Predicting the Impact of Climate Change on the Distribution of a Neglected Arboviruses Vector (Armigeres subalbatus) in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Occurrence Data
2.2. Environmental Predictor Variables
2.3. Species Distribution Modeling
2.4. Evaluation of Changes in Potential Suitable Areas
3. Results
3.1. Observed Distributions and Climate Factors Selected
3.2. Model Performance
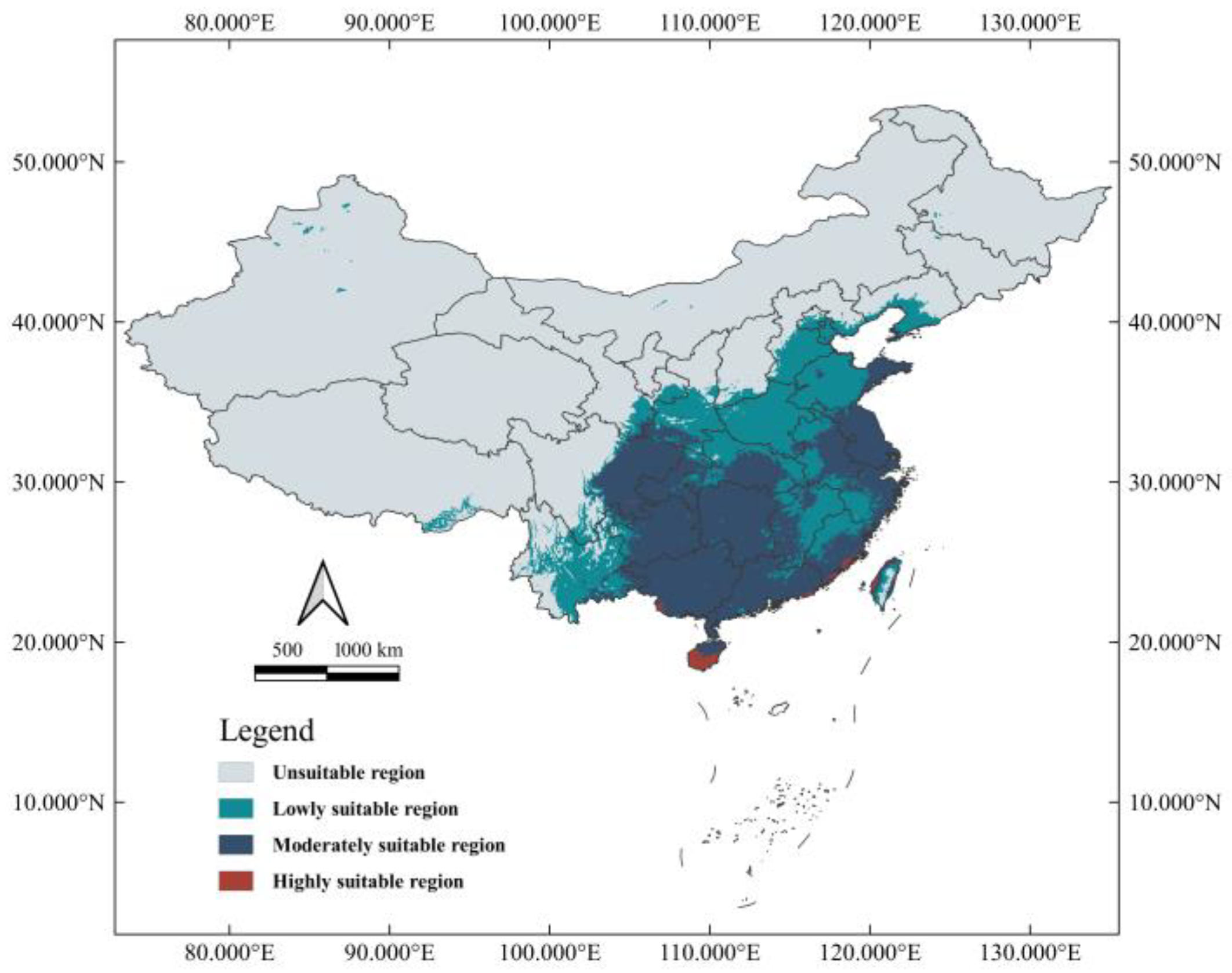
3.3. Current Distribution of Suitable Habitat
3.4. Important Bioclimatic Variables
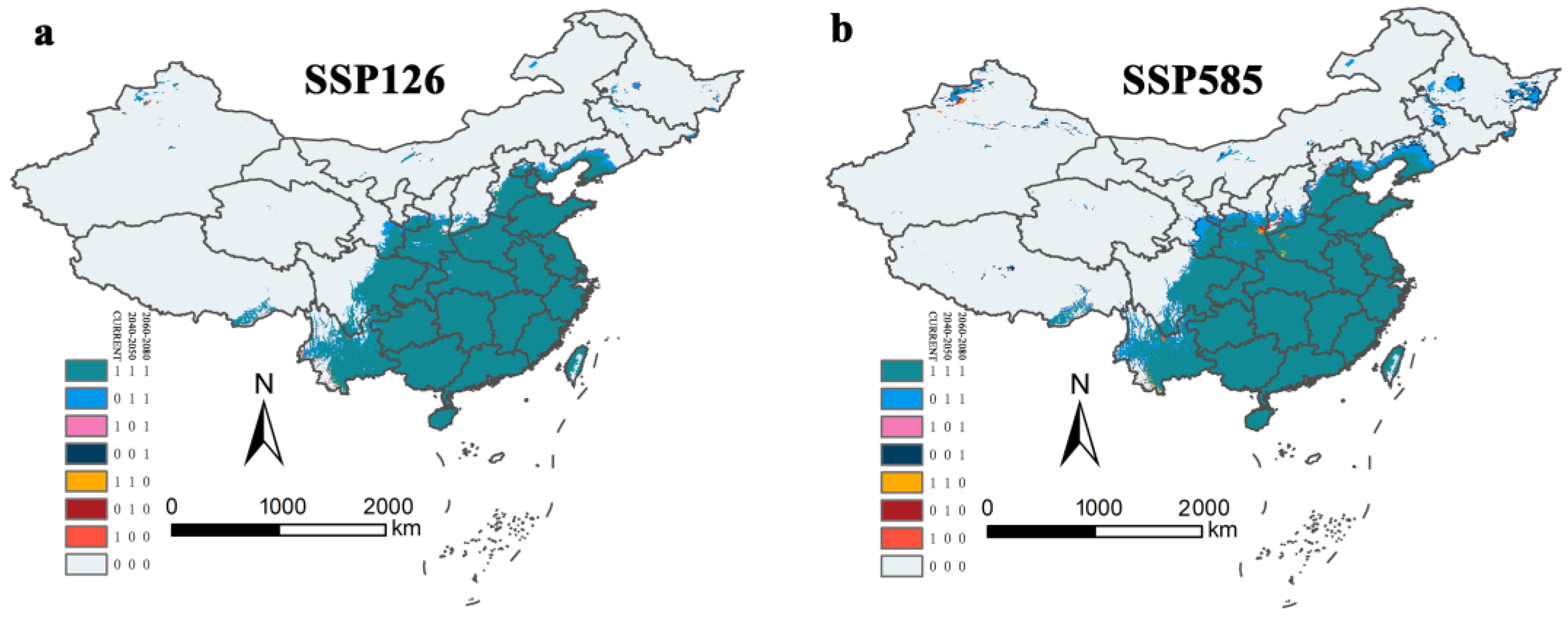
3.5. Potential Suitable Areas for Ar. subalbatus under Future Climate Scenarios
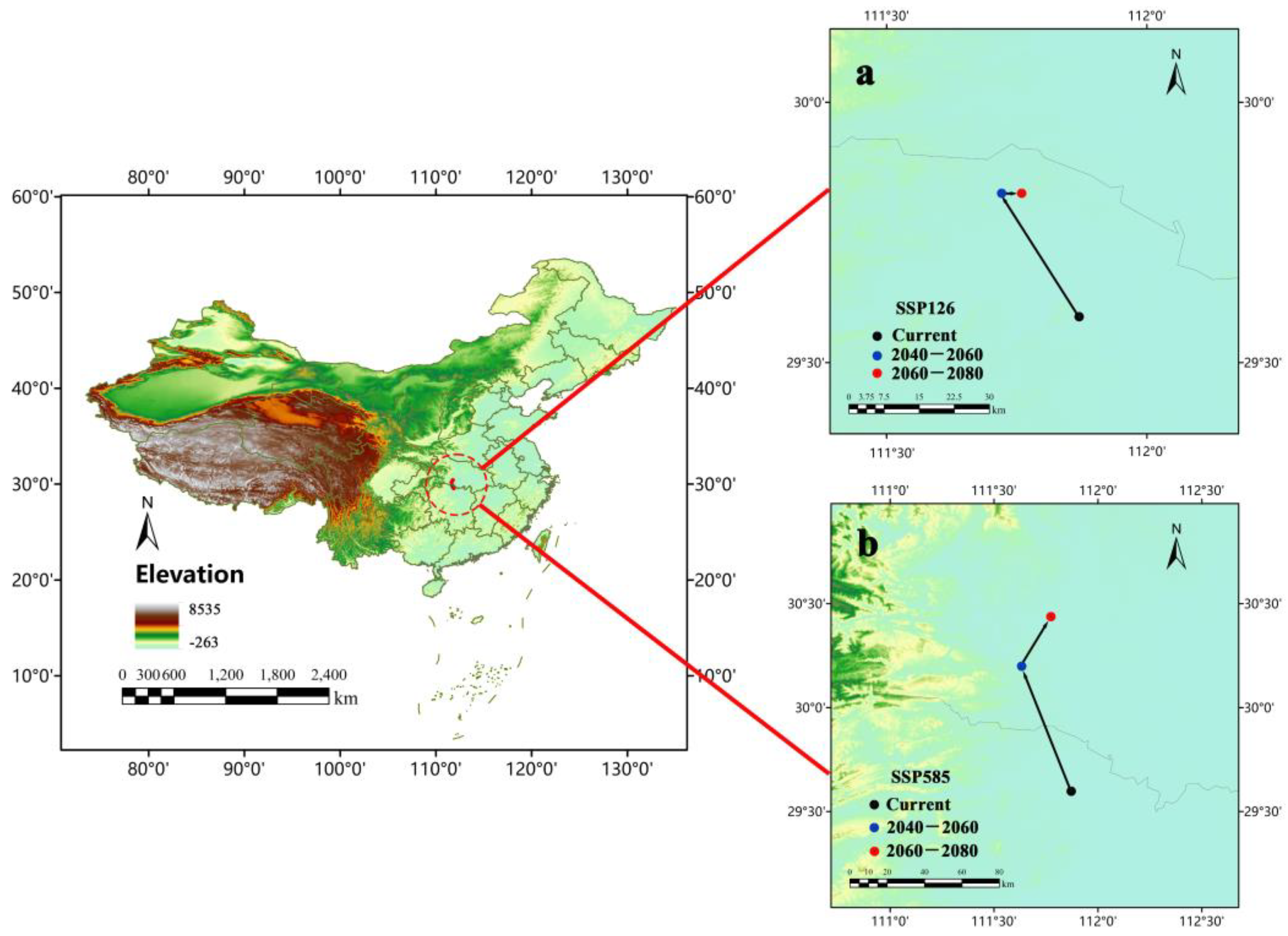
3.6. Centroid Shift and Potential Suitable Areas
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gage, K.L.; Burkot, T.R.; Eisen, R.J.; Hayes, E.B. Climate and Vectorborne Diseases. Am. J. Prev. Med. 2008, 35, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Lutambi, A.M.; Penny, M.A.; Smith, T.; Chitnis, N. Mathematical modelling of mosquito dispersal in a heterogeneous environment. Math. Biosci. 2013, 241, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.; Ternova, L.; Parasnis, S.A.; Kovaleva, M.; Nagy, G.J. Climate Change and Zoonoses: A Review of Concepts, Definitions, and Bibliometrics. Int. J. Environ. Res. Public Health 2022, 19, 893. [Google Scholar] [CrossRef]
- Outammassine, A.; Zouhair, S.; Loqman, S. Rift Valley Fever and West Nile virus vectors in Morocco: Current situation and future anticipated scenarios. Transbound. Emerg. Dis. 2022, 69, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef] [Green Version]
- Bueno-Mari, R.; Jimenez-Peydro, R. Global change and human vulnerability to vector-borne diseases. Front. Physiol. 2013, 4, 158. [Google Scholar] [CrossRef] [Green Version]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef] [Green Version]
- Ng, V.; Rees, E.E.; Lindsay, L.R.; Drebot, M.A.; Brownstone, T.; Sadeghieh, T.; Khan, S.U. Could exotic mosquito-borne diseases emerge in Canada with climate change? Can. Commun. Dis. Rep. 2019, 45, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R. Mosquitoes on the move. Science 2016, 354, 971–972. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Brooks, D.R. Evolution in action: Climate change, biodiversity dynamics and emerging infectious disease. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370, 20130553. [Google Scholar] [CrossRef]
- Heffernan, C. Climate change and multiple emerging infectious diseases. Vet. J. 2018, 234, 43–47. [Google Scholar] [CrossRef]
- Liang, L.; Gong, P. Climate change and human infectious diseases: A synthesis of research findings from global and spatio-temporal perspectives. Environ. Int. 2017, 103, 99–108. [Google Scholar] [CrossRef]
- Ogden, N.H.; Gachon, P. Climate change and infectious diseases: What can we expect? Can. Commun. Dis. Rep. 2019, 45, 76–80. [Google Scholar] [CrossRef]
- Garcia, E.; Yactayo, S.; Nishino, K.; Millo, V.; Perea, W.; Briand, S. Zika virus infection: Global update on epidemiology and potentially associated clinical manifestations. Wkly. Epidemiol. Rec. 2016, 91, 73–81. [Google Scholar]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenco-de-Oliveira, R.; Failloux, A.B. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef]
- Pei-Sze Jeslyn, W.; Mei-zhi Irene, L.; Chee-Seng, C.; Lee-Ching, N.; Cheong-Huat, T. Aedes (Stegomyia) albopictus (Skuse): A Potential Vector of Zika Virus in Singapore. PLoS Negl. Trop. Dis. 2013, 7, e2348. [Google Scholar]
- Li, C.X.; Guo, X.X.; Deng, Y.Q.; Liu, Q.M.; Xing, D.; Sun, A.J.; Wu, Q.; Dong, Y.D.; Zhang, Y.M.; Zhang, H.D.; et al. Susceptibility of Armigeres subalbatus Coquillett (Diptera: Culicidae) to Zika virus through oral and urine infection. PLoS Negl. Trop. Dis. 2020, 14, e0008450. [Google Scholar] [CrossRef]
- Lin, D.T.; Zheng, X.Y.; Sanogo, B.; Ding, T.; Sun, X.; Wu, Z.D. Bacterial composition of midgut and entire body of laboratory colonies of Aedes aegypti and Aedes albopictus from Southern China. Parasites Vectors 2021, 14, 586. [Google Scholar] [CrossRef]
- Diagne, C.T.; Diallo, D.; Faye, O.; Ba, Y.; Faye, O.; Gaye, A.; Dia, I.; Faye, O.; Weaver, S.C.; Sall, A.A.; et al. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect. Dis. 2015, 15, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braack, L.; Paulo Gouveia de Almeida, A.; Cornel, A.J.; Swanepoel, R.; De Jager, C. Mosquito-borne arboviruses of African origin: Review of key viruses and vectors. Parasites Vectors 2018, 11, 29. [Google Scholar] [CrossRef]
- Outammassine, A.; Zouhair, S.; Loqman, S. Global potential distribution of three underappreciated arboviruses vectors (Aedes japonicus, Aedes vexans and Aedes vittatus) under current and future climate conditions. Transbound. Emerg. Dis. 2022, 69, E1160–E1171. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Q.; Zhao, S.Y.; Xie, Y.G.; Liu, T.; Kong, L.; Guo, Y.J.; Xie, Z.S.; Liu, P.W.; Chen, X.G. Armigeres subalbatus is a potential vector for Zika virus but not dengue virus. Infect. Dis. Poverty 2022, 11, 62. [Google Scholar] [CrossRef]
- Fu, S.; Song, S.; Liu, H.; Li, Y.; Li, X.; Gao, X.; Xu, Z.; Liu, G.; Wang, D.; Tian, Z.; et al. ZIKA virus isolated from mosquitoes: A field and laboratory investigation in China, 2016. Sci. China-Life Sci. 2017, 60, 1364–1371. [Google Scholar] [CrossRef]
- Phumee, A.; Buathong, R.; Boonserm, R.; Intayot, P.; Aungsananta, N.; Jittmittraphap, A.; Joyjinda, Y.; Wacharapluesadee, S.; Siriyasatien, P. Molecular Epidemiology and Genetic Diversity of Zika Virus from Field-Caught Mosquitoes in Various Regions of Thailand. Pathogens 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Zhou, H.; Gong, Z. Insecta. VOIII. Diptera: Culicidae: Yunnan Mosquito Fauna; Yunnan Science & Technology Press: Kunming, China, 2009; p. 3. [Google Scholar]
- Zheng, Y.; Li, M.; Wang, H.; Liang, G. Japanese encephalitis and Japanese encephalitis virus in mainland China. Rev. Med. Virol. 2012, 22, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Fuchs, J.F.; Rocheleau, T.A.; Clark, A.K.; Hillyer, J.F.; Chen, C.; Christensen, B.M. Mosquito Transcriptome Profiles and Filarial Worm Susceptibility in Armigeres subalbatus. PLoS Negl. Trop. Dis. 2010, 4, e666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wang, Y.; Wang, Z.; Ding, W.; Xu, K.; Li, L.; Wang, Y.; Li, J.; Yang, M.; Liu, X.; et al. Modelling the current and future potential distribution of the bean bug Riptortus pedestris with increasingly serious damage to soybean. Pest Manag. Sci. 2020, 78, 4340–4352. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.J.; Hijmans, R.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.P.; Liu, G.Q.; Bu, W.J.; Gao, Y.B. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar]
- Fan, J.; Wu, P.; Tian, T.; Ren, Q.; Haseeb, M.; Zhang, R. Potential distribution and niche differentiation of Spodoptera frugiperda in Africa. Insects 2020, 11, 383. [Google Scholar] [CrossRef]
- Raffini, F.; Bertorelle, G.; Biello, R.; D’Urso, G.; Russo, D.; Bosso, L. From nucleotides to satellite imagery: Approaches to identify and manage the invasive patho-gen Xylella fastidiosa and its insect vectors in Europe. Sustainability 2020, 12, 4508. [Google Scholar] [CrossRef]
- Srivastava, V.; Roe, A.D.; Keena, M.A.; Hamelin, R.C.; Griess, V.C. Oh the places they’ll go: Improving species distribution modelling for invasive forest pests in an uncertain world. Biol. Invasions 2021, 23, 297–349. [Google Scholar] [CrossRef]
- Wiens, J.A.; Stralberg, D.; Jongsomjit, D.; Howell, C.A.; Snyder, M.A. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc. Natl. Acad. Sci. USA 2009, 106, 19729–19736. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.H.; Masuoka, P.; Klein, T.A.; Kim, H.C.; Somer, T.; Grieco, J. Ecological niche modeling to estimate the distribution of Japanese encephalitis virus in Asia. PLoS Negl. Trop. Dis. 2012, 6, 119–121. [Google Scholar] [CrossRef] [Green Version]
- González, C.; Wang, O.; Strutz, S.E.; González-Salazar, C.; Sánchez-Cordero, V.; Sarkar, S. Climate change and risk of leishmaniasis in North America: Predictions from ecological niche models of vector and reservoir species. PLoS Negl. Trop. Dis. 2010, 4, e585. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Gao, X.; Ma, J.; Jiao, Z.; Xiao, J.; Hayat, M.A.; Wang, H. Modeling the present and future distribution of arbovirus vectors Aedes aegypti and Aedes albopictus under climate change scenarios in Mainland China. Sci. Total Environ. 2019, 664, 203–214. [Google Scholar] [CrossRef]
- Chaves, L.F.; Imanishi, N.; Hoshi, T. Population dynamics of Armigeres subalbatus (Diptera: Culicidae) across a temperate altitudinal gradient. Bull. Entomol. Res. 2015, 105, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Kadmon, R.; Farber, O.; Danin, A. Effect of roadside bias on the accuracy of predictive maps produced by bioclimatic models. Ecol. Appl. 2004, 14, 401–413. [Google Scholar] [CrossRef]
- Edren, S.M.C.; Wisz, M.S.; Teilmann, J.; Dietz, R.; Söderkvist, T. Modelling spatial patterns in harbour porpoise satellite telemetry data using maximum entropy. Ecography 2010, 33, 698–708. [Google Scholar] [CrossRef]
- Jennings, A.P.; Veron, G. Predicted distributions and ecological niches of 8 civet and mongoose species in Southeast Asia. J. Mammal. 2011, 92, 316–327. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Brown, J.L.; Hill, D.J.; Dolan, A.M.; Carnaval, A.C.; Haywood, A.M. PaleoClim, high spatial resolution paleoclimate surfaces for global land areas. Sci. Data 2018, 5, 180254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef] [Green Version]
- Heikkinen, R.K.; Araújo, M.B.; Virkkala, R.; Thuiller, W.; Sykes, M.T. Methods and uncertainties in bioclimatic envelop modeling under climate change. Prog. Phys. Geogr. Earth Environ. 2006, 30, 751–777. [Google Scholar] [CrossRef] [Green Version]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Escobar, L.E.; Lira-Noriega, A.; Medina-Vogel, G.; Peterson, A.T. Potential for spread of the white-nose fungus (Pseudogymnoascus destructans) in the Americas: Use of Maxent and NicheA to assure strict model transference. Geospat. Health 2014, 9, 221–229. [Google Scholar] [CrossRef]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the global Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 2018, 13, e0210122. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, M.; Das, B.; Ramesh, R. Predicting climate change impacts on potential worldwide distribution of fall armyworm based on CMIP6 projections. J. Pest Sci. 2022, 95, 841–854. [Google Scholar] [CrossRef]
- Chen, S.; Ding, F.; Hao, M.; Jiang, D. Mapping the Potential Global Distribution of Red Imported Fire Ant (Solenopsis invicta Buren) Based on a Machine Learning Method. Sustainability 2020, 12, 10182. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, Z.; Ma, J.; Gao, X.; Xiao, J.; Hayat, M.A.; Wang, H. Modelling the potential distribution of arbovirus vector Aedes aegypti under current and future climate scenarios in Taiwan, China. Pest Manag. Sci. 2019, 75, 3076–3083. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for MAXENT ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Boyce, M.S.; Vernier, P.R.; Nielsen, S.E.; Schmiegelow, F.K.A. Evaluating resource selection functions. Ecol. Model. 2022, 157, 281–300. [Google Scholar] [CrossRef] [Green Version]
- Engler, R.; Guisan, A.; Rechsteiner, L. An improved approach for pre-dicting the distribution of rare and endangered species from occurrence and pseudo-absence data. J. Appl. Ecol. 2004, 41, 263–274. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011; p. 328. [Google Scholar]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunze, S.; Kochmann, J.; Klimpel, S. Global occurrence data improve potential distribution models for Aedes japonicus japonicus in non-native regions. Pest Manag. Sci. 2020, 76, 1814–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Wang, S.; Xie, W.; Yan, X. Evalution on CMIP6 Model Simulation of the Diurnal Temperature Range over China. Clim. Environ. Res. 2022, 27, 79–93. [Google Scholar]
- Barr, A.R.; Chellappah, W.T. The colonization and laboratory maintenance of Armigeres subalbatus (Coquillett). Bull. World Health Organ. 1964, 31, 439. [Google Scholar] [PubMed]
- Amerasinghe, F.P.; Munasingha, N.B. A predevelopment mosquito survey in the Mahaweli Development Project area, Sri Lanka: Adults. J. Med. Entomol. 1988, 25, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Scott, T.W.; Morrison, A.C.; Takada, T. Hot temperatures can force delayed mosquito outbreaks via sequential changes in Aedes aegypti demographic parameters in autocorrelated environments. Acta Trop. 2014, 129, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Ogden, N.H. Climate change and vector-borne diseases of public health significance. FEMS Microbiol. Lett. 2017, 364, fnx186. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Gao, X.; Zheng, K.; Ma, J.; Jiao, Z.; Xiao, J.; Wang, H. The potential distribution and dynamics of important vectors Culex pipiens pallens and Culex pipiens quinquefasciatus in China under climate change scenarios: An ecological niche modelling approach. Pest Manag. Sci. 2020, 76, 3096–3107. [Google Scholar] [CrossRef]







| Bioclimatic Variable | Description | Included |
|---|---|---|
| Bio01 | Annual mean temperature | |
| Bio02 | Mean diurnal temperature range | Yes |
| Bio03 | Isothermality | Yes |
| Bio04 | Temperature seasonality | |
| Bio05 | Max temperature of the warmest month | Yes |
| Bio06 | Min temperature of the coldest month | |
| Bio07 | Annual temperature range | |
| Bio08 | Mean temperature of the wettest quarter | |
| Bio09 | Mean temperature of the driest quarter | |
| Bio10 | Mean temperature of the warmest quarter | |
| Bio11 | Mean temperature of the coldest quarter | |
| Bio12 | Annual precipitation | Yes |
| Bio13 | Precipitation of the wettest month | |
| Bio14 | Precipitation of the driest month | Yes |
| Bio15 | Precipitation seasonality | Yes |
| Bio16 | Precipitation of the wettest quarter | |
| Bio17 | Precipitation of the driest quarter | |
| Bio18 | Precipitation of the warmest quarter | |
| Bio19 | Precipitation of the coldest quarter |
| Time Period | SSPS Scenarios | Area Change (%) | |||
|---|---|---|---|---|---|
| Range Expansion | No Change | Range Contraction | Net Change | ||
| 2040–2060 | SSP126 | 4.193% | 78.385% | 0.160% | 4.033% |
| 2040–2060 | SSP585 | 7.755% | 78.466% | 0.079% | 7.675% |
| 2060–2080 | SSP126 | 4.151% | 78.388% | 0.157% | 3.994% |
| 2060–2080 | SSP585 | 10.144% | 78.140% | 0.404% | 9.739% |
| Present or Absent in Different Periods | Areas under Climate Scenarios (km2) | |||
|---|---|---|---|---|
| CURRENT | 2045–2060 | 2060–2070 | SSP126 | SSP585 |
| 1 | 1 | 1 | 36,378.97 | 36,286.65 |
| 0 | 1 | 1 | 18,778.39 | 36,541.99 |
| 1 | 0 | 1 | 249.004 | 46.98122 |
| 0 | 0 | 1 | 17,030.69 | 15,327.62 |
| 1 | 1 | 0 | 2208.117 | 15,644.75 |
| 0 | 1 | 0 | 17,970.32 | 26,192.03 |
| 1 | 0 | 0 | 5496.802 | 26,192.03 |
| 0 | 0 | 0 | 91,431.79 | 3735.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Zhang, D.; Khan, J.; Guo, J.; Feng, Q.; Sun, Y.; Li, B.; Wu, Y.; Wu, Z.; Zheng, X. Predicting the Impact of Climate Change on the Distribution of a Neglected Arboviruses Vector (Armigeres subalbatus) in China. Trop. Med. Infect. Dis. 2022, 7, 431. https://doi.org/10.3390/tropicalmed7120431
Wang G, Zhang D, Khan J, Guo J, Feng Q, Sun Y, Li B, Wu Y, Wu Z, Zheng X. Predicting the Impact of Climate Change on the Distribution of a Neglected Arboviruses Vector (Armigeres subalbatus) in China. Tropical Medicine and Infectious Disease. 2022; 7(12):431. https://doi.org/10.3390/tropicalmed7120431
Chicago/Turabian StyleWang, Gang, Dongjing Zhang, Jehangir Khan, Jiatian Guo, Qingdeng Feng, Yan Sun, Beiqing Li, Yu Wu, Zhongdao Wu, and Xiaoying Zheng. 2022. "Predicting the Impact of Climate Change on the Distribution of a Neglected Arboviruses Vector (Armigeres subalbatus) in China" Tropical Medicine and Infectious Disease 7, no. 12: 431. https://doi.org/10.3390/tropicalmed7120431
APA StyleWang, G., Zhang, D., Khan, J., Guo, J., Feng, Q., Sun, Y., Li, B., Wu, Y., Wu, Z., & Zheng, X. (2022). Predicting the Impact of Climate Change on the Distribution of a Neglected Arboviruses Vector (Armigeres subalbatus) in China. Tropical Medicine and Infectious Disease, 7(12), 431. https://doi.org/10.3390/tropicalmed7120431






