Medicines for Malaria Venture Pandemic Box In Vitro Screening Identifies Compounds Highly Active against the Tachyzoite Stage of Toxoplasma gondii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Compounds of Pandemic Box
2.2. Plaque Assay
2.3. Cytotoxicity Assay
2.4. Forty-Eight-Hour Antiproliferative Assay
2.5. Post-Treatment Recovery (Washout) Assay
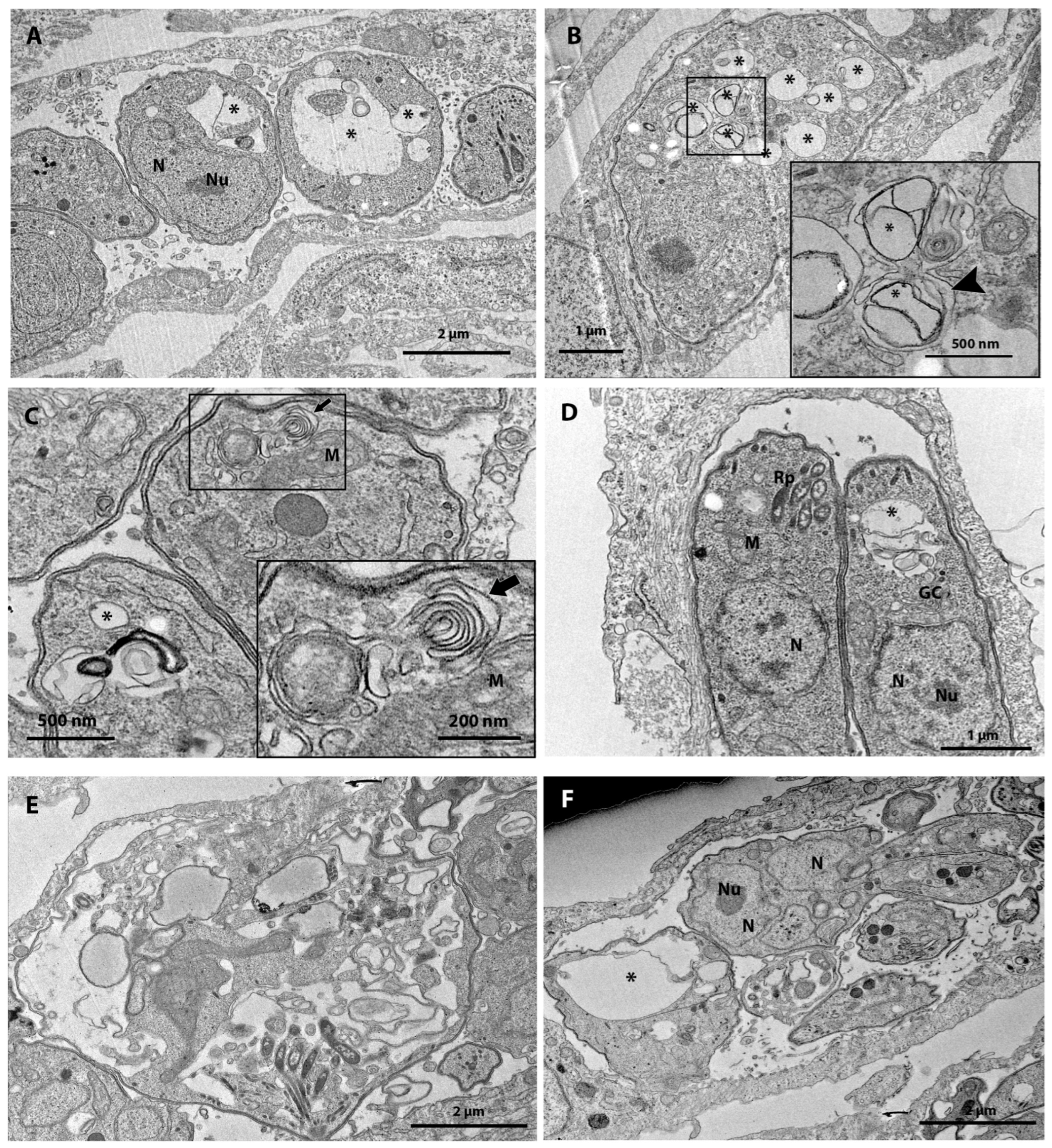
2.6. Ultrastructural Analysis through Transmission Electron Microscopy (TEM)
2.7. Immunofluorescence Assay
2.8. Statistical Analysis
3. Results
3.1. The Pandemic Box Compounds That Showed High Activity and Selectivity against T. gondii Tachyzoites
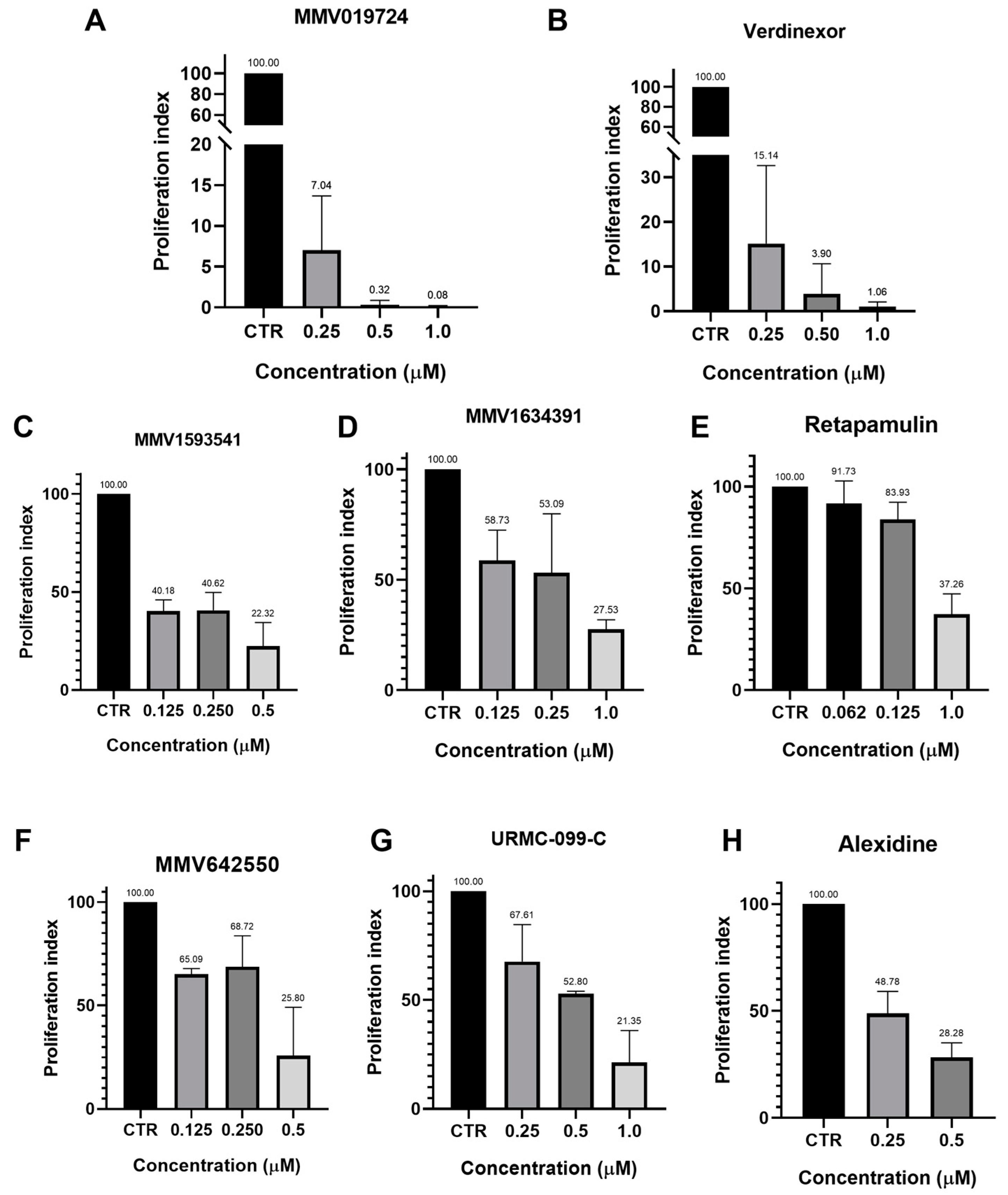
3.2. Forty-Eight-Hour Inhibition Assay
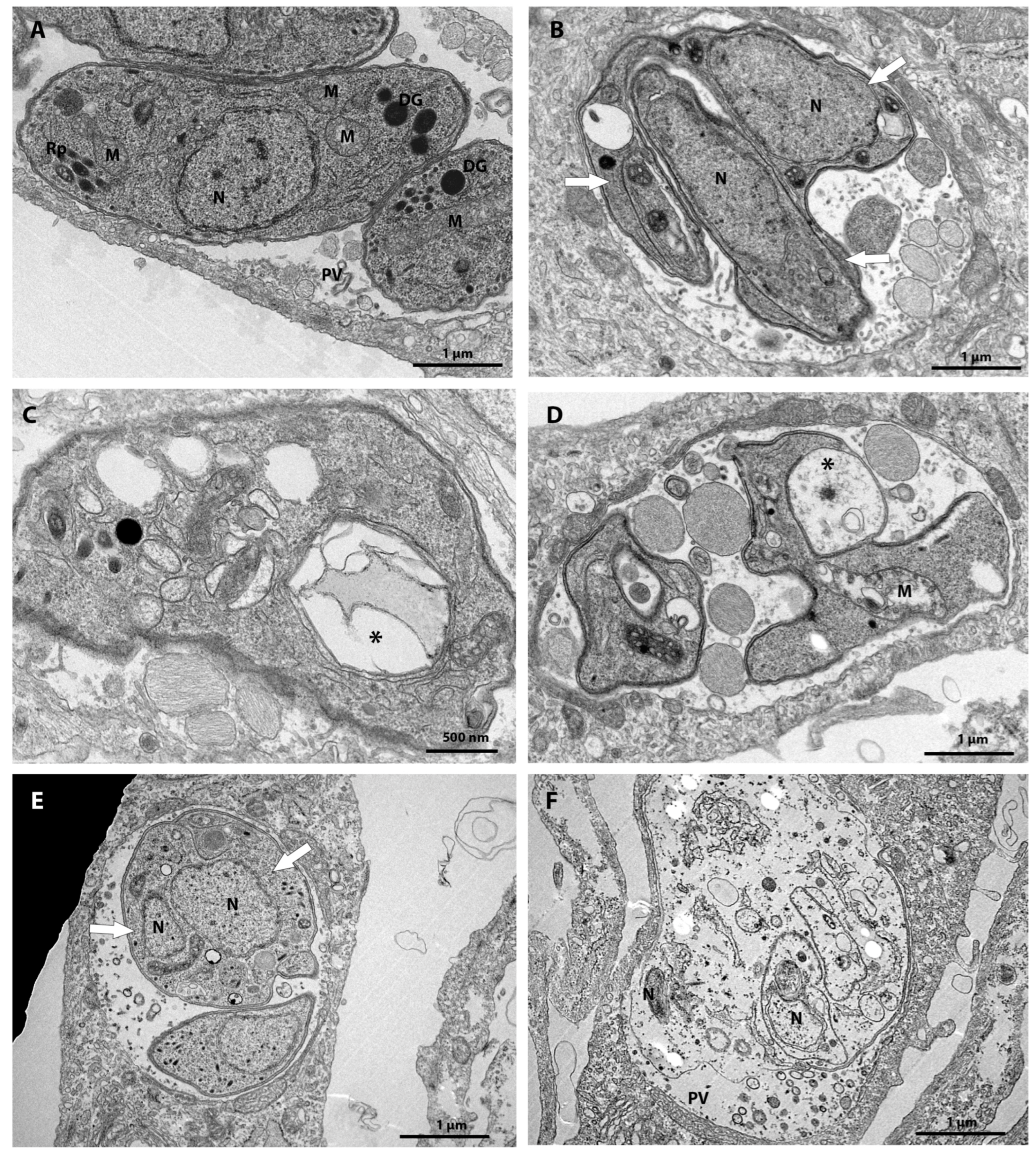
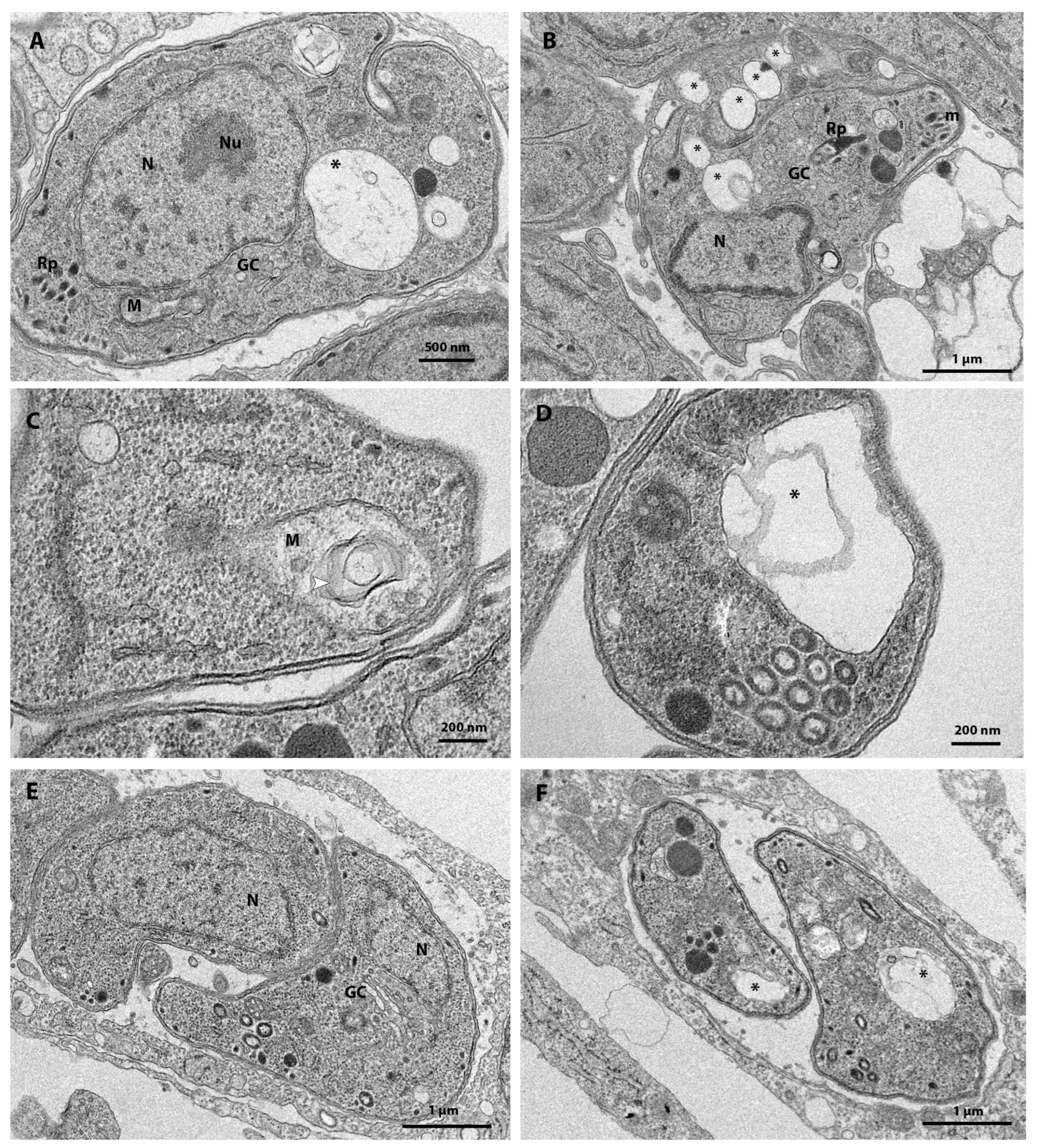
3.3. Ultrastructural Analysis after 48 h of Treatment
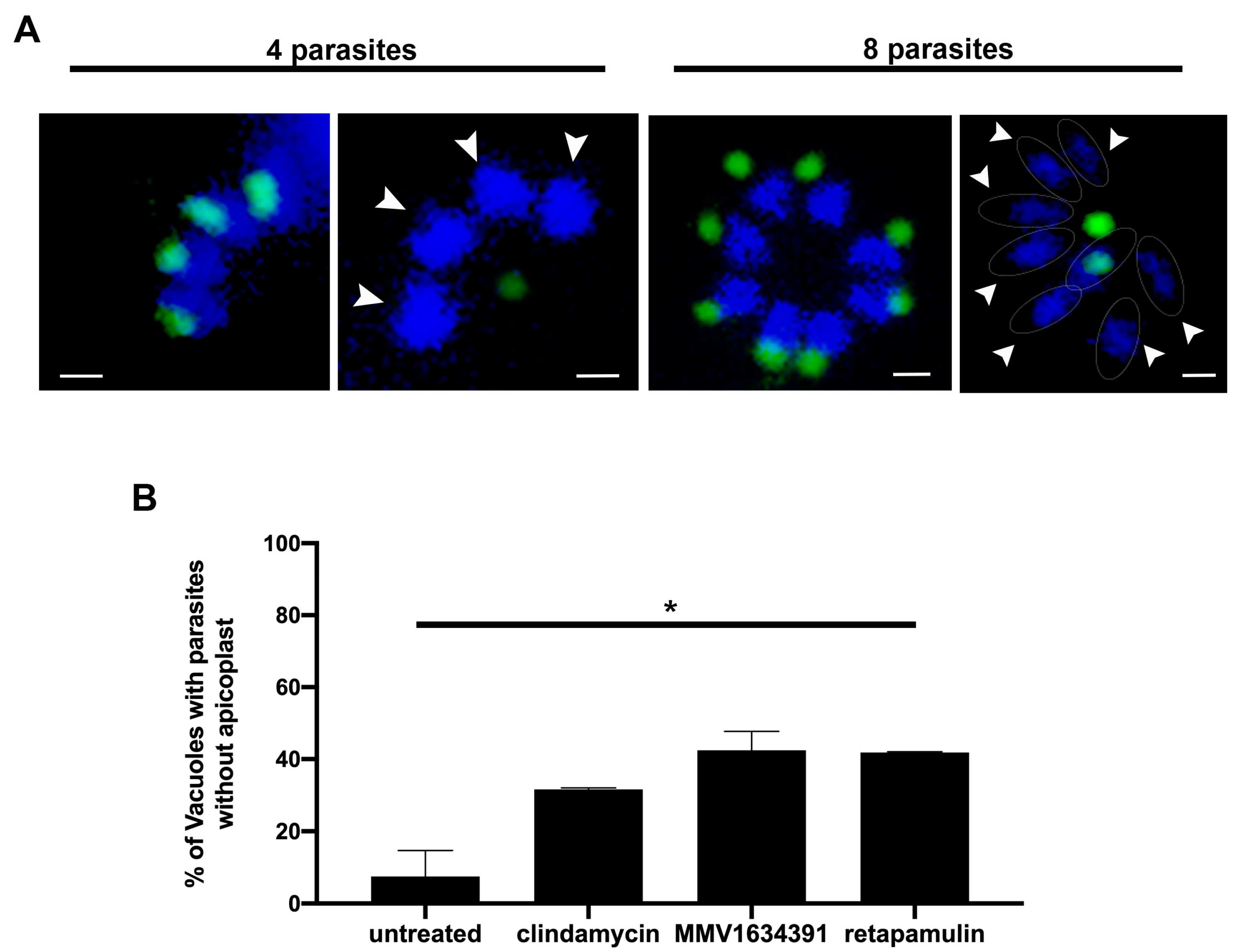
3.4. MMV163439 and Retapamulin Treatment Induce Apicoplast Loss in T. gondii
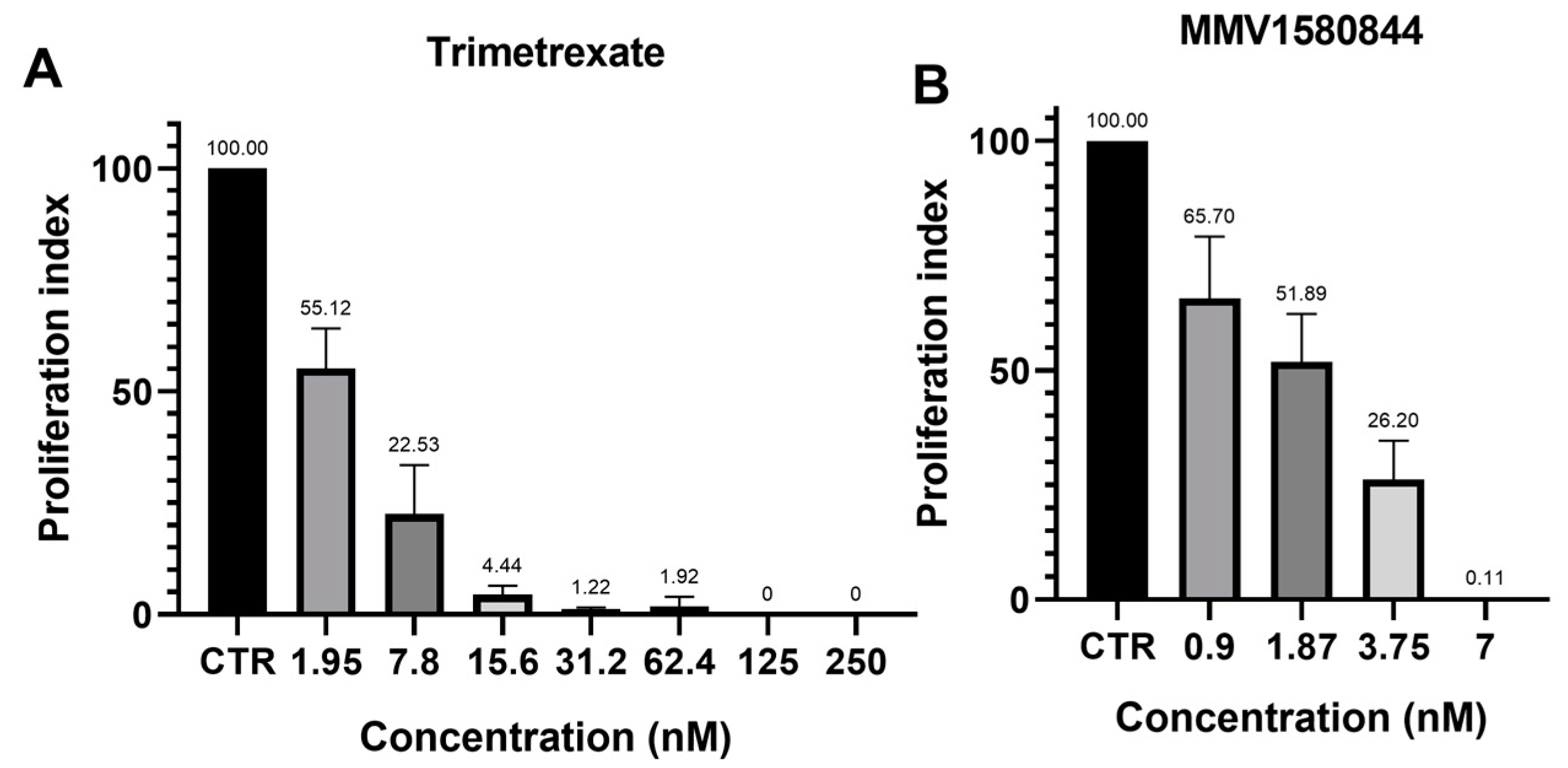
3.5. MMV1580844 and Trimetrexate Post-Treatment Recovery Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crabtree-Ramírez, B.; Caro-Vega, Y.; Shepherd, B.E.; Grinsztejn, B.; Wolff, M.; Cortes, C.P.; Padgett, D.; Carriquiry, G.; Fink, V.; Jayathilake, K.; et al. Time to HAART Initiation after Diagnosis and Treatment of Opportunistic Infections in Patients with AIDS in Latin America. PLoS ONE 2016, 11, e0153921. [Google Scholar] [CrossRef] [PubMed]
- Diesel, A.A.; Zachia, S.D.A.; Müller, A.L.L.; Perez, A.V.; Uberti, F.A.D.F.; Magalhães, J.A.D.A. Follow-up of Toxoplasmosis during Pregnancy: Ten-Year Experience in a University Hospital in Southern Brazil. Rev. Bras. Ginecol. Obs. 2019, 41, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Zangerle, R.; Allerberger, F.; Pohl, P.; Fritsch, P.; Dierich, M.P. High Risk of Developing Toxoplasmic Encephalitis in AIDS Patients Seropositive to Toxoplasma gondii. Med. Microbiol. Immunol. 1991, 180, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M. Neurologic Manifestations of HIV Infection. Ann. Intern. Med. 1994, 121, 769. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, F.F.; Mantelo, F.M.; Lima, K.K.D.; Marchioro, A.A.; Beletini, L.F.; Souza, A.H.D.; Santana, P.L.; Riedo, C.D.O.; Higa, L.T.; Guilherme, A.L.F. Prospective Evalution of Pregnant Women with Suspected Acute Toxoplasmosis Treated in a Reference Prenatal Care Clinic at a University Teaching Hospital in Southern Brazil. Rev. Inst. Med. Trop. São Paulo 2020, 62, e46. [Google Scholar] [CrossRef] [PubMed]
- de Lima Bessa, G.; de Almeida Vitor, R.W.; dos Santos Martins-Duarte, E. Toxoplasma gondii in South America: A Differentiated Pattern of Spread, Population Structure and Clinical Manifestations. Parasitol. Res. 2021, 120, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos-Santos, D.V.; Machado Azevedo, D.O.; Campos, W.R.; Oréfice, F.; Queiroz-Andrade, G.M.; Carellos, É.V.M.; Castro Romanelli, R.M.; Januário, J.N.; Resende, L.M.; Martins-Filho, O.A. Congenital Toxoplasmosis in Southeastern Brazil: Results of Early Ophthalmologic Examination of a Large Cohort of Neonates. Ophthalmology 2009, 116, 2199–2205.e1. [Google Scholar] [CrossRef] [PubMed]
- Baatz, H.; Mirshahi, A.; Puchta, J.; Gümbel, H.; Hattenbach, L.-O. Reactivation of Toxoplasma Retinochoroiditis Under Atovaquone Therapy in an Immunocompetent Patient. Ocul. Immunol. Inflamm. 2006, 14, 185–187. [Google Scholar] [CrossRef]
- Dannemann, B.; McCutchan, J.A.; Israelski, D.; Antoniskis, D.; Leport, C.; Luft, B.; Nussbaum, J.; Clumeck, N.; Morlat, P.; Chiu, J.; et al. Treatment of Toxoplasmic Encephalitis in Patients with AIDS: A Randomized Trial Comparing Pyrimethamine plus Clindamycin to Pyrimethamine plus Sulfadiazine. Ann. Intern. Med. 1992, 116, 33–43. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
- Silva, L.A.; Fernandes, M.D.; Machado, A.S.; Reis-Cunha, J.L.; Bartholomeu, D.C.; Almeida Vitor, R.W. Efficacy of Sulfadiazine and Pyrimetamine for Treatment of Experimental Toxoplasmosis with Strains Obtained from Human Cases of Congenital Disease in Brazil. Exp. Parasitol. 2019, 202, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Martins-Duarte, E.S.; de Araujo Portes, J.; da Silva, R.B.; Pires, H.S.; Garden, S.J.; de Souza, W. In Vitro Activity of N-Phenyl-1,10-Phenanthroline-2-Amines against Tachyzoites and Bradyzoites of Toxoplasma gondii. Bioorg. Med. Chem. 2021, 50, 116467. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, T.T.; Thor, K.B. Drug Repositioning: Identifying and Developing New Uses for Existing Drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, J.-P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug Repositioning: A Brief Overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Li, J.; Xie, H.; Wang, Y. Review of Drug Repositioning Approaches and Resources. Int. J. Biol. Sci. 2018, 14, 1232–1244. [Google Scholar] [CrossRef]
- Liu, N.; Wang, C.; Su, H.; Zhang, W.; Sheng, C. Strategies in the Discovery of Novel Antifungal Scaffolds. Future Med. Chem. 2016, 8, 1435–1454. [Google Scholar] [CrossRef]
- Boyom, F.F.; Fokou, P.V.T.; Tchokouaha, L.R.Y.; Spangenberg, T.; Mfopa, A.N.; Kouipou, R.M.T.; Mbouna, C.J.; Donfack, V.F.D.; Zollo, P.H.A. Repurposing the Open Access Malaria Box To Discover Potent Inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrob. Agents Chemother. 2014, 58, 5848–5854. [Google Scholar] [CrossRef]
- Subramanian, G.; Belekar, M.A.; Shukla, A.; Tong, J.X.; Sinha, A.; Chu, T.T.T.; Kulkarni, A.S.; Preiser, P.R.; Reddy, D.S.; Tan, K.S.W.; et al. Targeted Phenotypic Screening in Plasmodium falciparum and Toxoplasma gondii Reveals Novel Modes of Action of Medicines for Malaria Venture Malaria Box Molecules. mSphere 2018, 3, e00534-17. [Google Scholar] [CrossRef]
- Varberg, J.M.; LaFavers, K.A.; Arrizabalaga, G.; Sullivan, W.J. Characterization of Plasmodium Atg3-Atg8 Interaction Inhibitors Identifies Novel Alternative Mechanisms of Action in Toxoplasma gondii. Antimicrob. Agents Chemother. 2018, 62, e01489-17. [Google Scholar] [CrossRef]
- Spalenka, J.; Escotte-Binet, S.; Bakiri, A.; Hubert, J.; Renault, J.-H.; Velard, F.; Duchateau, S.; Aubert, D.; Huguenin, A.; Villena, I. Discovery of New Inhibitors of Toxoplasma gondii via the Pathogen Box. Antimicrob. Agents Chemother. 2018, 62, e01640-17. [Google Scholar] [CrossRef]
- Radke, J.B.; Burrows, J.N.; Goldberg, D.E.; Sibley, L.D. Evaluation of Current and Emerging Antimalarial Medicines for Inhibition of Toxoplasma gondii Growth in Vitro. ACS Infect. Dis. 2018, 4, 1264–1274. [Google Scholar] [CrossRef]
- Cajazeiro, D.C.; Toledo, P.P.M.; de Sousa, N.F.; Scotti, M.T.; Reimão, J.Q. Drug Repurposing Based on Protozoan Proteome: In Vitro Evaluation of In Silico Screened Compounds against Toxoplasma gondii. Pharmaceutics 2022, 14, 1634. [Google Scholar] [CrossRef]
- dos Santos, B.R.; da Silva Bellini Ramos, A.B.; de Menezes, R.P.B.; Scotti, M.T.; Colombo, F.A.; Marques, M.J.; Reimão, J.Q. Repurposing the Medicines for Malaria Venture’s COVID Box to Discover Potent Inhibitors of Toxoplasma gondii, and in Vivo Efficacy Evaluation of Almitrine Bismesylate (MMV1804175) in Chronically Infected Mice. PLoS ONE 2023, 18, e0288335. [Google Scholar] [CrossRef]
- Mayer, F.L.; Kronstad, J.W. Discovery of a Novel Antifungal Agent in the Pathogen Box. mSphere 2017, 2, e00120-17. [Google Scholar] [CrossRef]
- Vila, T.; Lopez-Ribot, J.L. Screening the Pathogen Box for Identification of Candida albicans Biofilm Inhibitors. Antimicrob. Agents Chemother. 2017, 61, e02006-16. [Google Scholar] [CrossRef]
- Mansour, N.R.; Paveley, R.; Gardner, J.M.F.; Bell, A.S.; Parkinson, T.; Bickle, Q. High Throughput Screening Identifies Novel Lead Compounds with Activity against Larval, Juvenile and Adult Schistosoma mansoni. PLoS Negl. Trop. Dis. 2016, 10, e0004659. [Google Scholar] [CrossRef]
- Hennessey, K.M.; Rogiers, I.C.; Shih, H.-W.; Hulverson, M.A.; Choi, R.; McCloskey, M.C.; Whitman, G.R.; Barrett, L.K.; Merritt, E.A.; Paredez, A.R.; et al. Screening of the Pathogen Box for Inhibitors with Dual Efficacy against Giardia lamblia and Cryptosporidium parvum. PLoS Negl. Trop. Dis. 2018, 12, e0006673. [Google Scholar] [CrossRef]
- Mbye, H.; Bojang, F.; Jaiteh, F.K.; Jawara, A.; Njie, B.; Correa, S.; D’Alessandro, U.; Amambua-Ngwa, A. Stepwise in Vitro Screening of MMV Pathogen Box Compounds against Plasmodium falciparum to Identify Potent Antimalarial Candidates. Int. J. Parasitol. Drugs Drug Resist. 2023, 22, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Pereira Filho, A.A.; Cunha, M.M.; Alves Stanton, M.; Fumiko Yamaguchi, L.; Jorge Kato, M.; Martins-Duarte, É.S. In Vitro Activity of Essential Oils from Piper Species (Piperaceae) against Tachyzoites of Toxoplasma gondii. Metabolites 2023, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Barltrop, J.A.; Owen, T.C.; Cory, A.H.; Cory, J.G. 5-(3-Carboxymethoxyphenyl)-2-(4,5-Dimethylthiazolyl)-3-(4-Sulfophenyl)Tetrazolium, Inner Salt (MTS) and Related Analogs of 3-(4,5-Dimethylthiazolyl)-2,5-Diphenyltetrazolium Bromide (MTT) Reducing to Purple Water-Soluble Formazans As Cell-Viability Indicators. Bioorg. Med. Chem. Lett. 1991, 1, 611–614. [Google Scholar] [CrossRef]
- de Araújo Jorge, T.C.; de Souza, W. Effect of Carbohydrates, Periodate and Enzymes in the Process of Endocytosis of Trypanosoma Cruzi by Macrophages. Acta Trop. 1984, 41, 17–28. [Google Scholar]
- Martins-Duarte, E.S.; Dubar, F.; Lawton, P.; da Silva, C.F.; Soeiro, M.d.N.C.; de Souza, W.; Biot, C.; Vommaro, R.C. Ciprofloxacin Derivatives Affect Parasite Cell Division and Increase the Survival of Mice Infected with Toxoplasma gondii. PLoS ONE 2015, 10, e0125705. [Google Scholar] [CrossRef]
- Piper, J.R.; Johnson, C.A.; Krauth, C.A.; Carter, R.L.; Hosmer, C.A.; Queener, S.F.; Borotz, S.E.; Pfefferkorn, E.R. Lipophilic Antifolates as Agents against Opportunistic Infections. 1. Agents Superior to Trimetrexate and Piritrexim against Toxoplasma gondii and Pneumocystis carinii in in Vitro Evaluations. J. Med. Chem. 1996, 39, 1271–1280. [Google Scholar] [CrossRef]
- Kovacs, J.A.; Allegra, C.J.; Chabner, B.A.; Swan, J.C.; Drake, J.; Lunde, M.; Parrillo, J.E.; Masur, H. Potent Effect of Trimetrexate, a Lipid-Soluble Antifolate, on Toxoplasma gondii. J. Infect. Dis. 1987, 155, 1027–1032. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.Y.; Fu, H.; Harvey, C.; Ravindran, S.; Roush, W.R.; Boothroyd, J.C.; Khosla, C. Chemistry and Biology of Macrolide Antiparasitic Agents. J. Med. Chem. 2011, 54, 2792–2804. [Google Scholar] [CrossRef]
- Wei, S.; Daniel, B.J.; Brumlik, M.J.; Burow, M.E.; Zou, W.; Khan, I.A.; Wadsworth, S.; Siekierka, J.; Curiel, T.J. Drugs Designed To Inhibit Human P38 Mitogen-Activated Protein Kinase Activation Treat Toxoplasma gondii and Encephalitozoon cuniculi Infection. Antimicrob. Agents Chemother. 2007, 51, 4324–4328. [Google Scholar] [CrossRef]
- Amberg-Johnson, K.; Yeh, E. Host Cell Metabolism Contributes to Delayed-Death Kinetics of Apicoplast Inhibitors in Toxoplasma gondii. Antimicrob. Agents Chemother. 2019, 63, e01646-18. [Google Scholar] [CrossRef]
- Fichera, M.E.; Roos, D.S. A Plastid Organelle as a Drug Target in Apicomplexan Parasites. Nature 1997, 390, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Anthony, N.G.; Breen, D.; Clarke, J.; Donoghue, G.; Drummond, A.J.; Ellis, E.M.; Gemmell, C.G.; Helesbeux, J.-J.; Hunter, I.S.; Khalaf, A.I.; et al. Antimicrobial Lexitropsins Containing Amide, Amidine, and Alkene Linking Groups. J. Med. Chem. 2007, 50, 6116–6125. [Google Scholar] [CrossRef] [PubMed]
- Dubois, E.A.; Cohen, A.F. Retapamulin. Br. J. Clin. Pharmacol. 2010, 69, 2–3. [Google Scholar] [CrossRef]
- dos Santos, B.R.; da Silva Bellini Ramos, A.B.; de Menezes, R.P.B.; Scotti, M.T.; Colombo, F.A.; Marques, M.J.; Reimão, J.Q. Anti-Toxoplasma gondii Screening of MMV Pandemic Response Box and Evaluation of RWJ-67657 Efficacy in Chronically Infected Mice. Parasitology 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Derouin, F.; Chastang, C. In Vitro Effects of Folate Inhibitors on Toxoplasma gondii. Antimicrob. Agents Chemother. 1989, 33, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.A.; Troth, E.V.; Russell, A.C.; Kyle, D.E. Discovery of Anti-Amoebic Inhibitors from Screening the MMV Pandemic Response Box on Balamuthia mandrillaris, Naegleria fowleri, and Acanthamoeba castellanii. Pathogens 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.J.; Khalaf, A.I.; Giordani, F.; Wong, P.E.; Duffy, S.; Barrett, M.; Avery, V.M.; Suckling, C.J. An Evaluation of Minor Groove Binders as Anti- Trypanosoma Brucei Brucei Therapeutics. Eur. J. Med. Chem. 2016, 116, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Reghunandanan, K.; Akhila, T.P.; Krishnan, N.; Darsana, K.M.; Prasad, R.; Nelson-Sathi, S.; Chandramohanadas, R. Search for Novel Plasmodium falciparum Pf ATP4 Inhibitors from the MMV Pandemic Response Box through a Virtual Screening Approach. J. Biomol. Struct. Dyn. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vanmechelen, B.; Stroobants, J.; Chiu, W.; Schepers, J.; Marchand, A.; Chaltin, P.; Vermeire, K.; Maes, P. Identification of Novel Ebola Virus Inhibitors Using Biologically Contained Virus. Antivir. Res. 2022, 200, 105294. [Google Scholar] [CrossRef] [PubMed]
- Fichera, M.E.; Bhopale, M.K.; Roos, D.S. In Vitro Assays Elucidate Peculiar Kinetics of Clindamycin Action against Toxoplasma gondii. Antimicrob. Agents Chemother. 1995, 39, 1530–1537. [Google Scholar] [CrossRef]
- Quinolinyl Pyrimidines: Potent Inhibitors of NDH-2 as a Novel Class of Anti-TB Agents. Available online: https://pubs.acs.org/doi/epdf/10.1021/ml300134b (accessed on 2 October 2023).
- Lin, S.S.; Gross, U.; Bohne, W. Two Internal Type II NADH Dehydrogenases of Toxoplasma gondii Are Both Required for Optimal Tachyzoite Growth. Mol. Microbiol. 2011, 82, 209–221. [Google Scholar] [CrossRef]
- Saleh, A.; Friesen, J.; Baumeister, S.; Gross, U.; Bohne, W. Growth Inhibition of Toxoplasma gondii and Plasmodium falciparum by Nanomolar Concentrations of 1-Hydroxy-2-Dodecyl-4(1H)Quinolone, a High-Affinity Inhibitor of Alternative (Type II) NADH Dehydrogenases. Antimicrob. Agents Chemother. 2007, 51, 1217–1222. [Google Scholar] [CrossRef]
- Widman, D.G.; Gornisiewicz, S.; Shacham, S.; Tamir, S. In Vitro Toxicity and Efficacy of Verdinexor, an Exportin 1 Inhibitor, on Opportunistic Viruses Affecting Immunocompromised Individuals. PLoS ONE 2018, 13, e0200043. [Google Scholar] [CrossRef]
- Perwitasari, O.; Johnson, S.; Yan, X.; Register, E.; Crabtree, J.; Gabbard, J.; Howerth, E.; Shacham, S.; Carlson, R.; Tamir, S.; et al. Antiviral Efficacy of Verdinexor In Vivo in Two Animal Models of Influenza A Virus Infection. PLoS ONE 2016, 11, e0167221. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Nyuykonge, B.; Eadie, K.; Konings, M.; Smeets, J.; Fahal, A.; Bonifaz, A.; Todd, M.; Perry, B.; Samby, K.; et al. Screening the Pandemic Response Box Identified Benzimidazole Carbamates, Olorofim and Ravuconazole as Promising Drug Candidates for the Treatment of Eumycetoma. PLoS Negl. Trop. Dis. 2022, 16, e0010159. [Google Scholar] [CrossRef] [PubMed]
- Borba-Santos, L.P.; Rollin-Pinheiro, R.; da Silva Fontes, Y.; dos Santos, G.M.P.; de Sousa Araújo, G.R.; Rodrigues, A.M.; Guimarães, A.J.; de Souza, W.; Frases, S.; Ferreira-Pereira, A.; et al. Screening of Pandemic Response Box Library Reveals the High Activity of Olorofim against Pathogenic Sporothrix Species. J. Fungi 2022, 8, 1004. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.C.; Castelli, R.F.; Reis, F.C.G.; Samby, K.; Nosanchuk, J.D.; Alves, L.R.; Rodrigues, M.L. Screening of the Pandemic Response Box Reveals an Association between Antifungal Effects of MMV1593537 and the Cell Wall of Cryptococcus neoformans, Cryptococcus deuterogattii, and Candida auris. Microbiol. Spectr. 2022, 10, e00601-22. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, S.A.; Cavender, D.E.; Beers, S.A.; Lalan, P.; Schafer, P.H.; Malloy, E.A.; Wu, W.; Fahmy, B.; Olini, G.C.; Davis, J.E.; et al. RWJ 67657, a Potent, Orally Active Inhibitor of P38 Mitogen-Activated Protein Kinase. J. Pharmacol. Exp. Ther. 1999, 291, 680. [Google Scholar]
- Agrawal, P.; Kumari, S.; Mohmmed, A.; Malhotra, P.; Sharma, U.; Sahal, D. Identification of Novel, Potent, and Selective Compounds against Malaria Using Glideosomal-Associated Protein 50 as a Drug Target. ACS Omega 2023, 8, 38506–38523. [Google Scholar] [CrossRef]
- Martins-Duarte, É.D.S.; De Souza, W.; Vommaro, R.C. Itraconazole Affects Toxoplasma gondii Endodyogeny: Itraconazole Affects Toxoplasma gondii Endodyogeny. FEMS Microbiol. Lett. 2008, 282, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Martins-Duarte, É.S.; Lemgruber, L.; de Souza, W.; Vommaro, R.C. Toxoplasma gondii: Fluconazole and Itraconazole Activity against Toxoplasmosis in a Murine Model. Exp. Parasitol. 2010, 124, 466–469. [Google Scholar] [CrossRef]
- Martins-Duarte, É.S.; de Souza, W.; Vommaro, R.C. Toxoplasma gondii: The Effect of Fluconazole Combined with Sulfadiazine and Pyrimethamine against Acute Toxoplasmosis in Murine Model. Exp. Parasitol. 2013, 133, 294–299. [Google Scholar] [CrossRef]
- Bag, S.; Tawari, N.R.; Queener, S.F.; Degani, M.S. Synthesis and Biological Evaluation of Biguanide and Dihydrotriazine Derivatives as Potential Inhibitors of Dihydrofolate Reductase of Opportunistic Microorganisms. J. Enzym. Inhib. Med. Chem. 2010, 25, 331–339. [Google Scholar] [CrossRef]
- Heaselgrave, W.; Hamad, A.; Coles, S.; Hau, S. In Vitro Evaluation of the Inhibitory Effect of Topical Ophthalmic Agents on Acanthamoeba Viability. Trans. Vis. Sci. Technol. 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, S. Reposition of the Fungicide Ciclopirox for Cancer Treatment. Recent Pat. Anti-Cancer Drug Discov. 2021, 16, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.J.; Hitchcock, C.A.; Sibley, C.M. Current and Emerging Azole Antifungal Agents. Clin. Microbiol. Rev. 1999, 12, 40–79. [Google Scholar] [CrossRef] [PubMed]

| Compound Identification | Previous Activity Reported against T. gondii? | a IC50 (nM) in Tachyzoites of RH Strain (7 Days) | Cytotoxicity against NHDF (7 Days) | ||||
|---|---|---|---|---|---|---|---|
| Plate Position | MMV ID | Trivial Name | Disease Area | b CC50 (nM) | c SI | ||
| A-A2 | MMV1634492 | Eberconazole | Antifungal | No | 723.6 ± 12.6 | d ND | ND |
| A-A3 | MMV002731 | Ciclopirox | Antifungal | No | 497.4 ± 18.1 | 4000 | 8 |
| A-H3 | MMV396785 | Alexidine | Antifungal | No | 271.9 ± 38.4 | 1627 | 6 |
| A-F9 | MMV1580173 | Trimetrexate | Antibacterial | Yes [33,34] | 3.9 ± 0.1 | 11,880 | 3046 |
| A-F10 | MMV000043 | Tafenoquine | Antibacterial | No | 896.9 ± 0.1 | 16,270 | 18 |
| A-G10 | MMV003137 | Erythromycin | Antibacterial | Yes [35] | 131.4 ± 15.8 | 11,930 | 91 |
| B-E5 | MMV1578890 | MMV1578890 | Antibacterial | No | 1100.0 ± 0.1 | 10,300 | 9 |
| B-B9 | MMV1593541 | MMV1593541 | Antibacterial | No | 37.9 ± 40.7 | 2541 | 67 |
| B-C9 | MMV1593537 | MMV1593537 | Antibacterial | No | 940.0 ± 38.6 | 5657 | 6 |
| B-D10 | MMV1580844 | MMV1580844 | Antibacterial | No | 1.5 ± 0.1 | 11,170 | 7447 |
| C-C2 | MMV1634391 | MMV1634391 | Antibacterial | No | 90.6 ± 39.6 | ND | ND |
| C-D2 | MMV1633674 | Retapamulin | Antibacterial | No | 37.3 ± 14.3 | 9979 | 270 |
| C-F5 | MMV1634399 | MMV1634399 | Antibacterial | No | 920.3 ± 0.1 | 24,120 | 26 |
| C-G5 | MMV000051 | Clindamycin | Antibacterial | Yes [29,32] | 3.3 ± 1.9 | 8841 | 2679 |
| D-E4 | MMV642550 | MMV642550 | Antiviral | No | 320.0 ± 17.0 | 6730 | 21 |
| D-B6 | MMV001793 | Fenretinide | Antiviral | No | 918.1 ± 29.1 | 26,670 | 29 |
| D-F8 | MMV1580797 | RWJ-67657 | Antiviral | Yes [36] | 411.7 ± 21.7 | 12,620 | 31 |
| D-A10 | MMV1782115 | MMV1782115 | Antiviral | No | 160.6 ± 60.8 | 9429 | 58 |
| E-D4 | MMV098836 | DNDI1417411 | Antiviral | No | 485.2 ± 43.8 | 14,510 | 32 |
| E-H5 | MMV1580493 | Verdinexor | Antiviral | No | 70.0 ± 24.4 | 2862 | 63 |
| E-A6 | MMV1580482 | URMC-099-C | Antiviral | No | 45.4 ± 31.9 | ND | ND |
| E-C6 | MMV1580496 | Triapine | Antiviral | No | 717.7 ± 0.1 | 2795 | 4 |
| E-H7 | MMV019724 | MMV019724 | Antiviral | No | 109.1 ± 47.6 | 2500 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, M.; Oliveira Costa, A.L.; Vaz, G.H.d.S.; de Souza, G.C.A.; Vitor, R.W.d.A.; Martins-Duarte, É.S. Medicines for Malaria Venture Pandemic Box In Vitro Screening Identifies Compounds Highly Active against the Tachyzoite Stage of Toxoplasma gondii. Trop. Med. Infect. Dis. 2023, 8, 510. https://doi.org/10.3390/tropicalmed8120510
dos Santos M, Oliveira Costa AL, Vaz GHdS, de Souza GCA, Vitor RWdA, Martins-Duarte ÉS. Medicines for Malaria Venture Pandemic Box In Vitro Screening Identifies Compounds Highly Active against the Tachyzoite Stage of Toxoplasma gondii. Tropical Medicine and Infectious Disease. 2023; 8(12):510. https://doi.org/10.3390/tropicalmed8120510
Chicago/Turabian Styledos Santos, Mike, Andréia Luiza Oliveira Costa, Guilherme Henrique de Souza Vaz, Gabriela Carolina Alves de Souza, Ricardo Wagner de Almeida Vitor, and Érica S. Martins-Duarte. 2023. "Medicines for Malaria Venture Pandemic Box In Vitro Screening Identifies Compounds Highly Active against the Tachyzoite Stage of Toxoplasma gondii" Tropical Medicine and Infectious Disease 8, no. 12: 510. https://doi.org/10.3390/tropicalmed8120510
APA Styledos Santos, M., Oliveira Costa, A. L., Vaz, G. H. d. S., de Souza, G. C. A., Vitor, R. W. d. A., & Martins-Duarte, É. S. (2023). Medicines for Malaria Venture Pandemic Box In Vitro Screening Identifies Compounds Highly Active against the Tachyzoite Stage of Toxoplasma gondii. Tropical Medicine and Infectious Disease, 8(12), 510. https://doi.org/10.3390/tropicalmed8120510








