Evaluation of Chiral Organosulfur Compounds on Their Activity against the Malaria Parasite Plasmodium falciparum
Abstract
:1. Introduction
2. Materials and Methods
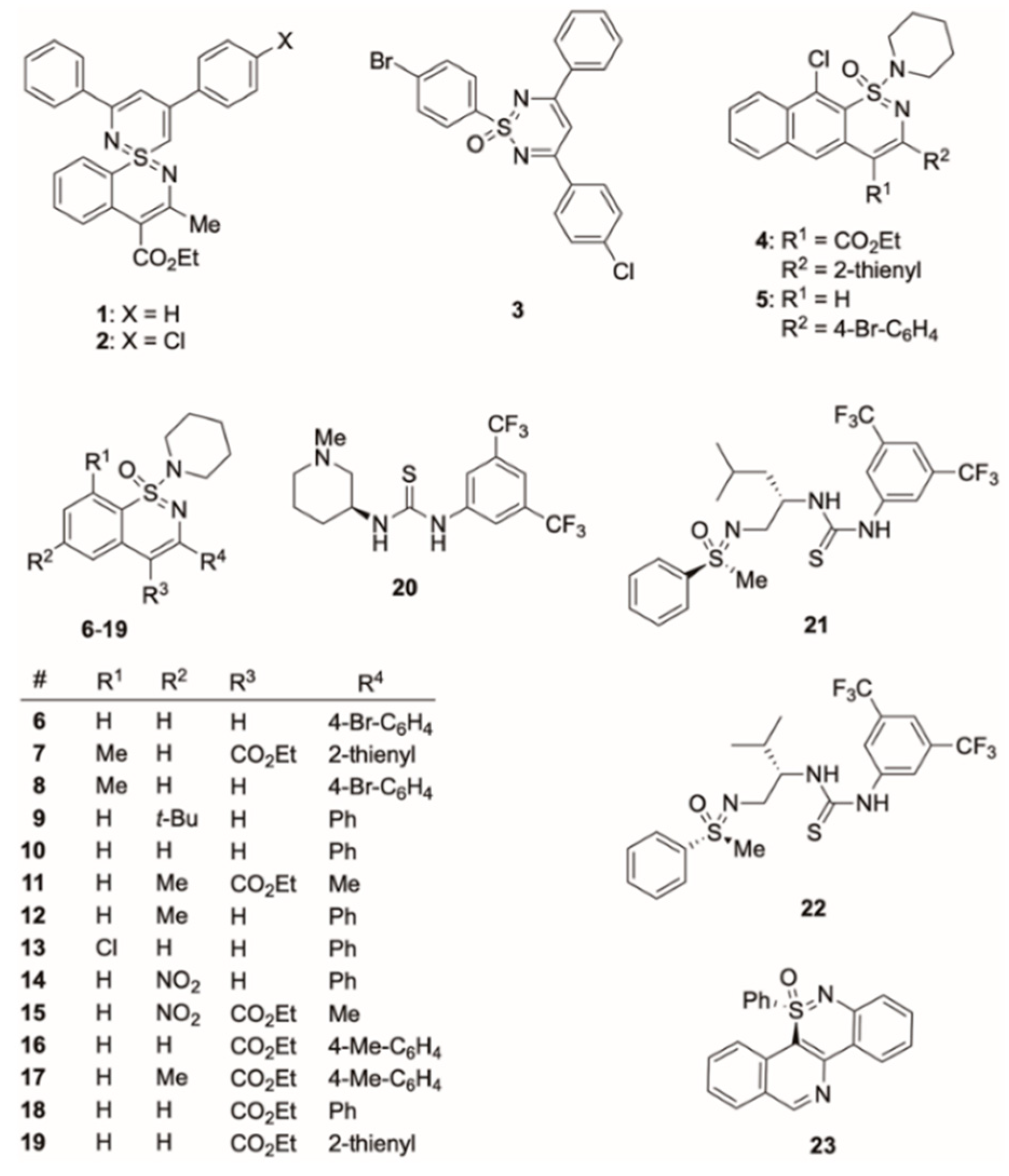
2.1. Organosulfur Compounds
2.2. Parasite Culture
2.3. Malstat Assay
2.4. Gametocyte Toxicity Test
2.5. Hemolytic Assay
2.6. Cytotoxicity Assay
3. Results
3.1. Organosulfur Compounds
3.2. The Organic Compounds Exhibit Antimalarial Activity
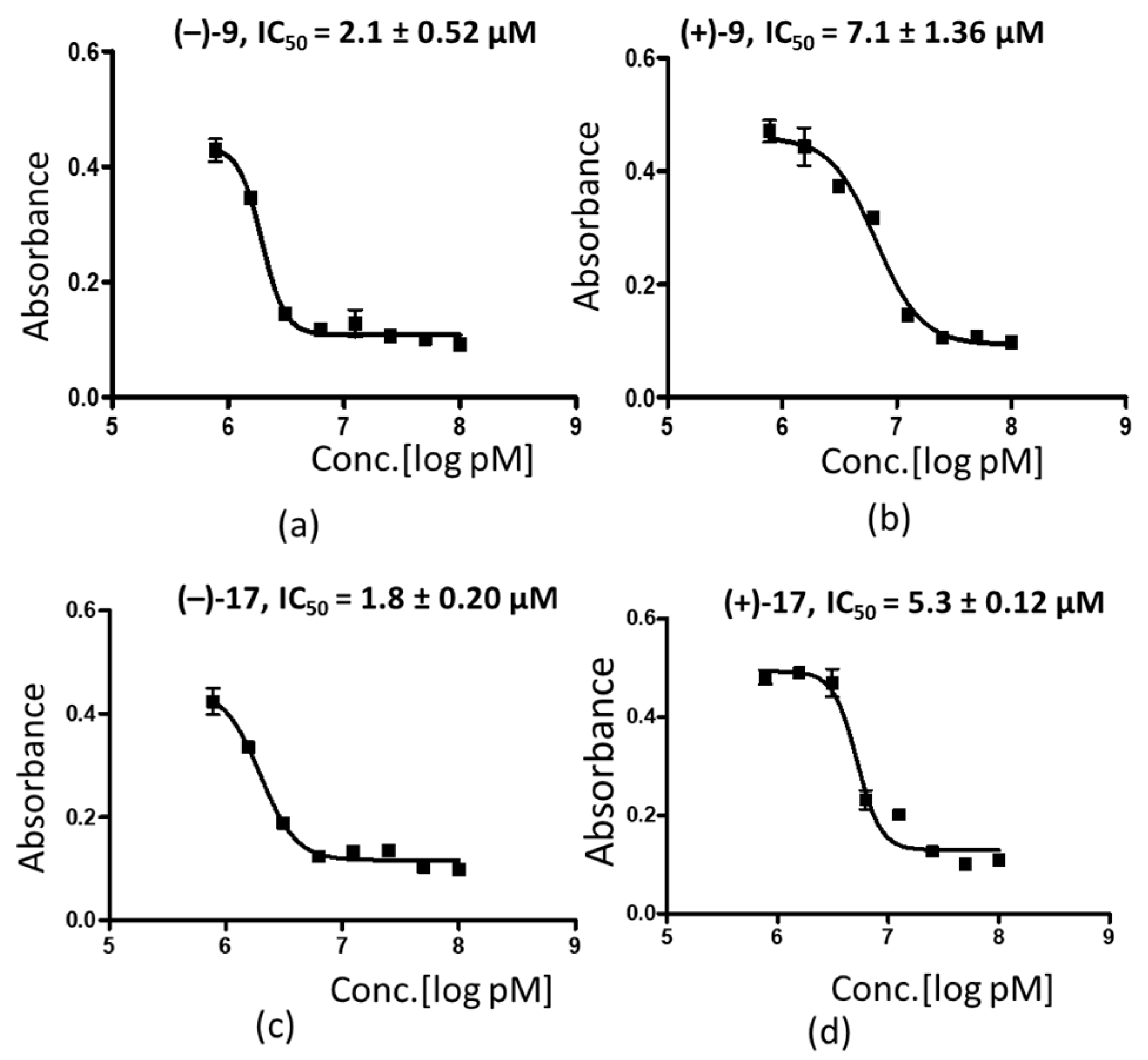
3.3. The (–) Enantiomers Exhibit Better Antiplasmodial Activity as Compared to Their (+) Counterparts
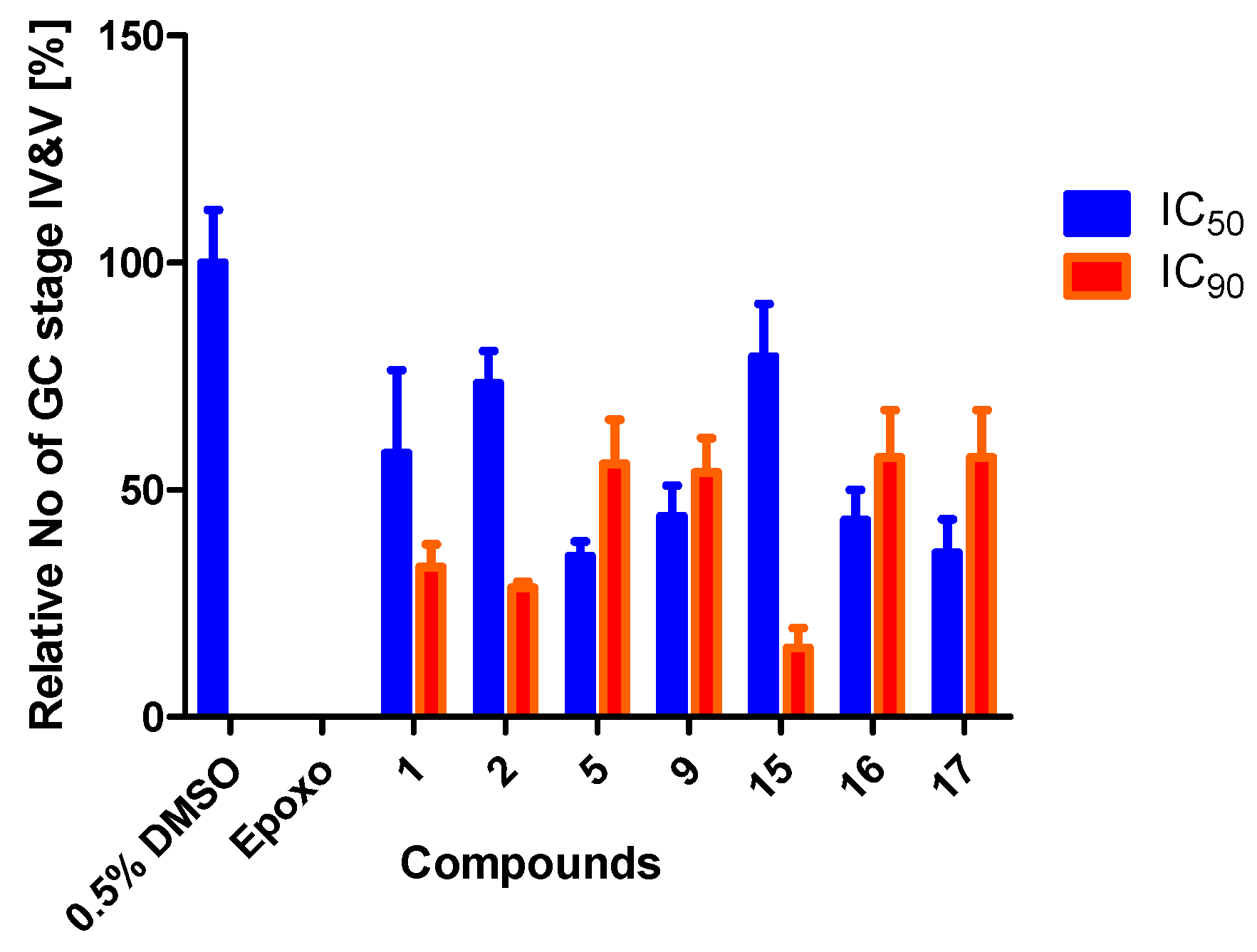
3.4. Treatment with Compounds Reduce Gametocyte Development
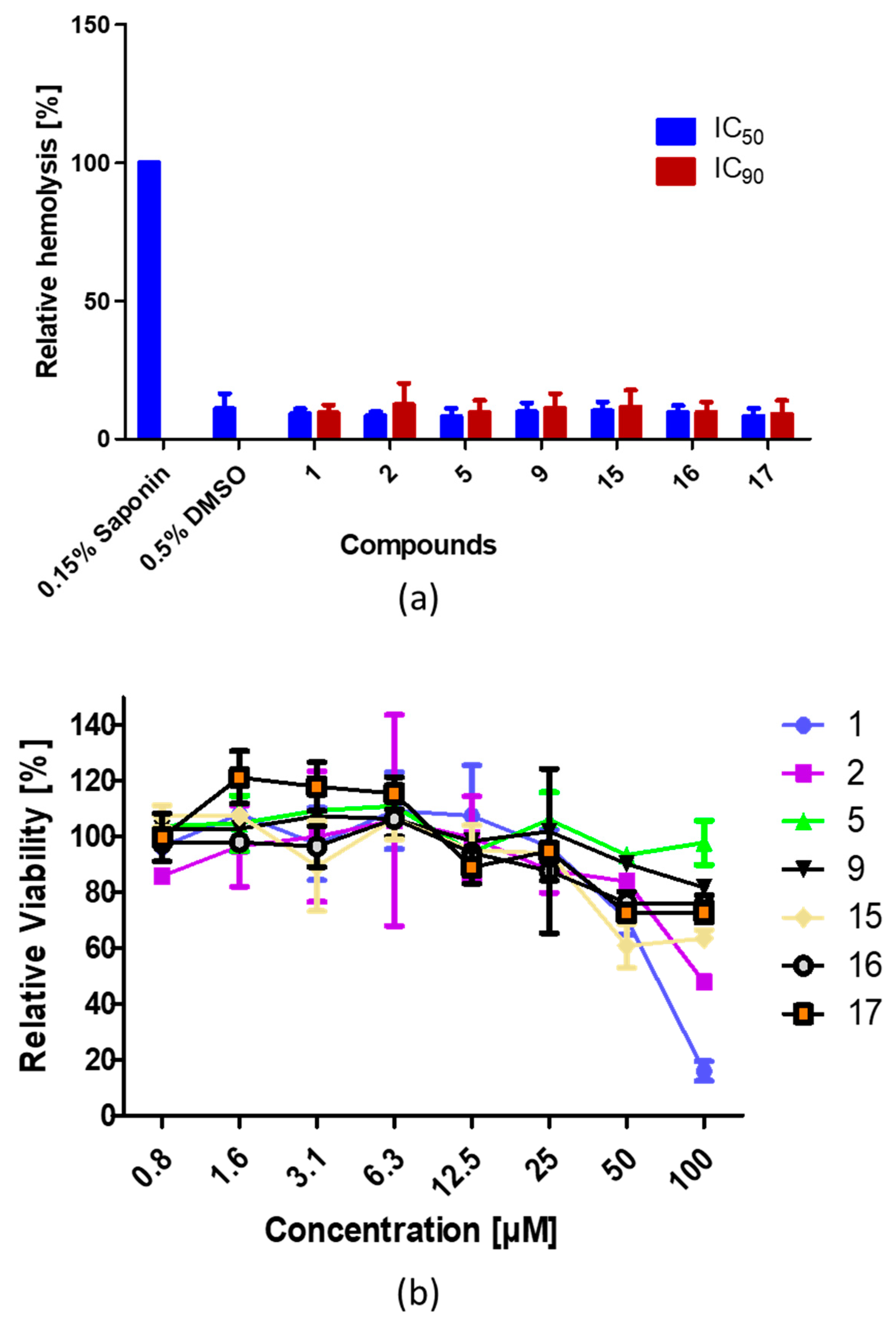
3.5. The Compounds Exhibit No Hemolytic or Less Cytotoxic Effect on Human Cells
3.6. Analyzed Enantiomers Were Not Toxic against HEL Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Malaria Report. 2021. Available online: https://www.who.int/publications/i/item/9789240040496 (accessed on 13 May 2022).
- Nsanzabana, C. Resistance to Artemisinin Combination Therapies (ACTs): Do Not Forget the Partner Drug! Trop. Med. Infect. Dis. 2019, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; van der Pluijm, R.W.; Kucharski, M.; Nayak, S.; Tripathi, J.; White, N.J.; Day, N.P.J.; Faiz, A.; Phyo, A.P.; Amaratunga, C.; et al. Artemisinin Resistance in the Malaria Parasite, Plasmodium Falciparum, Originates from Its Initial Transcriptional Response. Commun. Biol. 2022, 5, 274. [Google Scholar] [CrossRef]
- Phyo, A.P.; Nkhoma, S.; Stepniewska, K.; Ashley, E.A.; Nair, S.; McGready, R.; ler Moo, C.; Al-Saai, S.; Dondorp, A.M.; Lwin, K.M.; et al. Emergence of Artemisinin-Resistant Malaria on the Western Border of Thailand: A Longitudinal Study. Lancet 2012, 379, 1960–1966. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, D.B.; Jin, W.; Park, J.; Yoon, W.; Lee, Y.; Kim, S.; Lee, S.; Kim, S.; Lee, O.H.; et al. Comparative Studies of Bioactive Organosulphur Compounds and Antioxidant Activities in Garlic (Allium Sativum L.), Elephant Garlic (Allium Ampeloprasum L.) and Onion (Allium Cepa L.). Nat. Prod. Res. 2018, 32, 1193–1197. [Google Scholar] [CrossRef]
- Li, W.R.; Shi, Q.S.; Dai, H.Q.; Liang, Q.; Xie, X.B.; Huang, X.M.; Zhao, G.Z.; Zhang, L.X. Antifungal Activity, Kinetics and Molecular Mechanism of Action of Garlic Oil against Candida Albicans. Sci. Rep. 2016, 6, 22805. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Ruan, J.; Zhuang, X.; Zhang, X.; Li, Z. Phytochemicals of Garlic: Promising Candidates for Cancer Therapy. Biomed. Pharmacother. 2020, 123, 109730. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; Lopez-Roa, R.I.; Flores-Gutierrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuno-Sahagun, D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [Green Version]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium Sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Coppi, A.; Cabinian, M.; Mirelman, D.; Sinnis, P. Antimalarial Activity of Allicin, a Biologically Active Compound from Garlic Cloves. Antimicrob. Agents Chemother. 2006, 50, 1731–1737. [Google Scholar] [CrossRef]
- Palakkod Govindan, V.; Panduranga, A.N.; Krishna Murthy, P. Assessment of in Vivo Antimalarial Activity of Arteether and Garlic Oil Combination Therapy. Biochem. Biophys. Rep. 2016, 5, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Kumagai, H.; Seki, T.; Ariga, T. Biological and Chemical Stability of Garlic-Derived Allicin. J. Agric. Food Chem. 2008, 56, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Egen-Schwind, C.; Eckard, R.; Kemper, F.H. Metabolism of Garlic Constituents in the Isolated Perfused Rat Liver. Planta Med. 1992, 58, 301–305. [Google Scholar] [CrossRef]
- Bohmann, R.A.; Unoh, Y.; Miura, M.; Bolm, C. 1,2-Thiazines: One-Pot Syntheses Utilizing Mono and Diaza Analogs of Sulfones. Chem. A Eur. J. 2016, 22, 6783–6786. [Google Scholar] [CrossRef] [PubMed]
- Bohmann, R.A.; Schöbel, J.H.; Unoh, Y.; Miura, M.; Bolm, C. Regioselective Syntheses of 1,2-Benzothiazine 1-Imines by Rhodium-Catalyzed Annulation Reactions of Sulfondiimines. Adv. Synth. Catal. 2019, 361, 2000–2003. [Google Scholar] [CrossRef]
- Schöbel, J.H.; Elbers, P.; Truong, K.N.; Rissanen, K.; Bolm, C. 1,2-Benzothiazine Derivatives from Sulfonimidamides by Metal-Catalyzed Annulation Reactions in Solution and under Solvent-Free Mechanochemical Conditions. Adv. Synth. Catal. 2021, 363, 1322–1329. [Google Scholar] [CrossRef]
- Jörres, M.; Schiffers, I.; Atodiresei, I.; Bolm, C. Asymmetric Michael Additions of α-Nitrocyclohexanone to Aryl Nitroalkenes Catalyzed by Natural Amino Acid-Derived Bifunctional Thioureas. Org. Lett. 2012, 14, 4518–4521. [Google Scholar] [CrossRef]
- Frings, M.; Atodiresei, I.; Bolm, C. (R)-(−)-2-[(5-Oxido-5-phenyl-5λ4-isoquino[4,3-c][2,1]benzothiazin-12-yl)amino]benzonitrile. Molbank 2014, 2014, 834. [Google Scholar] [CrossRef] [Green Version]
- Frings, M.; Thomé, I.; Bolm, C. Synthesis of Chiral Sulfoximine-Based Thioureas and Their Application in Asymmetric Organocatalysis. Beilstein J. Org. Chem. 2012, 8, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Schöbel, J.-H. Bioaktive Und Heterocyclische Sulfondiimine und Sulfonimidamide. Ph.D. Thesis, RTWH Aachen University, Aachen, Germany, 2021. [Google Scholar] [CrossRef]
- Ifediba, T.; Vanderberg, J.P. Complete in Vitro Maturation of Plasmodium Falciparum Gametocytes. Nature 1981, 294, 364–366. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium Falciparum Erythrocytic Stages in Culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Basova, S.; Wilke, N.; Koch, J.C.; Prokop, A.; Berkessel, A.; Pradel, G.; Ngwa, C.J. Organoarsenic Compounds with in Vitro Activity against the Malaria Parasite Plasmodium Falciparum. Biomedicines 2020, 8, 260. [Google Scholar] [CrossRef]
- Ngwa, C.J.; Kiesow, M.J.; Orchard, L.M.; Farrukh, A.; Llinás, M.; Pradel, G. The G9a Histone Methyltransferase Inhibitor BIX-01294 Modulates Gene Expression during Plasmodium Falciparum Gametocyte Development and Transmission. Int. J. Mol. Sci. 2019, 20, 5087. [Google Scholar] [CrossRef] [Green Version]
- Ngwa, C.J.; Kiesow, M.J.; Papst, O.; Orchard, L.M.; Filarsky, M.; Rosinski, A.N.; Voss, T.S.; Llinás, M.; Pradel, G. Transcriptional Profiling Defines Histone Acetylation as a Regulator of Gene Expression during Human-to-Mosquito Transmission of the Malaria Parasite Plasmodium Falciparum. Front. Cell. Infect. Microbiol. 2017, 7, 320. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zavala, F. RTS,S: The First Malaria Vaccine. J. Clin. Investig. 2022, 132, e156588. [Google Scholar] [CrossRef] [PubMed]
- Bojang, K.A.; Milligan, P.J.M.; Pinder, M.; Vigneron, L.; Alloueche, A.; Kester, K.E.; Ballou, W.R.; Conway, D.J.; Reece, W.H.H.; Gothard, P.; et al. Efficacy of RTS,S/AS02 Malaria Vaccine against Plasmodium Falciparum Infection in Semi-Immune Adult Men in The Gambia: A Randomised Trial. Lancet 2001, 358, 1927–1934. [Google Scholar] [CrossRef]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over Half of Known Human Pathogenic Diseases Can Be Aggravated by Climate Change. Nat. Clim. Chang. 2022, 12, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur Compounds: A Review of Their Anti-Inflammatory Effects in Human Health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef]
- Rosenthal, A.S.; Chen, X.; Liu, J.O.; West, D.C.; Hergenrother, P.J.; Shapiro, T.A.; Posner, G.H. Malaria-Infected Mice Are Cured by a Single Oral Dose of New Dimeric Trioxane Sulfones Which Are Also Selectively and Powerfully Cytotoxic to Cancer Cells. J. Med. Chem. 2009, 52, 1198–1203. [Google Scholar] [CrossRef] [Green Version]
- Shenai, B.R.; Lee, B.J.; Alvarez-Hernandez, A.; Chong, P.Y.; Emal, C.D.; Neitz, R.J.; Roush, W.R.; Rosenthal, P.J. Structure-Activity Relationships for Inhibition of Cysteine Protease Activity and Development of Plasmodium falciparum by Peptidyl Vinyl Sulfones. Antimicrob. Agents Chemother. 2003, 47, 154–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernsdorfer, W.H.; Landgraf, B.; Kilimali, V.A.E.B.; Wernsdorfer, G. Activity of Benflumetol and Its Enantiomers in Fresh Isolates of Plasmodium falciparum from East Africa. Acta Trop. 1998, 70, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Souri, E.; Nateghpour, M.; Farsam, H.; Kaji, Z.; Hamedi, Y.; Amanlou, M. In Vitro Activity of Mefloquine and Its Enantiomers against Plasmodium falciparum. Iran. J. Pharmacol. Ther. 2002, 1, 17–19. [Google Scholar]
- Schöbel, J.-H.; Passia, M.T.; Wolter, N.A.; Puttreddy, R.; Rissanen, K.; Bolm, C. 1,2,6-Thiadiazine 1-Oxides: Unsaturated Three-Dimensional S,N-Heterocycles from Sulfonimidamides. Org. Lett. 2020, 22, 2702–2706. [Google Scholar] [CrossRef]
- Amundsen, L.H.; Nelson, L.S. Reduction of Nitriles to Primary Amines with Lithium Aluminum Hydride. J. Am. Chem. Soc. 1951, 73, 242–244. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]

| Compound | IC50 (µM) CQ-Sensitive Strain 3D7 | IC90 (µM) | IC50 (µM) CQ-Resistant Strain Dd2 |
|---|---|---|---|
| 1 | 3.8 ± 0.66 | 34.2 | |
| 2 | 3.12 ± 0.58 | 27.9 | |
| 3 | 22.1±1.06 | ||
| 4 | 9.1 ± 2.59 | ||
| 5 | 2.2 ± 0.64 | 19.8 | 6.8 ± 1.54 |
| 6 | 10.6 ± 1.53 | ||
| 7 | 35.8 ± 5.11 | ||
| 8 | 12.5 ± 3.27 | ||
| 9 | 3.3 ± 1.56 | 29.7 | 6.5 ± 0.87 |
| 10 | 46.4 ± 2.67 | ||
| 11 | 12 ± 4.28 | ||
| 12 | 21.3 ± 4.05 | ||
| 13 | 20.3 ± 1.69 | ||
| 14 | 10.3 ± 0.90 | ||
| 15 | 5.2 ± 1.95 | 46.8 | |
| 16 | 3.1 ± 0.26 | 27.9 | 4.4 ± 0.59 |
| 17 | 2.6 ± 0.70 | 23.4 | 4 ± 0.28 |
| 18 | 26.5 ± 1.19 | ||
| 19 | 31.5 ±4.9 | ||
| 20 | 8 ± 0.53 | ||
| 21 | 14.1 ± 1.50 | ||
| 22 | 15.9 ± 1.46 | ||
| 23 | 24.95 ± 1.06 | ||
| Chloroquine | 0.02 ± 0.004 | 0.14 ± 0.063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngwa, C.J.; Stratmann, R.; Musabyimana, J.P.; Pannen, K.; Schöbel, J.-H.; Frings, M.; Schiffers, I.; Quaranta, C.; Koschmieder, S.; Chatain, N.; et al. Evaluation of Chiral Organosulfur Compounds on Their Activity against the Malaria Parasite Plasmodium falciparum. Trop. Med. Infect. Dis. 2022, 7, 416. https://doi.org/10.3390/tropicalmed7120416
Ngwa CJ, Stratmann R, Musabyimana JP, Pannen K, Schöbel J-H, Frings M, Schiffers I, Quaranta C, Koschmieder S, Chatain N, et al. Evaluation of Chiral Organosulfur Compounds on Their Activity against the Malaria Parasite Plasmodium falciparum. Tropical Medicine and Infectious Disease. 2022; 7(12):416. https://doi.org/10.3390/tropicalmed7120416
Chicago/Turabian StyleNgwa, Che Julius, Rabea Stratmann, Jean Pierre Musabyimana, Kristina Pannen, Jan-Hendrik Schöbel, Marcus Frings, Ingo Schiffers, Calogero Quaranta, Steffen Koschmieder, Nicolas Chatain, and et al. 2022. "Evaluation of Chiral Organosulfur Compounds on Their Activity against the Malaria Parasite Plasmodium falciparum" Tropical Medicine and Infectious Disease 7, no. 12: 416. https://doi.org/10.3390/tropicalmed7120416
APA StyleNgwa, C. J., Stratmann, R., Musabyimana, J. P., Pannen, K., Schöbel, J.-H., Frings, M., Schiffers, I., Quaranta, C., Koschmieder, S., Chatain, N., Pradel, G., & Bolm, C. (2022). Evaluation of Chiral Organosulfur Compounds on Their Activity against the Malaria Parasite Plasmodium falciparum. Tropical Medicine and Infectious Disease, 7(12), 416. https://doi.org/10.3390/tropicalmed7120416








