Leukocyte and IgM Responses to Immunization with the CIDR1α-PfEMP1 Recombinant Protein in the Wistar Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Clearance
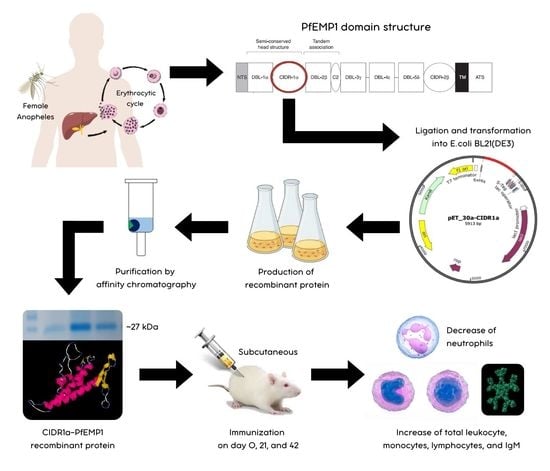
2.2. Production and Purification of CIDR1α-PfEMP1 Recombinant Protein
2.3. Immunization of CIDR1α-PfEMP1 Recombinant Protein in Wistar Rats
2.4. Measurement of Total Leukocyte and Leukocyte Differential Count
2.5. Measurement of IgM Concentration
2.6. Statistical Analysis
3. Results
3.1. Purification and Concentration of CIDR1α-PfEMP1 Recombinant Protein
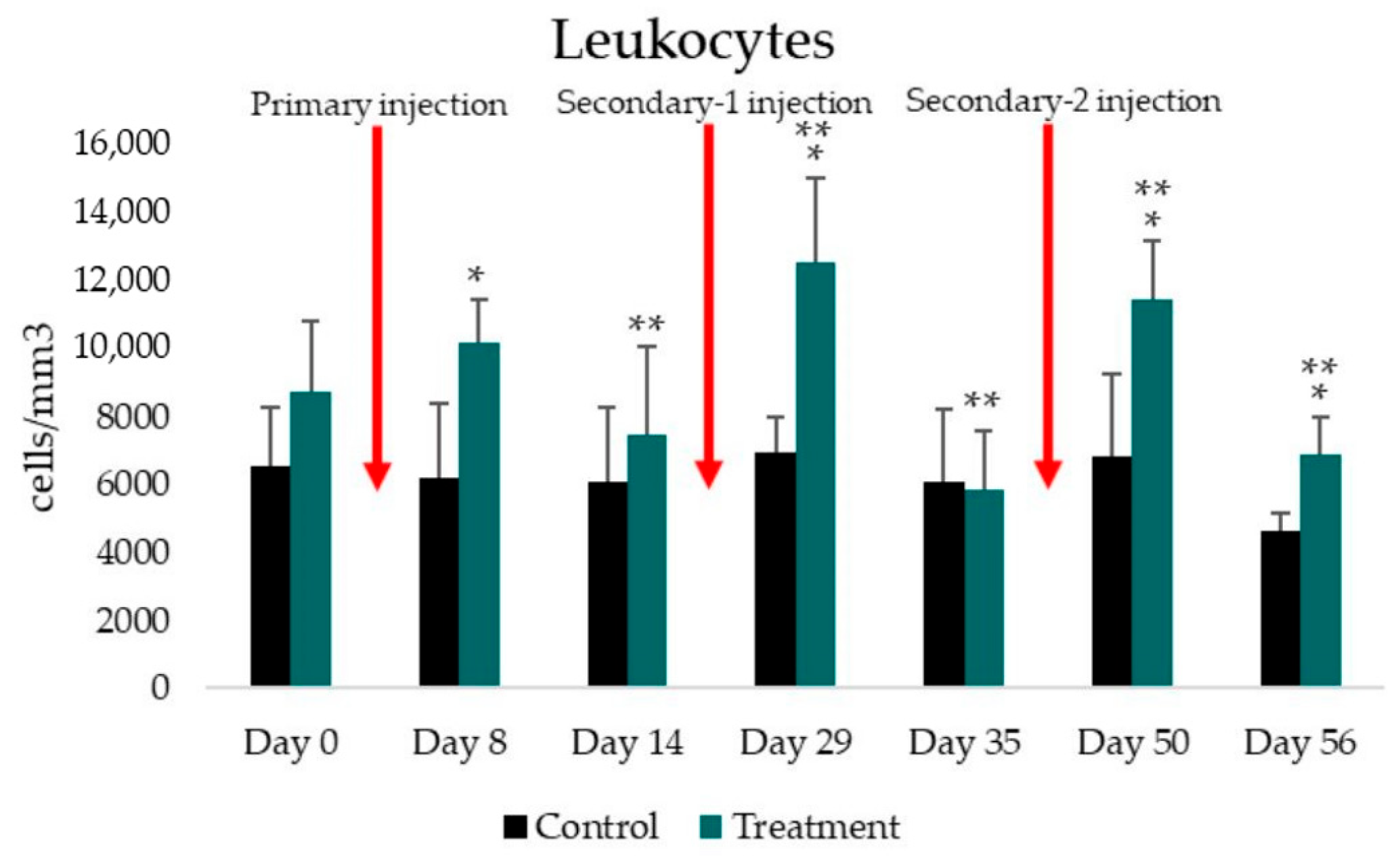
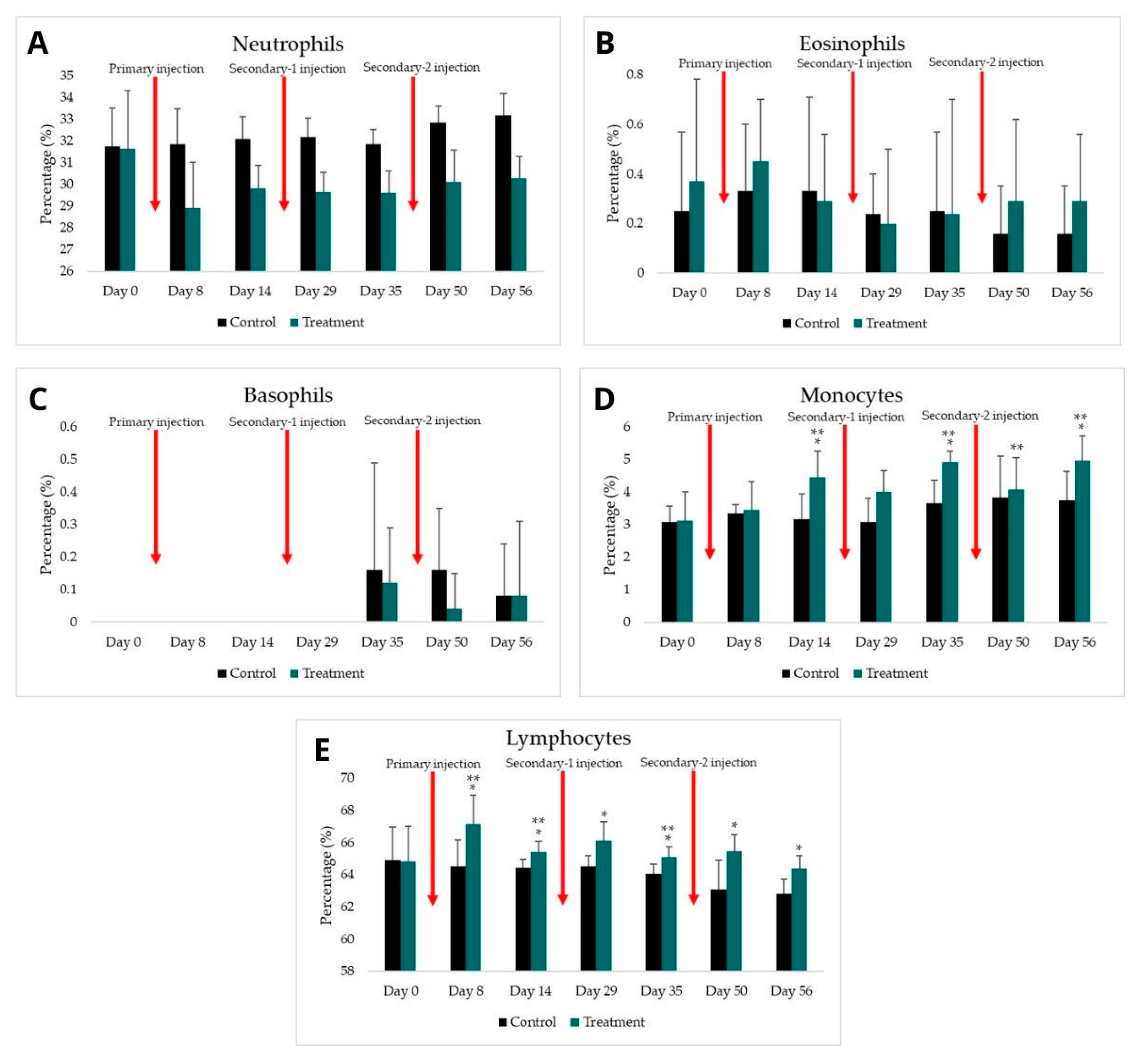
3.2. Total Leukocyte and Leukocyte Differential Count
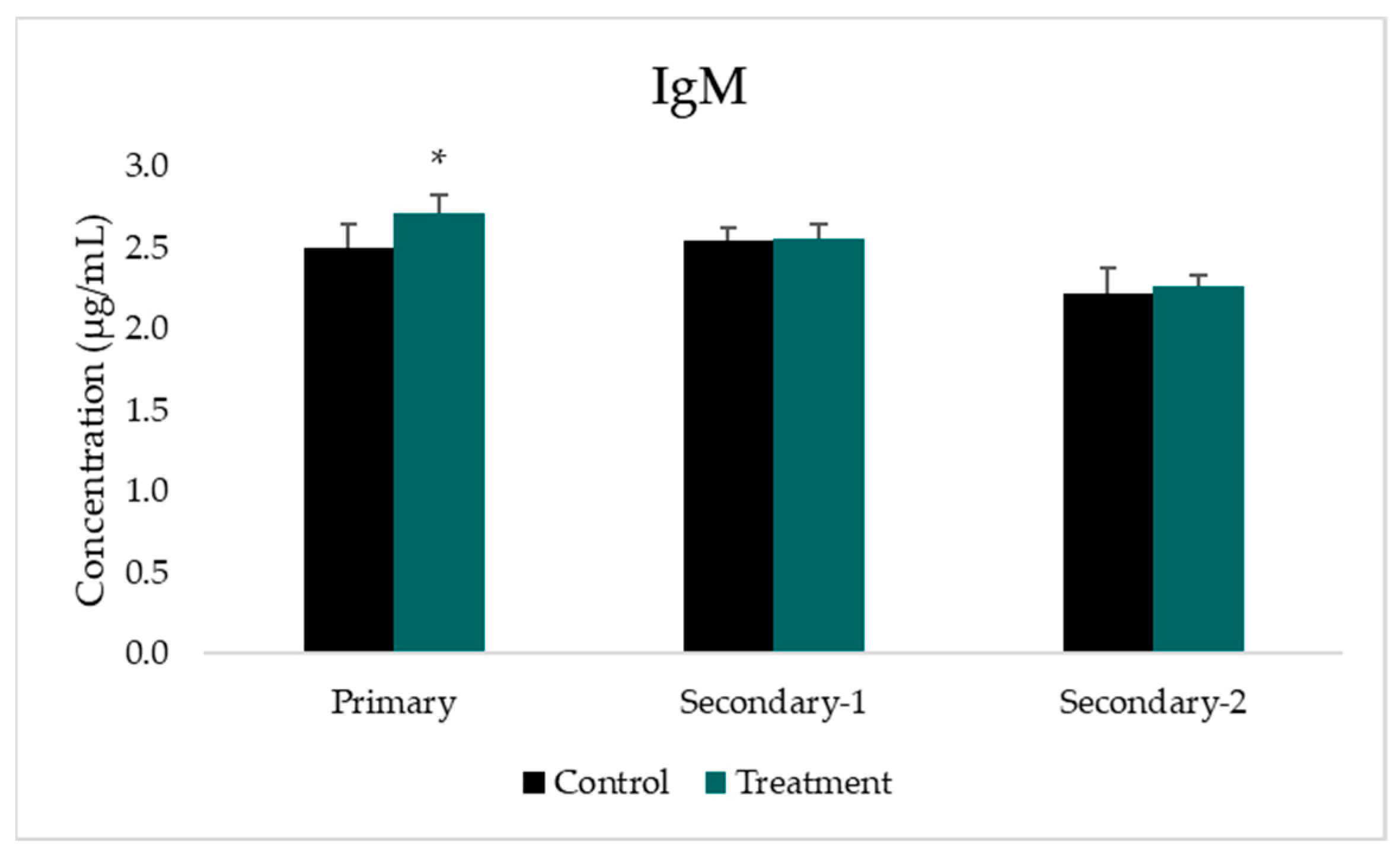
3.3. Analysis of IgM Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mer, M.; Dünser, M.W.; Giera, R.; Dondorp, A.M. Severe malaria. Current concepts and practical overview: What every intensivist should know. Intensive Care Med. 2020, 46, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and Disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Indonesian Ministry of Health. Health Profile of Indonesia 2020; Ministry of Health Repubic of Indonesia: Jakarta, Indonesia, 2021; Volume 48, pp. 200–204. Available online: https://www.scribd.com/document/526663412/Profil-Kesehatan-Indonesia-Tahun-2020 (accessed on 12 January 2022).
- Lambraño, J.; Curtidor, H.; Avendaño, C.; Díaz-Arévalo, D.; Roa, L.; Vanegas, M.; Patarroyo, M.E.; Patarroyo, M.A. Preliminary Evaluation of the Safety and Immunogenicity of an Antimalarial Vaccine Candidate Modified Peptide (IMPIPS) Mixture in a Murine Model. J. Immunol Res. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Parera, M.; Tiala, M.E. Potensi Vaksin Plasmodium Falciparum Fase Pra-Eritrositer RTS,S Sebagai Imunoprofilaksis pada Pelancong. Intisari Sains Medis. 2014, 1, 29–35. Available online: https://isainsmedis.id/index.php/ism/article/viewFile/93/94 (accessed on 16 November 2021). [CrossRef][Green Version]
- Sulistyaningsih, E. Vaksin Malaria: Perkembangan Generasi Vaksin, Kandidat Protein Untuk Vaksin, Dan Tantangannya; Jember University Press: Sumbersari, Indonesia, 2020. [Google Scholar]
- Jensen, A.R.; Adams, Y.; Hviid, L. Cerebral Plasmodium falciparum malaria: The role of PfEMP1 in its pathogenesis and immunity, and PfEMP1-based vaccines to prevent it. Immunol. Rev. 2020, 293, 230–252. [Google Scholar] [CrossRef]
- Sulistyaningsih, E.; Armiyanti, Y.; Dewi, R. The Cidr1α-Pfemp1 Sequence From Indonesian Plasmodium Falciparum And Its Potential Association With The Cerebral Outcome. MNJ 2021, 7, 34–39. [Google Scholar] [CrossRef]
- Aitken, E.H.; Alemu, A.; Rogerson, S.J. Neutrophils and Malaria. Front. Immunol. 2018, 9, 3005. [Google Scholar] [CrossRef]
- Ortega-Pajares, A.; Rogerson, S.J. The Rough Guide to Monocytes in Malaria Infection. Front. Immunol. 2018, 9, 2888. [Google Scholar] [CrossRef]
- Kumar, R.; Loughland, J.R.; Ng, S.S.; Boyle, M.J.; Engwerda, C.R. The regulation of CD4+ T cells during malaria. Immunol. Rev. 2020, 293, 70–87. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 8th ed.; Elsevier Inc.: Philadelphia, PA, USA, 2015. [Google Scholar]
- Pleass, R.J.; Moore, S.C.; Stevenson, L.; Hviid, L. Immunoglobulin M: Restrainer of Inflammation and Mediator of Immune Evasion by Plasmodium falciparum Malaria. Trends Parasitol. 2016, 32, 108–119. [Google Scholar] [CrossRef]
- Dewi, R.; Ratnadewi, A.A.I.; Sawitri, W.D.; Rachmania, S.; Sulistyaningsih, E. Cloning, sequence analysis, and expression of CIDR1α-pfEMP1 from Indonesian plasmodium falciparum isolate. Curr. Top. Pept. Protein. Res. 2018, 19, 95–104. [Google Scholar]
- Setyoadji, W.A.; Sulistyaningsih, E.; Kusuma, I.F. Optimized Expression Condition of CIDRα-PfEMP1 Recombinant Protein Production in Escherichia coli BL21(DE3): A Step to Develop Malaria Vaccine Candidate. Res. J. Life Sci. 2021, 8, 15–24. [Google Scholar] [CrossRef]
- Qiagen. The QIA Expressionist, 5th ed.; Qiagen: Hilden, Germany, 2003. [Google Scholar]
- BioRad. Mini-PROTEAN Precast Gels—Manual; Bio-Rad Laboratoties: Hercules, CA, USA, 2011; pp. 5–29. [Google Scholar]
- Greenfield, E.A. Standard immunization of mice, rats, and hamsters. Cold Spring Harb. Protoc. 2020, 2020, prot100297. [Google Scholar] [CrossRef] [PubMed]
- The Humane Society of the United States. Euthanasia Reference Manual, 2nd ed.; The Humane Society of the United States: Washington, DC, USA, 2013. [Google Scholar]
- Nurhayati, A.P.D.; Pratiwi, R.; Soekardiman, S. Total and Differential Cells of Leukocyte Mice (Mus musculus) on Evaluation in Vivo Anticancer Extracts Ethanol Marine Sponges Aaptos suberitoides. J. Phys. Conf. Ser. 2019, 1374, 012031. [Google Scholar] [CrossRef]
- Cahyani, D.D. Gambaran Hitung Jenis Leukosit pada Pekerja Perkebunan Sumber Wadung Kabupaten Jember yang Terinfeksi Soil-Transmitted Helminths. Repos. Univ. Jember. 2019. Available online: https://repository.unej.ac.id/bitstream/handle/123456789/90456/Desi%20Dwi%20Cahyani-152010101022.pdf?sequence=1&isAllowed=y (accessed on 20 February 2022).
- BT Laboratory. Rat Immunoglobulin M, IgM ELISA Kit. BT Laboratory; 2021. Available online: https://www.bt-laboratory.com/index.php/Shop/Index/productShijiheDetail/p_id/3471/cate/kit.html (accessed on 12 January 2022).
- Walker, J.M. (Ed.) The Proteomics Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2005; pp. 571–608. [Google Scholar] [CrossRef]
- Alassane, S.K.; Nicolau-Travers, M.-L.; Menard, S.; Andreoletti, O.; Cambus, J.-P.; Gaudre, N.; Wlodarczyk, M.; Blanchard, N.; Berry, A.; Abbes, S.; et al. Young Sprague Dawley rats infected by Plasmodium berghei: A relevant experimental model to study cerebral malaria. PLoS ONE 2017, 12, 0181300. [Google Scholar] [CrossRef]
- Kustiah, S.U.; Adrial, A.; Reza, M. Profil Hematologik Berdasarkan Jenis Plasmodium pada Pasien Malaria di Beberapa Rumah Sakit di Kota Padang. J. Kesehat. Andalas 2020, 9, 137–146. [Google Scholar] [CrossRef]
- Darlina, R.T.; Nurhayati, S. Perubahan jenis leukosit pada mencit yang diimunisasi dengan Plasmodium berghei yang diradiasi. Semin. Nas. SDM Teknol. Nukl. VII 2011, 434–439. Available online: https://digilib.batan.go.id/ppin/katalog/file/1978-0176-2011-434.pdf (accessed on 3 February 2022). [CrossRef]
- Oyewusi, H.A.; Akinyede, K.A.; Adeniyi, O.A. Evaluation of Biochemical and Heamatological Changes in Plasmodium-berghei-Infected Mice Administered With Aqueous Extract of Tithonia diversifolia. Int. J. Sci. Res. Publ. 2019, 9, 9438. [Google Scholar] [CrossRef]
- Zelter, T.; Strahilevitz, J.; Simantov, K.; Yajuk, O.; Jensen, A.R.; Dzikowski, R.; Granot, Z. Neutrophils impose strong selective pressure against PfEMP1 variants implicated in cerebral malaria. bioRxiv 2021. [Google Scholar] [CrossRef]
- McCracken, J.M.; Allen, L.-A.H. Regulation of Human Neutrophil Apoptosis and Lifespan in Health and Disease. J. Cell Death 2014, 7, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sumah, L.H.M.; Nindatu, M.; Kakisina, P. Efek pemberian ekstrak metanol kulit batang pohon pulai (Alstonia scholaris l. R. Br.) Terhadap hasil diferensiasi leukosit mencit (Mus musculus) yang diinfeksi plasmodium berghei anka. Molucca Med. 2012, 5, 39–53. [Google Scholar]
- Cahyaningsih, U.; Taher, D.M.; Gusdinar, R. Percentage of leukocytes type in mice after infected with Plasmodium berghei and given an ethyl acetate fraction of AFO varieties. Pros. Pokjanas. TOI Ke 57 2019, 57–61. Available online: https://ffs.uhamka.ac.id/wp-content/uploads/2020/07/Naskah-9.pdf (accessed on 27 March 2022).
- Calderón-Pérez, B.; Xoconostle-Cázares, B.; Lira-Carmona, R.; Hernández-Rivas, R.; Ortega-López, J.; Ruiz-Medrano, R. The Plasmodium falciparum translationally controlled tumor protein (TCTP) is incorporated more efficiently into B cells than its human homologue. PLoS ONE 2014, 9, e85514. [Google Scholar] [CrossRef][Green Version]
- Bain, B.J. Structure and function of red and white blood cells. Medicine 2017, 45, 187–193. [Google Scholar] [CrossRef]
- Prame, K.K.; Nicholls, A.J.; Wong, C.H.Y. Partners in crime: Neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef]
- Sampaio, N.G.; Eriksson, E.M.; Schofield, L. Plasmodium falciparum PfEMP1 modulates monocyte/macrophage transcription factor activation and cytokine and chemokine responses. Infect. Immun. 2017, 86, e00447-17. [Google Scholar] [CrossRef]
- Rachmania, S.; Sulistyaningsih, E.; Ratna, D.A.A.I. Recombinant DBL2β-PfEMP1 of the Indonesian Plasmodium falciparum induces immune responses in Wistar rats. J. Taibah. Univ. Med. Sci. 2021, 16, 422–430. [Google Scholar] [CrossRef]
- Widyastuti, D.A. Profil Darah Tikus Putih Wistar pada Kondisi Subkronis Pemberian Natrium Nitrit. J. Sain Vet. 2013, 31, 201–215. [Google Scholar]
- Suardana, I.B.K. Diktat Imunologi Dasar Sistem Imun. 2017. Available online: https://simdos.unud.ac.id/uploads/file_pendidikan_1_dir/284a0e69155751dc6c459b07f14bc03c.pdf (accessed on 28 March 2022).
- Scholzen, A.; Sauerwein, R.W. How malaria modulates memory: Activation and dysregulation of B cells in Plasmodium infection. Trends Parasitol. 2013, 29, 252–262. [Google Scholar] [CrossRef]
- Cawlfield, A.; Genito, C.; Beck, Z. Safety, Toxicity and Immunogenicity of a Malaria Vaccine Based on The Circumsporozoite Protein (FMP013) with the Adjuvant Army Liposome Formulation Containing QS21 (ALFQ). Vaccine 2019, 37, 3793–3803. [Google Scholar] [CrossRef] [PubMed]

| Indicator | Pre-Injection Day 0 | Primary Injection | Secondary-1 Injection | Secondary-2 Injection | ||||
|---|---|---|---|---|---|---|---|---|
| Day 8 | Day 14 | Day 29 | Day 35 | Day 50 | Day 56 | |||
| Total leukocyte number | ||||||||
| Control (cells/mm3) | 6562.52 ± 1752.31 | 6187.50 ± 2205.06 | 6112.50 ± 2177.29 | 6937.50 ± 1068.00 | 6087.50 ± 2125.78 | 6837.50 ± 2463.18 | 4637.50 ± 543.71 | |
| Treatment (cells/mm3) | 8756.25 ± 2066.30 | 10187.50 ± 1282.50 | 7481.25 ± 2598.89 | 12581.25 ± 2490.25 | 5881.25 ± 1739.84 | 11462.50 ± 1743.71 | 6900.00 ± 1115.15 | |
| p-value | to control group | 0.100 | 0.002 * | 0.389 | 0.002 * | 0.860 | 0.003 * | 0.004 |
| to previous treatment | - | 0.113 | 0.012 ** | 0.002 ** | 0.001 ** | 0.001 ** | 0.001 ** | |
| Neutrophils | ||||||||
| Control (%) | 31.75 ± 1.77 | 31.83 ± 1.66 | 32.08 ± 1.03 | 32.16 ± 0.88 | 31.83 ± 0.69 | 32.83 ± 0.79 | 33.16 ± 1.03 | |
| Treatment (%) | 31.66 ± 2.64 | 28.91 ± 2.09 | 29.83 ± 1.05 | 29.66 ± 0.87 | 29.62 ± 0.99 | 30.12 ± 1.45 | 30.29 ± 0.98 | |
| p-value | to control group | 0.954 | 0.036 * | 0.006 * | 0.001 * | 0.003 * | 0.007 * | 0.001 * |
| to previous treatment | - | 0.017 ** | 0.154 | 0.615 | 0.925 | 0.304 | 0.810 | |
| Eosinophils | ||||||||
| Control (%) | 0.25 ± 0.32 | 0.33 ± 0.27 | 0.33 ± 0.38 | 0.24 ± 0.16 | 0.25 ± 0.32 | 0.16 ± 0.19 | 0.16 ± 0.19 | |
| Treatment (%) | 0.37 ± 0.41 | 0.45 ± 0.25 | 0.29 ± 0.27 | 0.20 ± 0.30 | 0.24 ± 0.46 | 0.29 ± 0.33 | 0.29 ± 0.27 | |
| p-value | to control group | 0.644 | 0.409 | 0.856 | 0.578 | 0.702 | 0.580 | 0.463 |
| to previous treatment | - | 0.680 | 0.245 | 0.157 | 1.000 | 0.498 | 0.798 | |
| Basophils | ||||||||
| Control (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.16 ± 0.33 | 0.16 ± 0.19 | 0.08 ± 0.16 | |
| Treatment (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.12 ± 0.17 | 0.04 ± 0.11 | 0.08 ± 0.23 | |
| p-value | to control group | 1.000 | 1.000 | 1.000 | 1.000 | 0.919 | 0.176 | 0.695 |
| to previous treatment | - | 1.000 | 1.000 | 1.000 | 0.083 | 0.317 | 0.317 | |
| Monocytes | ||||||||
| Control (%) | 3.08 ± 0.49 | 3.33 ± 0.27 | 3.16 ± 0.79 | 3.08 ± 0.73 | 3.66 ± 0.71 | 3.83 ± 1.26 | 3.75 ± 0.87 | |
| Treatment (%) | 3.12 ± 0.89 | 3.45 ± 0.87 | 4.45 ± 0.81 | 4.00 ± 0.66 | 4.91 ± 0.34 | 4.08 ± 0.98 | 4.95 ± 0.76 | |
| p-value | to control group | 0.936 | 0.790 | 0.026 * | 0.055 | 0.002 * | 0.714 | 0.033 * |
| to previous treatment | - | 0.534 | 0.012 ** | 0.329 | 0.22 ** | 0.036 ** | 0.039 ** | |
| Lymphocytes | ||||||||
| Control (%) | 64.91 ± 2.07 | 64.50 ± 1.69 | 64.41 ± 0.56 | 64.50 ± 0.69 | 64.08 ± 0.57 | 63.00 ± 1.86 | 62.83 ± 0.88 | |
| Treatment (%) | 64.83 ± 2.19 | 67.16 ± 1.77 | 65.41 ± 0.68 | 66.12 ± 1.18 | 65.08 ± 0.63 | 65.45 ± 1.03 | 64.37 ± 0.80 | |
| p-value | to control group | 0.950 | 0.032 * | 0.031 * | 0.031 * | 0.024 * | 0.011 * | 0.013 * |
| to previous treatment | - | 0.031 ** | 0.019 ** | 0.131 | 0.006 ** | 0.369 | 0.017 ** | |
| IgM concentration | ||||||||
| Control (μg/mL) | - | - | 2.50 ± 0.15 | - | 2.54 ± 0.08 | - | 2.22 ± 0.15 | |
| Treatment (μg/mL) | - | - | 2.72 ± 0.12 | - | 2.56 ± 0.08 | - | 2.26 ± 0.07 | |
| p-value to control group | 0.006 * | 0.732 | 0.607 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulistyaningsih, E.; Wibisono, R.; Dewi, R. Leukocyte and IgM Responses to Immunization with the CIDR1α-PfEMP1 Recombinant Protein in the Wistar Rat. Trop. Med. Infect. Dis. 2022, 7, 222. https://doi.org/10.3390/tropicalmed7090222
Sulistyaningsih E, Wibisono R, Dewi R. Leukocyte and IgM Responses to Immunization with the CIDR1α-PfEMP1 Recombinant Protein in the Wistar Rat. Tropical Medicine and Infectious Disease. 2022; 7(9):222. https://doi.org/10.3390/tropicalmed7090222
Chicago/Turabian StyleSulistyaningsih, Erma, Renaldi Wibisono, and Rosita Dewi. 2022. "Leukocyte and IgM Responses to Immunization with the CIDR1α-PfEMP1 Recombinant Protein in the Wistar Rat" Tropical Medicine and Infectious Disease 7, no. 9: 222. https://doi.org/10.3390/tropicalmed7090222
APA StyleSulistyaningsih, E., Wibisono, R., & Dewi, R. (2022). Leukocyte and IgM Responses to Immunization with the CIDR1α-PfEMP1 Recombinant Protein in the Wistar Rat. Tropical Medicine and Infectious Disease, 7(9), 222. https://doi.org/10.3390/tropicalmed7090222