Molecular Detection and Genetic Characterization of Zoonotic Hookworm in Semi-Domesticated Cats Residing in Monasteries in Bangkok, Thailand
Abstract
:1. Introduction
2. Materials and Methods
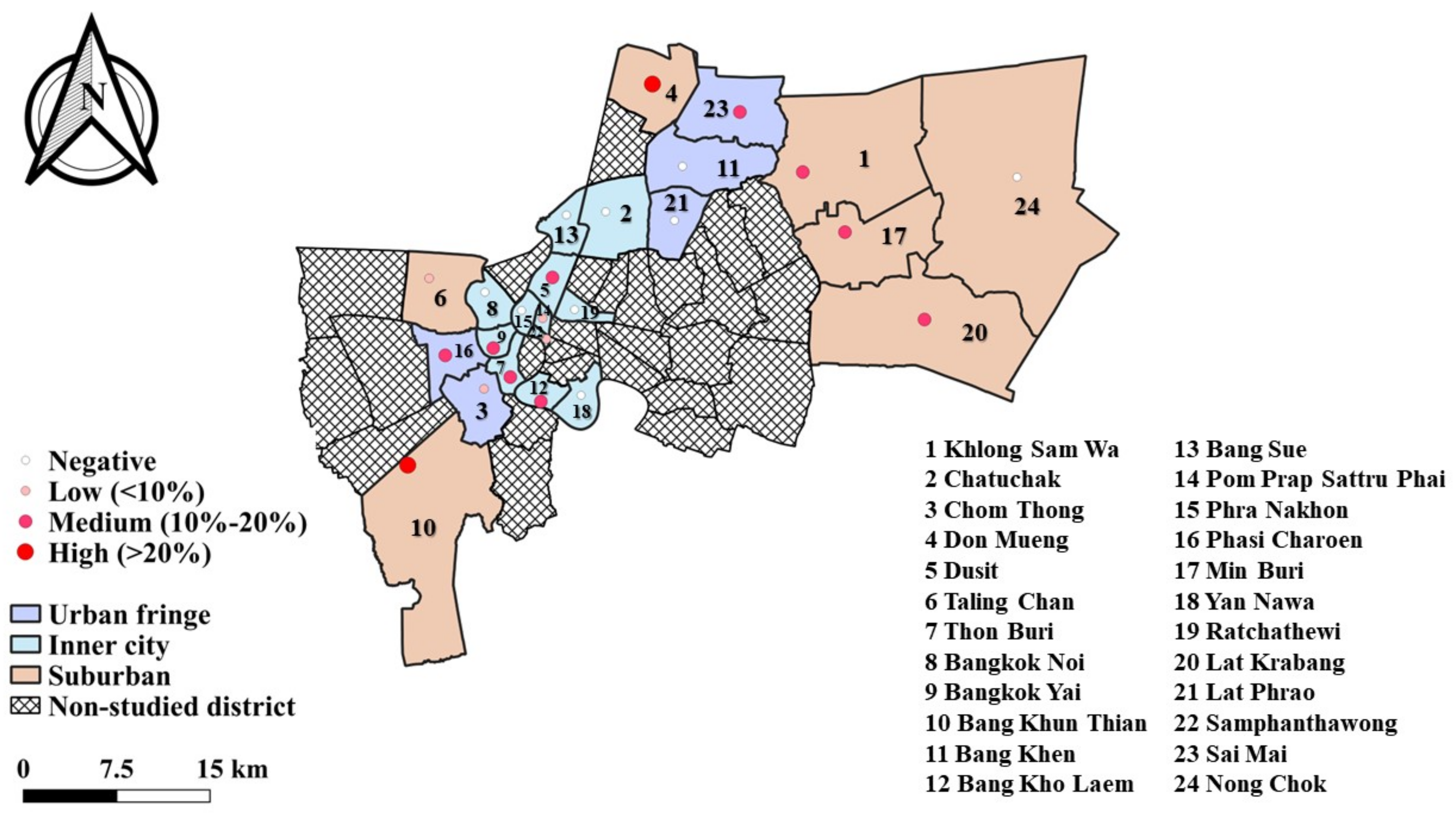
2.1. Study Areas, Samples, and Data Collection
2.2. DNA Extraction
2.3. Molecular Detection of Ancylostoma spp.
2.3.1. Amplification of the ITS Gene
2.3.2. Amplification of the cox 1 Gene
2.4. Sequencing and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Cat Population
3.2. Prevalence and Distribution of Ancylostoma spp. Infection in Cats
3.3. Genetic Characterization of Ancylostoma spp. Infection in Cats
3.4. Risk Factors Associated with Hookworm Infection in Cats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millán, J.; Blasco-Costa, I. Molecular evidence of shared hookworm Ancylostoma tubaeforme haplotypes between the critically endangered Iberian lynx and sympatric domestic cats. Vet. Parasitol. 2012, 186, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Feldmeier, H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect. Dis. 2008, 8, 302–309. [Google Scholar] [CrossRef]
- Kalkofen, U.P. Hookworms of dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 1987, 17, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Ngui, R.; Lim, Y.A.; Traub, R.; Mahmud, R.; Mistam, M.S. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl. Trop. Dis. 2012, 6, 1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niamnuy, N.; Kaewthamasorn, M.; Congpuong, K.; Phaytanavanh, B.; Lohsoonthorn, V. Prevalence and associated risk factors of intestinal parasites in humans and domestic animals across borders of Thailand and Lao PDR: Focus on hookworm and threadworm. Southeast Asian J. Trop. Med. Public Health 2016, 47, 901–911. [Google Scholar]
- Traub, R.J.; Robertson, I.D.; Irwin, P.; Mencke, N.; Thompson, R.C. Application of a species-specific PCR-RFLP to identify Ancylostoma eggs directly from canine faeces. Vet. Parasitol. 2004, 123, 245–255. [Google Scholar] [CrossRef]
- Inpankaew, T.; Schär, F.; Dalsgaard, A.; Khieu, V.; Chimnoi, W.; Chhoun, C.; Sok, D.; Marti, H.; Muth, S.; Odermatt, P.; et al. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg. Infect. Dis. 2014, 20, 976–982. [Google Scholar] [CrossRef]
- Ng-Nguyen, D.; Hii, S.F.; Nguyen, V.A.; Van Nguyen, T.; Van Nguyen, D.; Traub, R.J. Re-evaluation of the species of hookworms infecting dogs in Central Vietnam. Parasit. Vectors 2015, 8, 401. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; Zheng, G.C.; Zhang, P.; Alsarakibi, M.; Zhang, X.H.; Li, Y.W.; Liu, T.; Ren, S.N.; Chen, Z.X.; Liu, Y.L.; et al. Molecular identification of hookworms in stray and shelter dogs from Guangzhou city, China using ITS sequences. J. Helminthol. 2015, 89, 196–202. [Google Scholar] [CrossRef]
- Gasser, R.B. Molecular tools--advances, opportunities and prospects. Vet. Parasitol. 2006, 136, 69–89. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, G.; Alsarakibi, M.; Zhang, X.; Hu, W.; Lu, P.; Lin, L.; Tan, L.; Luo, Q.; Li, G. Molecular identification of Ancylostoma caninum isolated from cats in southern China based on complete ITS sequence. Biomed. Res. Int. 2013, 2013, 868050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngui, R.; Mahdy, M.A.; Chua, K.H.; Traub, R.; Lim, Y.A. Genetic characterization of the partial mitochondrial cytochrome oxidase c subunit I (cox 1) gene of the zoonotic parasitic nematode, Ancylostoma ceylanicum from humans, dogs and cats. Acta Trop. 2013, 128, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Furtado, L.F.V.; Alves, W.P.; Moreira, T.B.; Costa Junior, L.M.; Miranda, R.R.C.; Rabelo, É.M.L. Standardization and application of the tetraprimer ARMS-PCR technique for screening of the E198A SNP in the β-tubulin gene of hookworm populations in Brazil. Vet. Parasitol. 2016, 224, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Romstad, A.; Gasser, R.B.; Nansen, P.; Polderman, A.M.; Chilton, N.B. Necator americanus (Nematoda: Ancylostomatidae) from Africa and Malaysia have different ITS-2 rDNA sequences. Int. J. Parasitol. 1998, 28, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Papaiakovou, M.; Pilotte, N.; Grant, J.R.; Traub, R.J.; Llewellyn, S.; McCarthy, J.S.; Krolewiecki, A.J.; Cimino, R.; Mejia, R.; Williams, S.A. A novel, species-specific, real-time PCR assay for the detection of the emerging zoonotic parasite Ancylostoma ceylanicum in human stool. PLoS Negl. Trop. Dis. 2017, 11, 0005734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avise, J.C. Mitochondrial DNA and the evolutionary genetics of higher animals. Philos. Trans R. Soc. Lond. B Biol. Sci. 1986, 312, 325–342. [Google Scholar]
- Hu, M.; Chilton, N.B.; Zhu, X.; Gasser, R.B. Single-strand conformation polymorphism-based analysis of mitochondrial cytochrome c oxidase subunit 1 reveals significant substructuring in hookworm populations. Electrophoresis 2002, 23, 27–34. [Google Scholar] [CrossRef]
- Moser, J.M.; Carbone, I.; Arasu, P.; Gibson, G. Impact of population structure on genetic diversity of a potential vaccine target in the canine hookworm (Ancylostoma caninum). J. Parasitol. 2007, 93, 796–805. [Google Scholar] [CrossRef]
- Hu, M.; Rabelo, E.M.; Schindler, A.R.; Roberts, H.; Gasser, R.B. Extensive and complex sequence diversity in mitochondrial cytochrome c oxidase subunit 1 within Necator americanus from Colombia revealed by SSCP-coupled sequencing. Mol. Cell Probes. 2008, 22, 234–237. [Google Scholar] [CrossRef]
- Monteiro, K.J.L.; Jaeger, L.H.; Nunes, B.C.; Calegar, D.A.; Reis, E.R.C.D.; Bacelar, P.A.A.; Santos, J.P.D.; Bóia, M.N.; Carvalho-Costa, F.A. Mitochondrial DNA reveals species composition and phylogenetic relationships of hookworms in northeastern Brazil. Infect. Genet. Evol. 2019, 68, 105–112. [Google Scholar] [CrossRef]
- Coffeng, L.E.; Vaz Nery, S.; Gray, D.J.; Bakker, R.; de Vlas, S.J.; Clements, A.C.A. Predicted short and long-term impact of deworming and water, hygiene, and sanitation on transmission of soil-transmitted helminths. PLoS Negl. Trop. Dis. 2018, 12, 0006758. [Google Scholar] [CrossRef] [PubMed]
- Pumidonming, W.; Salman, D.; Gronsang, D.; Abdelbaset, A.E.; Sangkaeo, K.; Kawazu, S.I.; Igarashi, M. Prevalence of gastrointestinal helminth parasites of zoonotic significance in dogs and cats in lower Northern Thailand. J. Vet. Med. Sci. 2017, 78, 1779–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jitsamai, W.; Khrutkham, N.; Hunprasit, V.; Chandrashekar, R.; Bowman, D.; Sukhumavasi, W. Prevalence of endoparasitic and viral infections in client-owned cats in metropolitan Bangkok, Thailand, and the risk factors associated with feline hookworm infections. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100584. [Google Scholar] [CrossRef]
- Yamane, T. Statistics, An Introductory Analysis, 2nd ed.; Harper and Row: New York, NY, USA, 1967; pp. 129–162. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 9 December 2022).
- Setasuban, P.; Vajrasthira, S.; Muennoo, C. Prevalence and zoonotic potential of Ancylostoma ceylanicum in cats in Thailand. Southeast Asian J. Trop. Med. Public Health 1976, 7, 534–539. [Google Scholar] [PubMed]
- Traub, R.J.; Inpankaew, T.; Sutthikornchai, C.; Sukthana, Y.; Thompson, R.C. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet. Parasitol. 2008, 155, 67–73. [Google Scholar] [CrossRef]
- Kladkempetch, D.; Tangtrongsup, S.; Tiwananthagorn, S. Ancylostoma ceylanicum: The Neglected Zoonotic Parasite of Community Dogs in Thailand and Its Genetic Diversity among Asian Countries. Animals 2020, 10, 2154. [Google Scholar] [CrossRef]
- Wongwigkan, J.; Inpankaew, T. Semi-domesticated dogs as a potential reservoir for zoonotic hookworms in Bangkok, Thailand. Vet. World 2020, 13, 909–915. [Google Scholar] [CrossRef]
- Bradbury, R.S.; Hii, S.F.; Harrington, H.; Speare, R.; Traub, R. Ancylostoma ceylanicum Hookworm in the Solomon Islands. Emerg. Infect. Dis. 2017, 23, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Traversa, D. Pet roundworms and hookworms: A continuing need for global worming. Parasit. Vectors 2012, 5, 91. [Google Scholar] [CrossRef] [Green Version]
- Balassiano, B.C.; Campos, M.R.; Menezes Rde, C.; Pereira, M.J. Factors associated with gastrointestinal parasite infection in dogs in Rio de Janeiro, Brazil. Prev. Vet. Med. 2009, 91, 234–240. [Google Scholar] [CrossRef]
- Pinyopanuwat, N.; Kengradomkij, C.; Kamyingkird, K.; Chimnoi, W.; Suraruangchai, D.; Inpankaew, T. Stray animals (dogs and cats) as sources of soil-transmitted parasite eggs/cysts in temple grounds of Bangkok metropolitan, Thailand. J. Trop. Med. Parasitol. 2018, 41, 15–20. [Google Scholar]

| Target Gene | Primer Name | Product Size | Reference |
|---|---|---|---|
| ITS | RTGHF1: 5′-CGTGCTAGTCTTCAGGACTTTG-3′ RTGHR1: 5′-CGTTGTCATACTAGCCACTGC-3′ | 679–690 | [6] |
| cox 1 | Aceycox1F: 5′-GCTTTTGGTATTGTA-AGACAG-3′ Aceycox1R: 5′-CTAACAACATAATAAG-TATCATG-3′ | 377 | [7] |
| Factor | Number of Cats | Hookworm Positive (%) | Chi-Square χ2 | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|
| Sex | 0.14 | 0.708 | |||
| Male | 287 | 36 (12.5) | 1.0 | ||
| Female | 213 | 30 (14.1) | 1.1 (0.7–1.9) | ||
| Age | 4.80 | 0.029 | |||
| Less than one year | 104 | 7 (6.7) | 1.0 | ||
| More than one year | 396 | 59 (14.9) | 2.4 (1.1–5.5) | ||
| Free-roaming | 1.67 | 0.196 | |||
| Yes | 268 | 30 (11.2) | 1.0 | ||
| No | 232 | 36 (15.5) | 1.5 (0.9–2.5) | ||
| Veterinary attention | 15.04 | 0.0001 | |||
| Yes | 361 | 34 (9.4) | 1.0 | ||
| No | 139 | 32 (23.0) | 2.9 (1.7–4.9) | ||
| Previous deworming | 0.72 | 0.403 | |||
| Yes | 123 | 13 (10.6) | 1.0 | ||
| No | 377 | 53 (14.0) | 1.4 (0.7–2.6) | ||
| Bangkok Zone | 12.00 | 0.0005 | |||
| Inner city | 218 | 18 (8.3) | 1.0 | ||
| Urban fringe | 105 | 11 (10.5) | 2.3 (1.1–4.7) | ||
| Suburban | 177 | 37 (20.9) | 2.9 (1.6–5.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phoosangwalthong, P.; Kamyingkird, K.; Kengradomkij, C.; Chimnoi, W.; Odermatt, P.; Inpankaew, T. Molecular Detection and Genetic Characterization of Zoonotic Hookworm in Semi-Domesticated Cats Residing in Monasteries in Bangkok, Thailand. Trop. Med. Infect. Dis. 2023, 8, 122. https://doi.org/10.3390/tropicalmed8020122
Phoosangwalthong P, Kamyingkird K, Kengradomkij C, Chimnoi W, Odermatt P, Inpankaew T. Molecular Detection and Genetic Characterization of Zoonotic Hookworm in Semi-Domesticated Cats Residing in Monasteries in Bangkok, Thailand. Tropical Medicine and Infectious Disease. 2023; 8(2):122. https://doi.org/10.3390/tropicalmed8020122
Chicago/Turabian StylePhoosangwalthong, Pornkamol, Ketsarin Kamyingkird, Chanya Kengradomkij, Wissanuwat Chimnoi, Peter Odermatt, and Tawin Inpankaew. 2023. "Molecular Detection and Genetic Characterization of Zoonotic Hookworm in Semi-Domesticated Cats Residing in Monasteries in Bangkok, Thailand" Tropical Medicine and Infectious Disease 8, no. 2: 122. https://doi.org/10.3390/tropicalmed8020122
APA StylePhoosangwalthong, P., Kamyingkird, K., Kengradomkij, C., Chimnoi, W., Odermatt, P., & Inpankaew, T. (2023). Molecular Detection and Genetic Characterization of Zoonotic Hookworm in Semi-Domesticated Cats Residing in Monasteries in Bangkok, Thailand. Tropical Medicine and Infectious Disease, 8(2), 122. https://doi.org/10.3390/tropicalmed8020122







