Abstract
Delayed diagnosis of tuberculosis (TB) increases mortality and extends the duration of disease transmission. This study aimed to identify significant ICD-10 admission diagnoses preceding TB. All hospital electronic medical records from fiscal year 2015 to 2020 in the Songkhla Province, Thailand were retrieved. After excluding diabetes and HIV patients, a case-control analysis was performed. Exposures of interest were ICD-10 diagnoses on admissions 1–12 months prior to the visit during which TB was detected. Incident cases of respiratory tuberculosis (A15.0–A16.9) that had been admitted with at least one such exposure were chosen. For every case, controls were retrieved from weekly concurrent OPD patients who had the same 10-year interval of age, sex, and preceding admission and discharge week as the case. The 10 most common comorbidities during hospitalization preceding TB with their relative odds ratios (RORs) and 95% confidence intervals were identified. These included five significant exposures related to lower respiratory infection without adequate TB investigation. Significant RORs ranged from 3.10 (unspecified pneumonia) to 34.69 (hemoptysis). Full TB investigation was not performed due to problems with health insurance. In conclusion, the physicians should be informed about this pitfall, and the insurance system should be revised accordingly.
1. Introduction
Tuberculosis (TB) is a chronic infectious disease that is one of the 10 leading causes of death in lower- and middle-income countries [1]. Since TB is an airborne disease, aerosolization of droplet nuclei is the most likely mode of exposure from an infected individual to others [2]. Once a person is infected, TB can affect any body part, particularly the lungs. TB is active if the disease manifests in the affected part. If the immune response inhibits the development of TB and no disease manifestation occurs, the infected person has latent TB infection (LTBI) [3]. LTBI is not contagious, but it can become active TB if the immune response is unable to stop TB from growing [4]. The incubation period of active TB usually ranges from 1 month to 2 years (90% within 1–12 months), after which the chance of developing active TB wanes with time [5]. Additionally, without a potential screening system, by the time that TB is diagnosed, is often too late to prevent transmission [6].
Thailand was one of the 30 countries with the highest TB burden in 2020 [2]. More than 100,000 active TB cases are reported annually [7]. A root cause of this high incidence is delayed TB diagnosis, which is not easily remedied, especially in poor socioeconomic settings [8]. TB can mimic many diseases in the meninges, lungs, and abdomen [9,10,11], thereby leading to misdiagnosis. Moreover, a review of TB admission in 2009 showed that approximately 20% of TB cases were admitted without knowing that the patients had active TB, and TB disease proved fatal for 20% of those without TB detection [12]. For the remainder of the patients, TB was usually detected when the patients were scheduled for follow-up at the outpatient department (OPD). It is hypothesized that missing TB detection at this stage leads to in-hospital and household transmission.
Most previous studies on disease-associated TB were case-control or cohort studies assessing the effect of well-known diseases, such as human immunodeficiency virus (HIV), diabetes mellitus (DM), and other diseases or behavioral factors based on medical conjectures [13,14,15,16,17,18,19,20,21]. In Thailand, TB screening in DM and HIV patients has been practiced, but efforts to detect active TB in the general population are lacking [22,23]. Additional strategies for early diagnosis of active TB, isolation, and initiation of treatment to minimize disease severity are therefore required. Hence, additional knowledge regarding the early diagnosis of TB in Thailand should be specifically assimilated, and the application of this knowledge in poor socioeconomic settings is essential.
Using electronic health records (EHRs), we introduced an approach to identify comorbidities or manifestations that precede TB based on a case-control study to develop a novel policy for early diagnosis of TB. Thus, this study aimed to identify the 10 most commonly hospitalized comorbidities preceding TB disease presentation and measure the significance of these covariates.
2. Materials and Methods
2.1. Concept and Study Design
The concept of this study is based on a case-control design. Hospitalized comorbidities predicting TB could be more common among subsequent TB cases than among hospital controls who were not subsequently identified with TB disease.
The comorbidities of DM and HIV are well known among practicing physicians to be clinical risk factors for TB [22,23] and any TB disease diagnosis accompanying these comorbidities is less likely to be missed during hospital admission [12]. Therefore, we investigated what other comorbid conditions could predict subsequent active TB.
2.2. Data Collection and Retrieval
Relational data from in-patient (IP) and outpatient (OP) electronic medical records from 17 public hospitals (2 tertiary and 15 secondary levels) in Songkhla Province covering the time period April 1, 2015 to October 28, 2020 were deidentified and encrypted for personal information and retrieved from e-claim data of the 12th Regional National Health Security Office (NHSO). Data were previously audited for claim and medical expense information and were collected without missing information on age, sex, service codes, date of services, and ICD-10 diagnoses. Data of patients who lived outside Songkhla Province were excluded. IP and OP records with ICD-10 diagnoses in the categories F, H, O-Q, S, T, and V-Z were eliminated according to the reasons explained in Table 1. Patients without hospital admission history and those aged under 1 year or over 80 years were excluded. The remaining data were used to select cases and controls.

Table 1.
Categories for the exclusion of irrelevant diseases.
2.3. Case Ascertainment
Cases were OP TB patients coded as A15.0–A16.9 from 1 January 2016 to 28 October 2020. Cases without any preceding admission within 1–12 months before the date of diagnosis or with HIV and or DM were excluded. Besides ICD-10 codes, TB diagnosis was confirmed based on available pharmacy data for the allocation of prescribed anti-TB drug treatment regimens for smear-positive patients [24]. Individuals having a culture-negative TB confirmation were excluded.
2.4. Control Selection
For every case, controls without HIV or DM were retrieved from weekly concurrent OP who had the same 10-year interval of age, sex, and preceding admission and discharge week as the case. If many admissions could be matched, only the oldest one was selected. Those who had been receiving any anti-TB drugs were excluded.
2.5. Matching Ratio and Matching Process
The ratio between cases and controls in each matched set varied depending on the number of cases and available controls for each particular week. The number of controls was limited to 4 per case (1:1, 1:2, 1:3, or 1:4). When more than four eligible controls were available, only four subjects were randomly selected. The looping process for matching was used until all cases were selected. Each case was randomly selected to match with eligible controls. The controls that were chosen could not be matched with the next case.
2.6. Outcome
The outcome variable was automatically defined from the subject selection as the TB case (outcome = 1) and control (outcome = 0).
2.7. Exposures
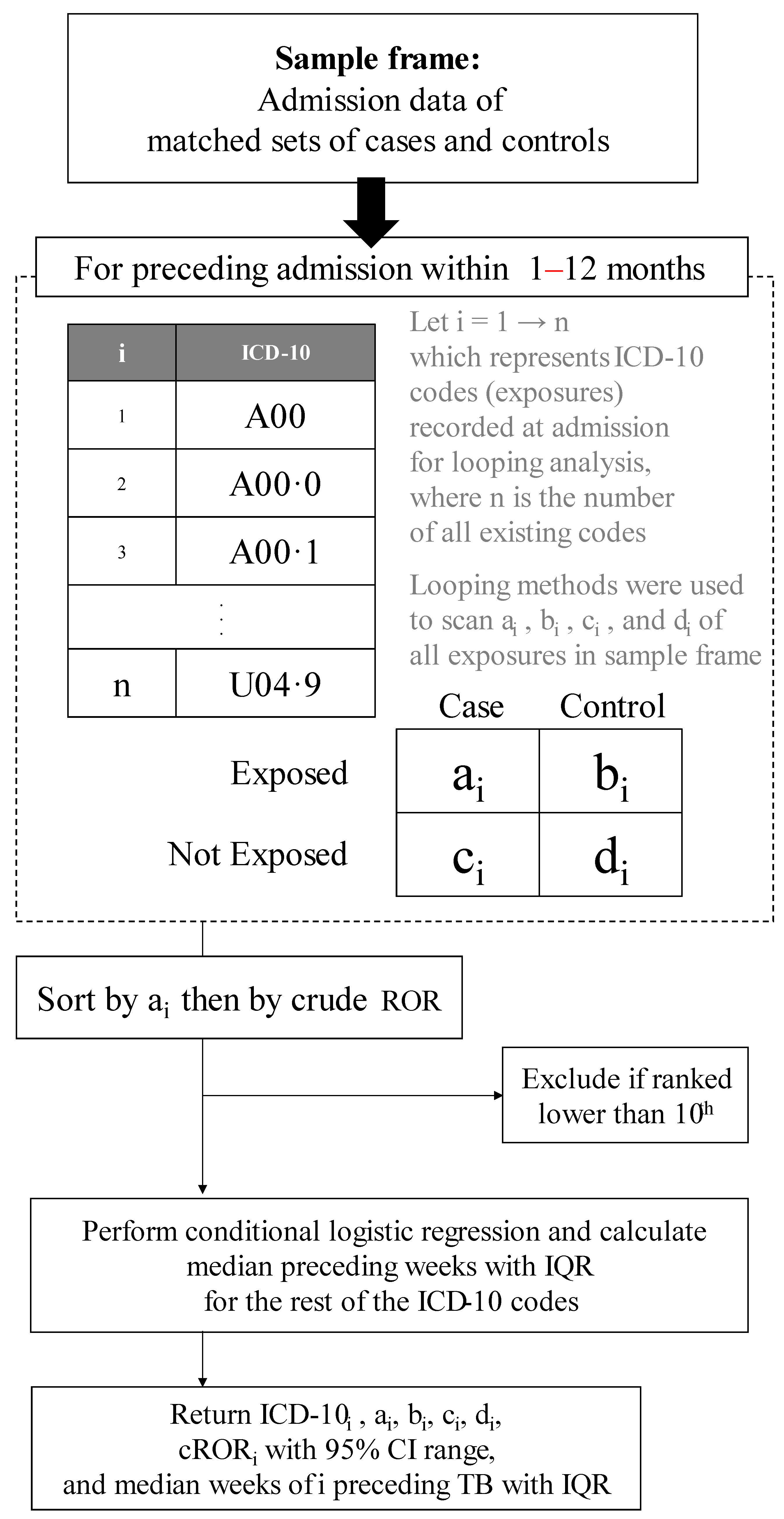
The period of available exposure for our exploration was 1 to 12 months before the initial diagnosis week of cases. Since 90% of active TB takes place after this period, we did not include admission beyond 12 months to reduce noise from irrelevant hospitalized comorbidities. The looping method was used to perform a 2 by 2 discordant tables can through all ICD-10 codes to select only the 10 most common ones, as shown in Figure 1. Each of these diseases was used as an exposure variable, one at a time: exposure = 1 for that disease, and 0 otherwise.
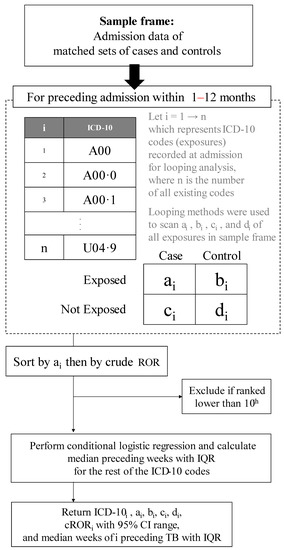
Figure 1.
Algorithm to rank the 10 commonly hospitalized comorbidities preceding tuberculosis. CI, confidence interval; cROR, conditional relative odds ratio; IQR, interquartile range; ROR, relative odds ratio; TB, tuberculosis.
2.8. Statistical Analysis
Matched sets were constructed based on perfect matching and the age-sex distribution was summarized in tables. The cases and controls who had the same matched variables were placed in the same match set. Due to the matching nature of the study design, the conditional relative odds ratio (cROR) was computed with a 95% confidence interval for each exposure. The preceding period since discharge of each exposure was also computed and reported as its median and interquartile range (IQR). This was repeated until the top 10 diagnoses were analyzed. All analyses were performed using the epiDisplay and tidyverse packages on R language and environment version 4.1.1 (R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). A p-value of ≤0.05 was considered statistically significant.
2.9. Further Exploration for Hospitalized Comorbidities Preceding
For statistically significant exposures, IP and OP records of all cases were re-reviewed to verify whether and how cases were investigated for bacteriologic evidence of TB.
3. Results
3.1. Matching Results and Their Characteristics
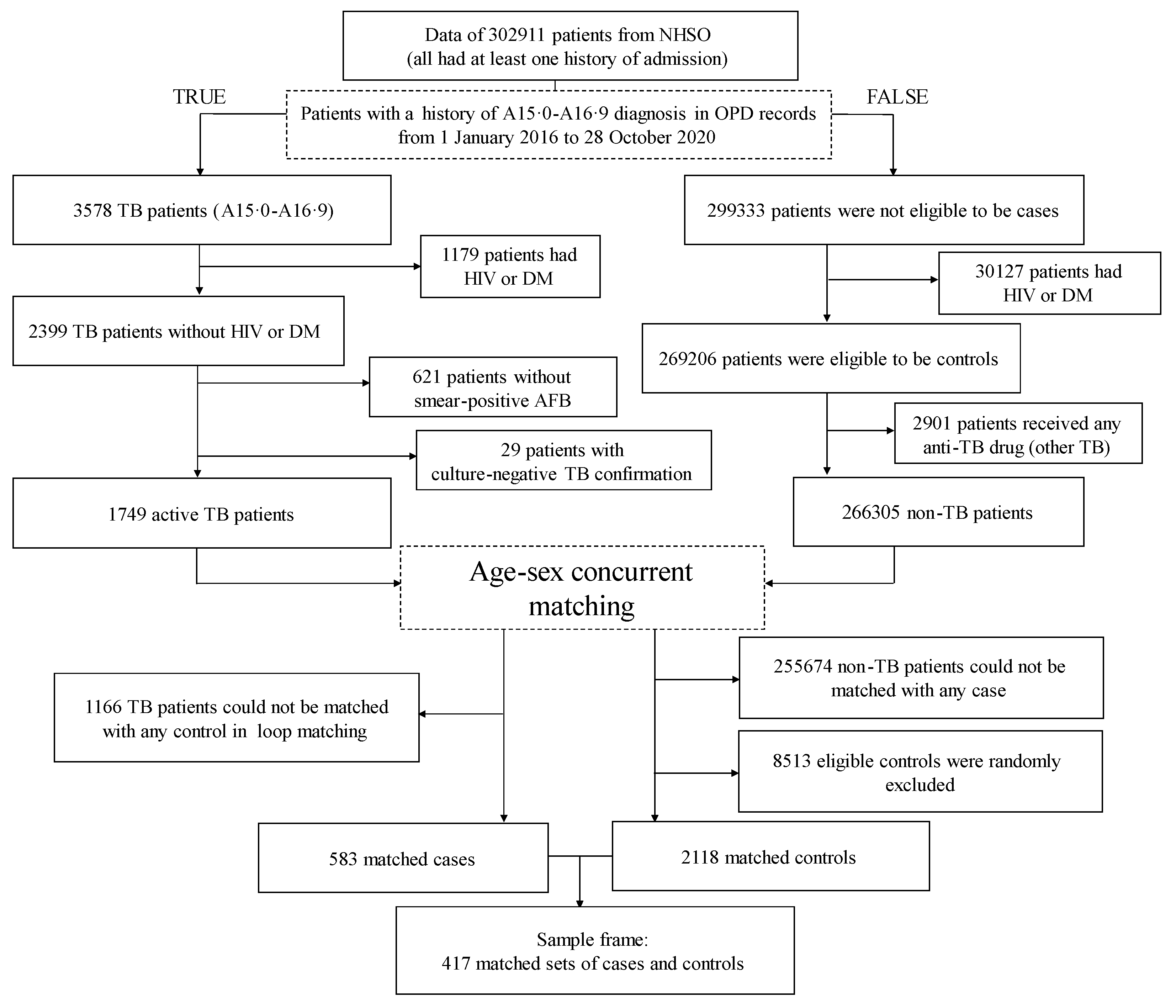
The numbers of patients included and excluded in the different steps of the case and control selection are shown in Figure 2. Eventually, we identified 417 matched sets (583 cases and 2118 controls). According to Table 2, over two-thirds of the cases were men. TB diagnosis was least common among individuals aged <20 years.
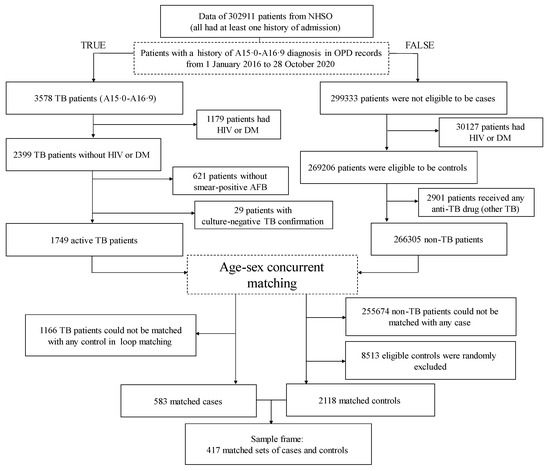
Figure 2.
Study flow chart. AFB, acid-fast bacilli; DM, diabetes mellitus; HIV, human immunodeficiency virus; NHSO, National Health Security Office; OPD, outpatient department; TB, tuberculosis.

Table 2.
Age and sex distribution of the matched sets.
3.2. Preceding Admitted Diseases Returned from the Looping Analysis
Table 3 shows the 10 most common exposures preceding TB among patients without HIV and DM. They were J18.9, E87.6, J15.9, E87.1, J90, R04.2, J44.1, J18.1, A09.9, and R50.9, respectively. The significant exposures were unspecified pneumonia (J18.9), unspecified bacterial pneumonia (J15.9), hypo-osmolality and hyponatremia (E87.1), unspecified pleural effusion (J90), hemoptysis (R04.2), and unspecified lobar pneumonia (J18.1). J18.9, J15.9, J90, R04.2, and J18.1 were mutually exclusive of each other. Of note, these 5 diagnoses were principal diagnoses of admission while E87.1 was not a principal diagnosis of any admission. Therefore, we listed the principal diagnoses in Table 4, and 75% of them were pneumonia. Each one had only one principal diagnosis.

Table 3.
Ten most frequent hospitalized comorbidities preceding tuberculosis.

Table 4.
Principal diagnoses accompanied by E87.1 among the TB cases.
3.3. Bacteriological Evidence for TB
All admissions with J18.9, J15.9, J90, R04.2, or J18.1 were investigated using sputum AFB. None of them showed a positive result. Neither the cartridge-based nucleic acid amplification test (Gene-Xpert, Cepheid, Sunnyvale, CA, USA) nor TB culture were used as they were not covered by insurance for patients with pneumonia.
For J90, with 10 (58.8%) of 17 cases having thoracentesis, pleural fluid samples were tested for AFB and all of them were negative. None of the fluid samples underwent adenosine deaminase (ADA) testing. Other profile data of the pleural fluid were not available.
4. Discussion
After excluding HIV, DM, and known LTBI, only one-quarter of the TB cases could be matched for this data mining. The 10 most common comorbidities preceding TB were mostly related to lower respiratory problems. All cases with lower respiratory symptoms had negative sputum AFB results. Additionally, hyponatremia was the most common accompanying diagnosis with pneumonia.
Our data re-confirmed the inadequate sensitivity of the sputum AFB test to detect TB among the hospitalized patients who manifested with lower respiratory problems. Pneumonic TB is a common TB manifestation; however, early TB is usually misdiagnosed especially without bacteriological evidence [25]. Without cavity formation, TB may grow slowly, resulting in a small number of Mycobacterium tuberculosis that cannot be detected by AFB [26].
Pleural effusion is an even more difficult clinical entity regarding obtaining its etiologic agent. A low yield for AFB and even Gene-Xpert is well known [27,28]. With the optimal cut-off point (>40 IU.L−1), the ADA test is more useful, with both the sensitivity and specificity being 80–100% [29]. However, this test is usually performed at a referral hospital along with other investigations. At rural hospitals, where TB is common, these suspected cases are either referred or discharged and followed up. A delay in TB diagnosis is therefore often inevitable.
Hemoptysis has been classically described as a manifestation of pulmonary TB [30]. The sensitivity of AFB to detect TB with hemoptysis is approximately 30% [31]. The finding that some patients with hemoptysis and negative AFB were later confirmed to have TB warrants further investigation.
The role of hyponatremia in the early manifestation of TB should be further investigated. Its presence may be a clue for the diagnosis of TB. A plausible mechanism may include a syndrome of inappropriate antidiuretic hormone secretion [32,33] and poor intake, which are associated with active TB [34].
Although universal health coverage in Thailand covers full TB investigation for suspects, hospitalized lower respiratory infections have not yet been included. A lack of awareness of TB’s ability to mimic these diseases combined with the lack of support regarding insurance has increased the pitfalls of missing TB in hospitalized patients. The problems should therefore be solved on both sides.
Our data may not have been adequate for the detection of small effect sizes of many comorbidities. The exclusion of HIV and DM patients may have eliminated other significant comorbidities. Our analysis did not take into account confounders because socioeconomic variables were not available in the current EHRs. ROR in this study compared the exposure of interest with other exposures. Hence, ROR could not be interpreted as a conventional odds ratio.
This study was conducted in Songkhla Province, a southern province of Thailand. These findings should be interpreted with caution before being applied to other settings.
5. Conclusions
The most common hospitalized comorbidities preceding TB were related to lower respiratory infection. This suggests that TB was missed in diagnosis as it often mimics other lower respiratory infections. In lower- and middle-income countries, physicians treating patients with lower respiratory infections should be reminded about this pitfall. Full investigation for possible pulmonary TB was not performed by the health system due to problems with health insurance. Therefore, physicians should be informed about this pitfall, and the insurance system should be revised accordingly.
Author Contributions
Conceptualization, P.K. and V.C.; Methodology, P.K. and V.C.; Software, P.K. and V.C.; Formal analysis, P.K.; Investigation, V.C.; Data curation, P.K. and V.C.; Writing—original draft preparation, P.K.; Writing—review and editing, V.C.; Visualization, P.K. and V.C.; Supervision, V.C.; Funding acquisition, P.K. and V.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fogarty International Center and the National Institute of Allergy and Infectious Diseases, of the National Institutes of Health under Award Number D43 TW009522. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Human Research Ethics Committee, Prince of Songkla University (REC 64555-18-4). Patient data were encrypted for personalized anonymization according to the Thai Personal Data Protection Act 2019, Thailand (PDPA). Data were received from the 12th Regional NHSO under a project approval granted by the Human Research Ethics Committee, Prince of Songkla University (REC 64555-18-4). Informed consent was not required.
Informed Consent Statement
Patient consent was waived because the study was based on a retrospective review of anonymized data.
Data Availability Statement
The data of the sample frame and codes used in the analysis are available in a GitHub repository. The full data sets of all patients in Songkhla Province could not be shared according to PDPA. For the GitHub repository of this study see at https://github.com/ponlagrit/tb_data_mining (accessed on 31 March 2022).
Acknowledgments
We thank the NHSO for their kind provision of the data sets.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 29 March 2022).
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed on 31 March 2022).
- Getahun, H.; Matteelli, A.; Chaisson, R.E.; Raviglione, M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med. 2015, 372, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Kiazyk, S.; Ball, T. Latent tuberculosis infection: An overview. Can. Commun. Dis. Rep. 2017, 43, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.A.; Edelstein, P.H.; Ramakrishnan, L. Revisiting the timetable of tuberculosis. BMJ 2018, 362, k2738. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.; Jarvis, W.R. Administrative, Engineering, and Personal Protective Measures for Controlling Mycobacterium tuberculosis. In Multidrug-Resistant Tuberculosis; Bastian, I., Portaels, F., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 269–284. [Google Scholar]
- Thailand Department of Disease Control, Tuberculosis Division. Tuberculosis Case Management Dashboard. Available online: http://tbcmthailand.ddc.moph.go.th/UIForm/Tableau/TEST_tbcm.php (accessed on 31 March 2022).
- Getnet, F.; Demissie, M.; Assefa, N.; Mengistie, B.; Worku, A. Delay in diagnosis of pulmonary tuberculosis in low-and middle-income settings: Systematic review and meta-analysis. BMC Pulm. Med. 2017, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.S.; Goyal, A.; Guleria, R.; Gupta, A.K. Chest tuberculosis: Radiological review and imaging recommendations. Indian J. Radiol. Imaging 2015, 25, 213–225. [Google Scholar] [CrossRef]
- Török, M.E. Tuberculous meningitis: Advances in diagnosis and treatment. Br. Med. Bull. 2015, 113, 117–131. [Google Scholar] [CrossRef]
- Ladumor, H.; Al-Mohannadi, S.; Ameerudeen, F.S.; Ladumor, S.; Fadl, S. TB or not TB: A comprehensive review of imaging manifestations of abdominal tuberculosis and its mimics. Clin. Imaging 2021, 76, 130–143. [Google Scholar] [CrossRef]
- Bhuket, J.R.N.; Pungrassami, P.; Chongsuvivatwong, V. Hospital tuberculosis control activities and treatment success in Thailand during the implementation year of the admission policy for new smear positive pulmonary tuberculosis patients. J. Med. Assoc. Thai. 2013, 96, 782–785. [Google Scholar]
- Silva, D.R.; Muñoz-Torrico, M.; Duarte, R.; Galvão, T.; Bonini, E.H.; Arbex, F.F.; Arbex, M.A.; Augusto, V.M.; Rabahi, M.F.; de Mello, F.C.Q. Risk factors for tuberculosis: Diabetes, smoking, alcohol use, and the use of other drugs. J. Bras. Pneumol. 2018, 44, 145–152. [Google Scholar] [CrossRef]
- Melsew, Y.A.; Doan, T.N.; Gambhir, M.; Cheng, A.C.; McBryde, E.; Trauer, J.M. Risk Factors for infectiousness of patients with tuberculosis: A systematic review and meta-analysis. Epidemiol. Infect. 2018, 146, 345–353. [Google Scholar] [CrossRef]
- Ai, J.W.; Ruan, Q.L.; Liu, Q.H.; Zhang, W.H. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg. Microbes Infect. 2016, 5, e10. [Google Scholar] [CrossRef] [PubMed]
- Wagnew, F.; Eshetie, S.; Alebel, A.; Dessie, G.; Tesema, C.; Abajobir, A.A. Meta-analysis of the prevalence of tuberculosis in diabetic patients and its association with cigarette smoking in African and Asian countries. BMC Res. Notes 2018, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Gelaw, Y.; Getaneh, Z.; Melku, M. Anemia as a risk factor for tuberculosis: A systematic review and meta-analysis. Environ. Health Prev. Med. 2021, 26, 13. [Google Scholar] [CrossRef]
- Rees, D.; Murray, J. Silica, silicosis and tuberculosis. Int. J. Tuberc. Lung Dis. 2007, 11, 474–484. [Google Scholar] [PubMed]
- Simou, E.; Britton, J.; Leonardi-Bee, J. Alcohol consumption and risk of tuberculosis: A systematic review and meta-analysis. Int. J. Tuberc. Lung. Dis. 2018, 22, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.L.; Marais, B.J.; Mitnick, C.D.; Lecca, L.; Marks, G.B. Tuberculosis and chronic respiratory disease: A systematic review. Int. J. Infect. Dis. 2015, 32, 138–146. [Google Scholar] [CrossRef]
- Tavares, A.M.; Fronteira, I.; Couto, I.; Machado, D.; Viveiros, M.; Abecasis, A.B.; Dias, S. HIV and tuberculosis co-infection among migrants in Europe: A systematic review on the prevalence, incidence and mortality. PLoS ONE 2017, 12, e0185526. [Google Scholar] [CrossRef]
- Imsanguan, W.; Bupachat, S.; Wanchaithanawong, V.; Luangjina, S.; Thawtheong, S.; Nedsuwan, S.; Pungrassami, P.; Mahasirimongkol, S.; Wiriyaprasobchok, A.; Kaewmamuang, K.; et al. Contact tracing for tuberculosis, Thailand. Bull. World Health Organ. 2020, 98, 212–218. [Google Scholar] [CrossRef]
- Oo, M.M.; Tassanakijpanich, N.; Phyu, M.H.; Safira, N.; Kandel, S.; Chumchuen, K.; Zhang, L.M.; Kyu, H.A.; Sriwannawit, P.; Bilmumad, B.; et al. Coverage of tuberculosis and diabetes mellitus screening among household contacts of tuberculosis patients: A household-based cross-sectional survey from Southern Thailand. BMC Public Health 2020, 20, 957. [Google Scholar] [CrossRef]
- Bureau of Tuberculosis, Department of Disease Control, Thailand. National Tuberculosis Control Program Guideline, Thailand. 2020. Available online: https://www.thainapci.org/2021/2021/11/30/ (accessed on 31 March 2022).
- Wei, M.; Zhao, Y.; Qian, Z.; Yang, B.; Xi, J.; Wei, J.; Tang, B. Pneumonia caused by Mycobacterium tuberculosis. Microbes Infect. 2020, 22, 278–284. [Google Scholar] [CrossRef]
- Gengenbacher, M.; Kaufmann, S.H.E. Mycobacterium tuberculosis: Success through dormancy. FEMS Microbiol. Rev. 2012, 36, 514–532. [Google Scholar] [CrossRef] [PubMed]
- Gopi, A.; Madhavan, S.M.; Sharma, S.K.; Sahn, S.A. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest 2007, 131, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Peng, L. Is Xpert MTB/RIF Appropriate for diagnosing tuberculous pleurisy with pleural fluid samples? A systematic review. BMC Infect. Dis. 2018, 18, 284. [Google Scholar] [CrossRef]
- Castro, D.J.; Nuevo, G.D.; Pérez-Rodríguez, E.; Light, R.W. Diagnostic value of adenosine deaminase in nontuberculous lymphocytic pleural effusions. Eur. Respir. J. 2003, 21, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Allan, W.G.; Girling, D.J.; Fayers, P.M.; Fox, W. The symptoms of newly diagnosed pulmonary tuberculosis and patients’ attitudes to the disease and to its treatment in Hong Kong. Tubercle 1979, 60, 211–223. [Google Scholar] [CrossRef]
- Fidan, A.; Ozdoğan, S.; Oruç, O.; Salepçi, B.; Ocal, Z.; Cağlayan, B. Hemoptysis: A retrospective analysis of 108 cases. Respir. Med. 2002, 96, 677–680. [Google Scholar] [CrossRef][Green Version]
- Jonaidi Jafari, N.; Izadi, M.; Sarrafzadeh, F.; Heidari, A.; Ranjbar, R.; Saburi, A. Hyponatremia due to pulmonary tuberculosis: Review of 200 Cases. Nephrourol. Mon. 2013, 5, 687–691. [Google Scholar] [CrossRef]
- Shalhoub, R.J.; Antoniou, L.D. The mechanism of hyponatremia in pulmonary tuberculosis. Ann. Intern. Med. 1969, 70, 943–962. [Google Scholar] [CrossRef]
- Mupere, E.; Parraga, I.M.; Tisch, D.J.; Mayanja, H.K.; Whalen, C.C. Low nutrient intake among adult women and patients with severe tuberculosis disease in Uganda: A cross-sectional study. BMC Public Health 2012, 12, 1050. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).