting Human and Leishmania Arginases Using Cannabis sativa as a Potential Therapy for Cutaneous Leishmaniasis: A Molecular Docking Study. Tropical Medicine and Infectious Disease. 2022; 7(12):400. https://doi.org/10.3390/tropicalmed7120400
Chicago/Turabian StyleAssouab, Aicha, Hajar El Filaly, and Khadija Akarid. 2022. "Inhibiting Human and Leishmania Arginases Using Cannabis sativa as a Potential Therapy for Cutaneous Leishmaniasis: A Molecular Docking Study" Tropical Medicine and Infectious Disease 7, no. 12: 400. https://doi.org/10.3390/tropicalmed7120400
APA StyleAssouab, A., El Filaly, H., & Akarid, K. (2022). Inhibiting Human and Leishmania Arginases Using Cannabis sativa as a Potential Therapy for Cutaneous Leishmaniasis: A Molecular Docking Study. Tropical Medicine and Infectious Disease, 7(12), 400. https://doi.org/10.3390/tropicalmed7120400






