A Risk Prediction Model and Risk Score of SARS-CoV-2 Infection Following Healthcare-Related Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Protocol
2.2. Data Collection and Preparation
2.3. Study Definition
2.3.1. Vaccine Formula and Potency Grouping
2.3.2. Laboratory Analysis and Case Definition
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Factors Associated with SARS-CoV-2 Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Definition
- -
- Healthcare workers or healthcare personnel include but are not limited to emergency medical service personnel, nurses, nursing assistants, physicians, technicians, therapists, phlebotomists, pharmacists, students and trainees, contractual staff not employed by the healthcare facility, and persons not directly involved in patient care, but who could be exposed to infectious agents that can be transmitted in the healthcare setting (e.g., clerical, dietary, environmental services, laundry, security, engineering and facilities management, administrative, billing, and volunteer personnel).
- -
- Aerosol-generating procedure: a procedure that could generate more infectious aerosols than coughing, sneezing, talking, or breathing:
- ○
- Open suctioning of airways;
- ○
- Sputum induction;
- ○
- Cardiopulmonary resuscitation;
- ○
- Endotracheal intubation and extubation;
- ○
- Non-invasive ventilation (e.g., BiPAP or CPAP);
- ○
- Bronchoscopy;
- ○
- Manual ventilation;
- ○
- Nebulizer administration and high-flow oxygen delivery.
- -
- Symptoms related to SARS-CoV-2 infection:
- ○
- Fever or chill;
- ○
- Fatigue;
- ○
- Muscle ache;
- ○
- Headache;
- ○
- Cough;
- ○
- Runny nose;
- ○
- Sore throat;
- ○
- Loss in the sense of smell or taste;
- ○
- Shortness of breath;
- ○
- Nausea;
- ○
- Vomiting;
- ○
- Diarrhea.
Appendix B. Mathematical Component of Risk Score
- ○
- For each independent risk factor:
- ○
- For protective factor: education:
- ○
- For protective factor: vaccination:
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Branch-Elliman, W.; Savor Price, C.; McGeer, A.; Perl, T.M. Protecting the frontline: Designing an infection prevention platform for preventing emerging respiratory viral illnesses in healthcare personnel. Infect. Control Hosp. Epidemiol. 2015, 36, 336–345. [Google Scholar] [CrossRef] [PubMed]
- COVID-19: Occupational Health and Safety for Health Workers: Interim Guidance. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-HCW_advice-2021-1 (accessed on 29 May 2022).
- Ashinyo, M.E.; Dubik, S.D.; Duti, V.; Amegah, K.E.; Ashinyo, A.; Larsen-Reindorf, R.; Kaba Akoriyea, S.; Kuma-Aboagye, P. Healthcare Workers Exposure Risk Assessment: A Survey among Frontline Workers in Designated COVID-19 Treatment Centers in Ghana. J. Prim. Care Community Health 2020, 11, 2150132720969483. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Dedoukou, X.; Tseroni, M.; Tsonou, P.; Raftopoulos, V.; Papadima, K.; Mouratidou, E.; Poufta, S.; Panagiotakopoulos, G.; Hatzigeorgiou, D.; et al. SARS-CoV-2 Infection in Healthcare Personnel with High-risk Occupational Exposure: Evaluation of 7-Day Exclusion From Work Policy. Clin. Infect. Dis. 2020, 71, 3182–3187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Zhao, X.; Zhang, J.; Ma, W.; Zhao, H.; Han, X. A Semi-Quantitative Risk Assessment and Management Strategies on COVID-19 Infection to Outpatient Health Care Workers in the Post-Pandemic Period. Risk Manag. Healthc. Policy 2021, 14, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic—A narrative review. Anaesthesia 2020, 75, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Pienthong, T.; Khawcharoenporn, T.; Apisarnthanarak, P.; Weber, D.J.; Apisarnthanarak, A. Factors Associated with COVID-19 Infection Among Thai Health Care Personnel with High Risk Exposures: The Important Roles of Double Masking and Physical Distancing while Eating. Infect. Control Hosp. Epidemiol. 2022, 1–9. [Google Scholar] [CrossRef]
- Gragnani, C.M.; Fernandes, P.; Waxman, D.A. Validation of Centers for Disease Control and Prevention level 3 risk classification for healthcare workers exposed to severe acute respiratory coronavirus virus 2 (SARS-CoV-2). Infect. Control Hosp. Epidemiol. 2021, 42, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Vargese, S.S.; Dev, S.S.; Soman, A.S.; Kurian, N.; Varghese, V.A.; Mathew, E. Exposure risk and COVID-19 infection among frontline health-care workers: A single tertiary care centre experience. Clin. Epidemiol. Glob. Health 2022, 13, 100933. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.S.; Tok, P.S.K.; Yoga Ratnam, K.K.; Aziz, N.; Isahak, M.; Ahmad Zaki, R.; Nik Farid, N.D.; Hairi, N.N.; Rampal, S.; Ng, C.W.; et al. Implementation of a COVID-19 surveillance programme for healthcare workers in a teaching hospital in an upper-middle-income country. PLoS ONE 2021, 16, e0249394. [Google Scholar] [CrossRef] [PubMed]
- Interim Guidance for Managing Healthcare Personnel with SARS-CoV-2 Infection or Exposure to SARS-CoV-2. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html (accessed on 13 May 2022).
- Interim Infection Prevention and Control Recommendations for Healthcare Personnel during the Coronavirus Disease 2019 (COVID-19) Pandemic. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html (accessed on 13 May 2022).
- Contact Tracing in the European Union: Public Health Management of Persons, Including Healthcare Workers, Who Have Had Contact with COVID-19 Cases—Fourth Update. Available online: https://www.ecdc.europa.eu/en/covid-19-contact-tracing-public-health-management (accessed on 13 May 2022).
- Risk Assessment and Management of Exposure of Health Care Workers in the Context of COVID-19: Interim Guidance. Available online: https://www.who.int/publications/i/item/risk-assessment-and-management-of-exposure-of-health-care-workers-in-the-context-of-covid-19-interim-guidance (accessed on 15 May 2022).
- Guidelines for Surveillance and Investigation of Coronavirus Disease 2019 (COVID-19). Available online: https://ddc.moph.go.th/viralpneumonia/eng/file/guidelines/g_GSI_22Dec21.pdf (accessed on 15 May 2022).
- Sritipsukho, P.; Khawcharoenporn, T.; Siribumrungwong, B.; Damronglerd, P.; Suwantarat, N.; Satdhabudha, A.; Chaiyakulsil, C.; Sinlapamongkolkul, P.; Tangsathapornpong, A.; Bunjoungmanee, P.; et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: A test-negative case-control study. Emerg. Microbes Infect. 2022, 11, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Public Health’s Guidelines for Vaccination against COVID-19. Available online: https://ddc.moph.go.th/vaccine-covid19/getFiles/14/1639630757714.pdf (accessed on 21 May 2022).
- Ministry of Public Health’s Guidelines for COVID-19 Vaccination as a Booster Shot. Available online: https://ddc.moph.go.th/vaccine-covid19/getFiles/14/1640232499139.pdf (accessed on 21 May 2022).
- Report on the Results of Surveillance for COVID-19 Strains during 24–30 July 2021. Available online: https://www3.dmsc.moph.go.th/post-view/1234 (accessed on 20 May 2022).
- Report on the Results of Surveillance for COVID-19 Strains during 2–8 October 2021. Available online: https://www3.dmsc.moph.go.th/post-view/1328 (accessed on 20 May 2022).
- Report on the Results of Surveillance for COVID-19 Strains during 1 November–11 February 2022. Available online: https://www3.dmsc.moph.go.th/post-view/1481 (accessed on 20 May 2022).
- Infection Prevention and Control during Health Care When Coronavirus Disease (COVID-19) Is Suspected or Confirmed: Interim Guidance. Available online: https://apps.who.int/iris/handle/10665/332879 (accessed on 20 May 2022).
- Bahl, P.; Doolan, C.; de Silva, C.; Chughtai, A.A.; Bourouiba, L.; MacIntyre, C.R. Airborne or Droplet Precautions for Health Workers Treating Coronavirus Disease 2019? J. Infect. Dis. 2022, 225, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D. Why the WHO took two years to say COVID is airborne. Nature 2022, 604, 26–31. [Google Scholar] [CrossRef] [PubMed]
- French, N.; Jones, G.; Heuer, C.; Hope, V.; Jefferies, S.; Muellner, P.; McNeill, A.; Haslett, S.; Priest, P. Creating symptom-based criteria for diagnostic testing: A case study based on a multivariate analysis of data collected during the first wave of the COVID-19 pandemic in New Zealand. BMC Infect. Dis. 2021, 21, 1119. [Google Scholar] [CrossRef] [PubMed]
- Struyf, T.; Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Leeflang, M.M.; Spijker, R.; Hooft, L.; Emperador, D.; Domen, J.; et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst. Rev. 2021, 2, CD013665. [Google Scholar] [CrossRef] [PubMed]
- Chadeau-Hyam, M.; Bodinier, B.; Elliott, J.; Whitaker, M.D.; Tzoulaki, I.; Vermeulen, R.; Kelly-Irving, M.; Delpierre, C.; Elliott, P. Risk factors for positive and negative COVID-19 tests: A cautious and in-depth analysis of UK biobank data. Int. J. Epidemiol. 2020, 49, 1454–1467. [Google Scholar] [CrossRef] [PubMed]
- Lu, M. The Front Line: Visualizing the Occupations with the Highest COVID-19 Risk. Available online: https://www.visualcapitalist.com/the-front-line-visualizing-the-occupations-with-the-highest-covid-19-risk/ (accessed on 20 May 2022).
- Eisenstein, M. What’s your risk of catching COVID? Nature 2020, 589, 158–159. [Google Scholar] [CrossRef]

| Characteristics | Subsequent COVID-19 Infection within 14 Days after Last Exposure | Total | p Value | |||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| n = 6847 | n = 299 | Event Rate | n = 7146 | % of Total | ||
| Demographic | ||||||
| Age at exposure, year | ||||||
| Mean, standard deviation | 34.95, 10.49 | 35.72, 10.64 | 34.98, 10.50 | 0.216 | ||
| Median (interquartile range) | 32 (27–42) | 35 (26–44) | 32 (27–42) | 0.186 | ||
| Gender | 0.067 | |||||
| Male | 1781 | 92 | 4.9% | 1873 | 26.2% | |
| Female | 5066 | 207 | 3.9% | 5273 | 73.8% | |
| The highest education attainment | <0.001 § | |||||
| Primary or secondary school | 1599 | 133 | 7.7% | 1732 | 24.2% | |
| Associate’s degree | 1296 | 69 | 5.1% | 1365 | 19.1% | |
| Bachelor’s degree | 2846 | 80 | 2.7% | 2926 | 40.9% | |
| Master’s degree | 762 | 12 | 1.6% | 774 | 10.8% | |
| Doctoral degree | 344 | 5 | 1.4% | 349 | 4.9% | |
| Role of hospital worker | 0.620 | |||||
| Healthcare personnel | 5864 | 253 | 4.1% | 6117 | 86.6% | |
| Non-healthcare personnel | 983 | 46 | 4.5% | 1029 | 14.4% | |
| COVID-19 vaccination status | ||||||
| Vaccines | <0.001 | |||||
| CoronaVac–CoronaVac | 3684 | 190 | 4.9% | 3874 | 54.2% | |
| CoronaVac–CoronaVac–ChAdOx-1 | 1203 | 47 | 3.8% | 1250 | 17.5% | |
| CoronaVac–CoronaVac–BNT162b2 | 1070 | 18 | 1.7% | 1088 | 15.2% | |
| ChAdOx-1 | 284 | 10 | 3.4% | 294 | 4.1% | |
| ChAdOx-1–ChAdOx-1 | 219 | 9 | 3.9% | 228 | 3.2% | |
| None | 117 | 19 | 14.0% | 136 | 1.9% | |
| ChAdOx-1–BNT162b2 | 116 | 1 | 0.9% | 117 | 1.6% | |
| Others | 154 | 5 | 3.1% | 159 | 2.2% | |
| Potency of COVID-19 Vaccines * | <0.001 § | |||||
| None | 117 | 19 | 14.0% | 136 | 1.9% | |
| Low-potency vaccines | 4025 | 202 | 4.8% | 4227 | 59.2% | |
| Moderate-potency vaccines | 2537 | 77 | 2.9% | 2614 | 37.6% | |
| High-potency vaccines | 168 | 1 | 0.6% | 169 | 2.4% | |
| The interval between the last dose of COVID-19 vaccines and exposure, day | ||||||
| Mean, standard deviation | 72.07, 33.36 | 73.78, 29.68 | 72.14, 33.22 | 0.351 | ||
| Median (interquartile range) | 72 (47–93) | 75 (57–95) | 72 (48–93) | 0.302 | ||
| Missing data | 207 | 21 | 228 | 3.2% | ||
| Previous COVID-19 infection | 0.755 # | |||||
| Absence | 6564 | 290 | 4.2% | 6854 | 99.1% | |
| Presence | 62 | 3 | 4.6% | 65 | 0.9% | |
| Exposure characteristics | ||||||
| Infected person was wearing a mask/N95 respirator during exposure | <0.001 | |||||
| Yes | 2897 | 61 | 2.1% | 2958 | 41.4% | |
| No | 3950 | 238 | 5.7% | 4188 | 58.6% | |
| Distance of contact | <0.001 | |||||
| More than 1 m | 1510 | 40 | 2.6% | 1550 | 21.7% | |
| Less than 1 m | 5337 | 259 | 4.6% | 5596 | 78.3% | |
| Duration of exposure | <0.001 | |||||
| Less than 15 min | 3380 | 53 | 1.5% | 3433 | 48.0% | |
| More than 15 min | 3467 | 246 | 6.6% | 3713 | 52.0% | |
| Exposed hospital worker was wearing a mask/N95 respirator during exposure | <0.001 | |||||
| Yes | 4535 | 91 | 2.0% | 4626 | 64.7% | |
| No | 2312 | 208 | 8.3% | 2520 | 35.3% | |
| Exposed hospital worker was wearing a face shield during exposure | <0.001 | |||||
| Yes | 1941 | 38 | 1.9% | 1979 | 27.7% | |
| No | 4906 | 261 | 5.1% | 5167 | 72.3% | |
| Infected person was undergoing aerosol-generating procedures | 0.186 | |||||
| No | 6465 | 277 | 4.1% | 6742 | 94.3% | |
| Yes; exposed hospital worker was wearing N95 respirator/PAPR and face shield | 77 | 2 | 2.5% | 79 | 1.1% | |
| Yes; exposed hospital worker was not wearing N95 respirator/PAPR and face shield | 305 | 20 | 6.2% | 325 | 4.5% | |
| Exposed hospital worker had direct contact with the aerodigestive secretion of the infected person | <0.001 | |||||
| No | 6549 | 249 | 3.7% | 6798 | 95.1% | |
| Yes | 298 | 50 | 14% | 348 | 4.9% | |
| Exposure risk category by infectious disease physicians | <0.001 | |||||
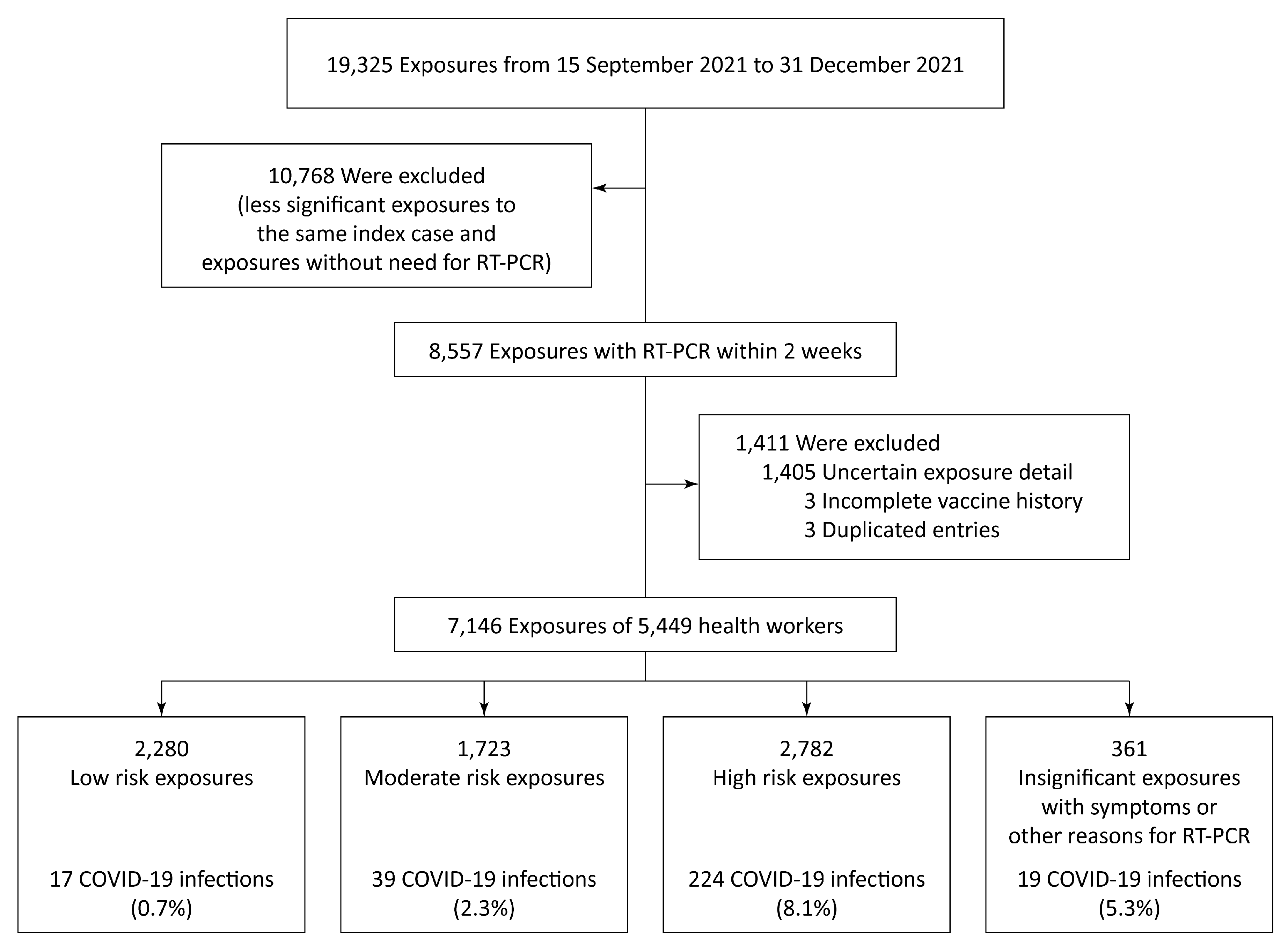
| Low risk | 2263 | 17 | 0.7% | 2280 | 31.9% | |
| Moderate risk | 1684 | 39 | 2.3% | 1723 | 24.1% | |
| High risk | 2558 | 224 | 8.1% | 2782 | 38.9% | |
| Insignificant exposure with symptom(s) or reason(s) for RT-PCR | 342 | 19 | 5.3% | 361 | 5.1% | |
| Symptom of exposed hospital worker | ||||||
| Fever or other COVID-19-related symptoms | <0.001 | |||||
| Absence | 5073 | 103 | 2.0% | 5176 | 79.1% | |
| Presence | 1174 | 196 | 14.3% | 1370 | 20.9% | |
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| Crude OR | (95% CI) | p Value | Adjusted OR | (95% CI) | p Value | |
| Demographic | ||||||
| Age (year) | 1.01 | (1–1.02) | 0.216 | 1.01 | (1–1.02) | 0.053 |
| Male gender | 1.26 | (0.98–1.63) | 0.068 | 1.11 | (0.83–1.48) | 0.480 |
| The highest education attainment | <0.001 | <0.001 | ||||
| Primary or secondary school (reference) | ||||||
| Associate’s | 0.64 | (0.47–0.86) | 0.004 | 0.76 | (0.54–1.06) | 0.106 |
| Bachelor’s | 0.34 | (0.25–0.45) | <0.001 | 0.44 | (0.32–0.61) | <0.001 |
| Master’s | 0.19 | (0.1–0.34) | <0.001 | 0.31 | (0.17–0.58) | <0.001 |
| Doctoral | 0.18 | (0.07–0.43) | <0.001 | 0.36 | (0.14–0.92) | 0.033 |
| Role of worker: Healthcare personnel | 0.92 | (0.67–1.27) | 0.620 | |||
| Exposure characteristics | ||||||
| Infected person was not wearing a mask/N95 respirator during exposure | 2.86 | (2.15–3.81) | <0.001 | 1.45 | (1–2.1) | 0.048 |
| Distance of exposure less than 1 m | 1.83 | (1.31–2.57) | <0.001 | 1.4 | (0.97–2) | 0.069 |
| Duration of exposure more than 15 min | 4.53 | (3.35–6.11) | <0.001 | 2.51 | (1.81–3.48) | <0.001 |
| Exposed hospital worker not wearing a mask/N95 respirator during exposure | 4.48 | (3.49–5.77) | <0.001 | 2.54 | (1.72–3.76) | <0.001 |
| Exposed hospital worker not wearing face shield or goggles during exposure | 2.72 | (1.93–3.83) | <0.001 | 1.25 | (0.78–1.98) | 0.353 |
| Infected person was undergoing aerosol-generating procedures | 0.156 | 0.001 | ||||
| No (reference) | ||||||
| Yes; exposed HCP was wearing N95 respirator/PAPR and face shield | 0.61 | (0.15–2.48) | 0.486 | 1.28 | (0.29–5.66) | 0.748 |
| Yes; exposed HCP was not wearing N95 respirator/PAPR and face shield | 1.53 | (0.96–2.44) | 0.075 | 2.86 | (1.64–5) | <0.001 |
| Exposed hospital worker had direct contact with aerodigestive secretion of the infected person | 4.41 | (3.19–6.11) | <0.001 | 1.48 | (1.02–2.15) | 0.038 |
| Symptoms of exposed hospital worker | ||||||
| Fever or other COVID-19-related symptoms | 5.44 | (4.26–6.95) | <0.001 | 4.9 | (3.78–6.34) | <0.001 |
| COVID-19 vaccination status | ||||||
| Potency of COVID-19 vaccines * | <0.001 | <0.001 | ||||
| None (reference) | ||||||
| Low-potency vaccines | 0.31 | (0.19–0.51) | <0.001 | 0.31 | (0.18–0.54) | <0.001 |
| Moderate-potency vaccines | 0.19 | (0.11–0.32) | <0.001 | 0.16 | (0.09–0.3) | <0.001 |
| High-potency vaccines | 0.04 | (0.01–0.28) | 0.001 | 0.05 | (0.01–0.41) | 0.005 |
| The interval between the last dose of COVID-19 vaccines and exposure (day) | (1–1.01) | 0.402 | ||||
| Previous COVID-19 infection: Yes | 1.1 | (0.34–3.51) | 0.878 | |||
| Risk Factor | β | Odds Ratio (95% CI) | p Value | Point |
|---|---|---|---|---|
| The highest education attainment | <0.001 | |||
| Primary or secondary school (reference) | 3 | |||
| Undergraduate (associate’s or bachelor’s) | −0.64 | 0.53 (0.4–0.68) | <0.001 | 1 |
| Postgraduate (master’s or doctoral) | −1.13 | 0.32 (0.19–0.55) | <0.001 | 0 |
| Infected person was not wearing a mask/N95 respirator during exposure | 0.37 | 1.44 (1.01–2.07) | 0.046 | 1 |
| Distance of exposure less than 1 m without a face shield | 0.33 | 1.39 (1.02–1.89) | 0.038 | 1 |
| Duration of exposure more than 15 min | 0.93 | 2.52 (1.82–3.49) | <0.001 | 3 |
| Exposed hospital worker was not wearing a mask/N95 respirator during exposure | 0.91 | 2.49 (1.75–3.54) | <0.001 | 3 |
| Exposed hospital worker was not wearing an N95 respirator and face shield/goggles while the infected person was undergoing aerosol-generating procedure | 1.05 | 2.87 (1.66–4.96) | <0.001 | 3 |
| Exposed hospital worker had direct contact with the aerodigestive secretion of the infected person | 0.40 | 1.5 (1.03–2.17) | 0.033 | 1 |
| Fever or other COVID-19-related symptoms | 1.60 | 4.94 (3.83–6.39) | <0.001 | 5 |
| Potency of COVID-19 vaccines * | <0.001 | |||
| None (reference) | 9 | |||
| Low-potency vaccines | −1.19 | 0.3 (0.17–0.53) | <0.001 | 5 |
| Moderate-potency vaccines | −1.79 | 0.17 (0.09–0.3) | <0.001 | 4 |
| High-potency vaccines | −2.98 | 0.05 (0.01–0.4) | 0.004 | 0 |
| Constant | −3.69 | <0.001 |
| Total Point | Predicted Probability of COVID-19 Infection (%) |
|---|---|
| 0–9 | 0.05–0.93 |
| 10–14 | 1.28–4.60 |
| 15–16 | 6.28–8.51 |
| 17–19 | 11.44–19.94 |
| 20–23 | 25.70–48.09 |
| 24–29 | 56.27–86.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sripanidkulchai, K.; Rattanaumpawan, P.; Ratanasuwan, W.; Angkasekwinai, N.; Assanasen, S.; Werarak, P.; Navanukroh, O.; Phatharodom, P.; Tocharoenchok, T. A Risk Prediction Model and Risk Score of SARS-CoV-2 Infection Following Healthcare-Related Exposure. Trop. Med. Infect. Dis. 2022, 7, 248. https://doi.org/10.3390/tropicalmed7090248
Sripanidkulchai K, Rattanaumpawan P, Ratanasuwan W, Angkasekwinai N, Assanasen S, Werarak P, Navanukroh O, Phatharodom P, Tocharoenchok T. A Risk Prediction Model and Risk Score of SARS-CoV-2 Infection Following Healthcare-Related Exposure. Tropical Medicine and Infectious Disease. 2022; 7(9):248. https://doi.org/10.3390/tropicalmed7090248
Chicago/Turabian StyleSripanidkulchai, Kantarida, Pinyo Rattanaumpawan, Winai Ratanasuwan, Nasikarn Angkasekwinai, Susan Assanasen, Peerawong Werarak, Oranich Navanukroh, Phatharajit Phatharodom, and Teerapong Tocharoenchok. 2022. "A Risk Prediction Model and Risk Score of SARS-CoV-2 Infection Following Healthcare-Related Exposure" Tropical Medicine and Infectious Disease 7, no. 9: 248. https://doi.org/10.3390/tropicalmed7090248
APA StyleSripanidkulchai, K., Rattanaumpawan, P., Ratanasuwan, W., Angkasekwinai, N., Assanasen, S., Werarak, P., Navanukroh, O., Phatharodom, P., & Tocharoenchok, T. (2022). A Risk Prediction Model and Risk Score of SARS-CoV-2 Infection Following Healthcare-Related Exposure. Tropical Medicine and Infectious Disease, 7(9), 248. https://doi.org/10.3390/tropicalmed7090248










