A Strategy Formulation Framework for Efficient Screening during the Early Stage of a Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Proposed Strategy Formulation Framework (SFF)
2.1.1. The First Question: Who Will Be Screened Based on Which Priorities?
2.1.2. The Second Question: How Many People Need to Be Screened?
2.1.3. The Third Question: How Is the Efficiency of a Screening Strategy Evaluated?
2.2. Proposed Screening Strategy
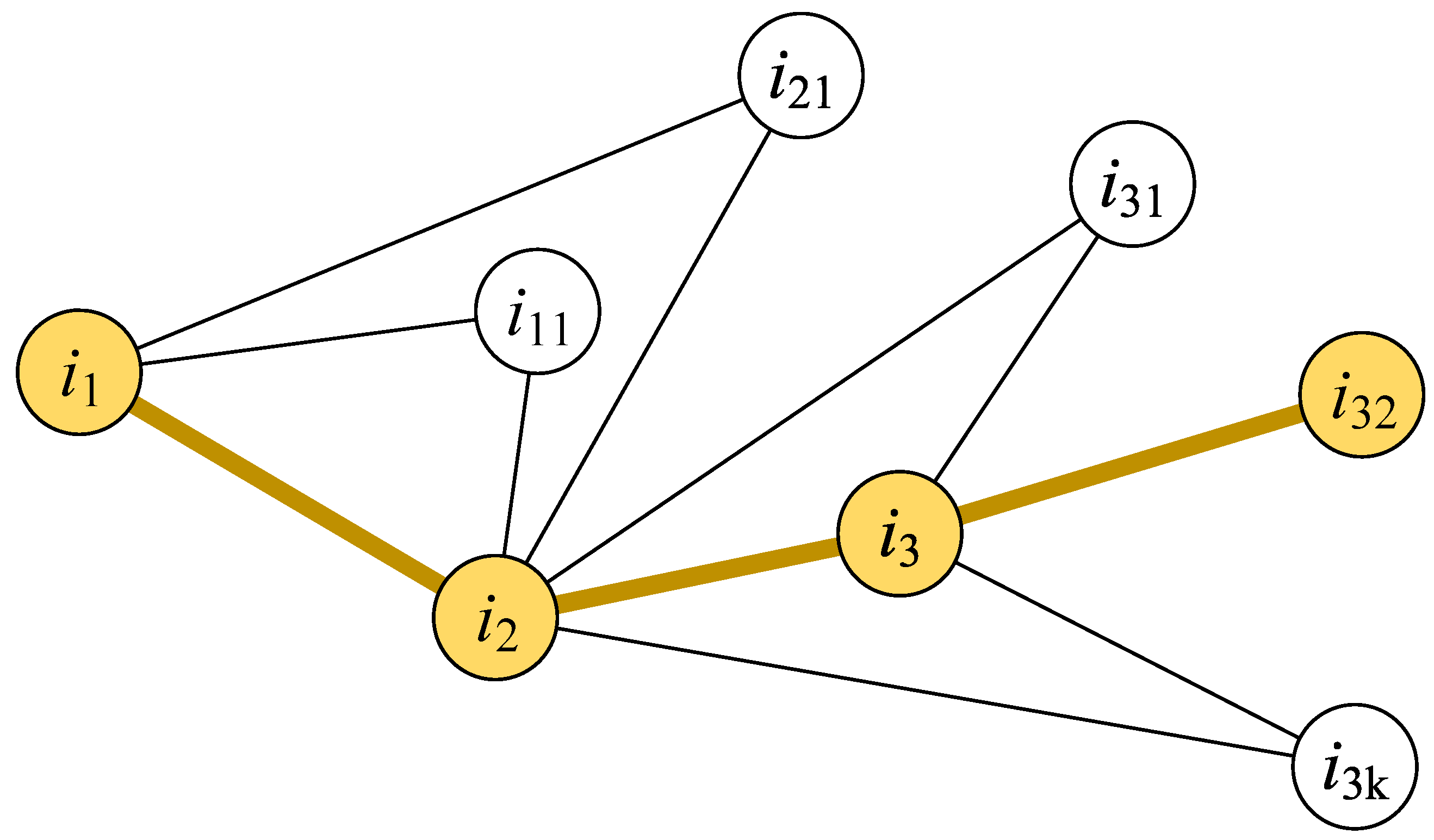
2.2.1. Two Metrics for Identifying Influential People
2.2.2. Setting Screening Priorities for Influential People
Setting Screening Priorities Based on Individual Infection Risk
Setting Screening Priorities Based on the Individual Diffusion Influence
2.2.3. Screening Number in a Screening Period
The Base Screening Value in a Screening Period
The Screening Floating Value in a Screening Period
2.3. Proposed Model
2.3.1. The Activity Features and Behaviors of People in Normal Life
2.3.2. Activity Features and Behaviors of People during Disease Diffusion
2.3.3. Activity Features and Behaviors of People during Information Spreading
2.4. Experimental Designations
3. Results
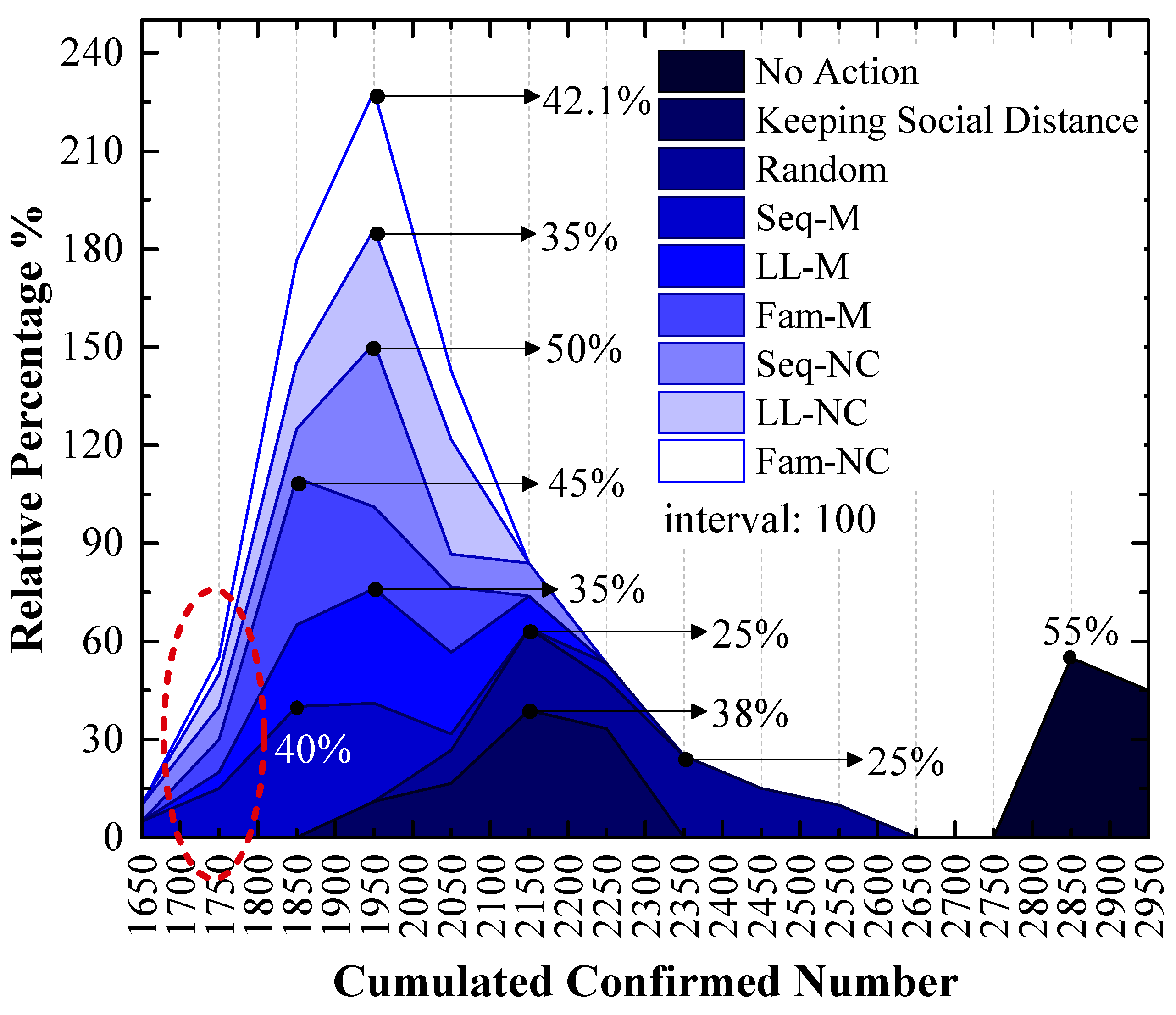
3.1. Experiment Results of the 1st Scenario
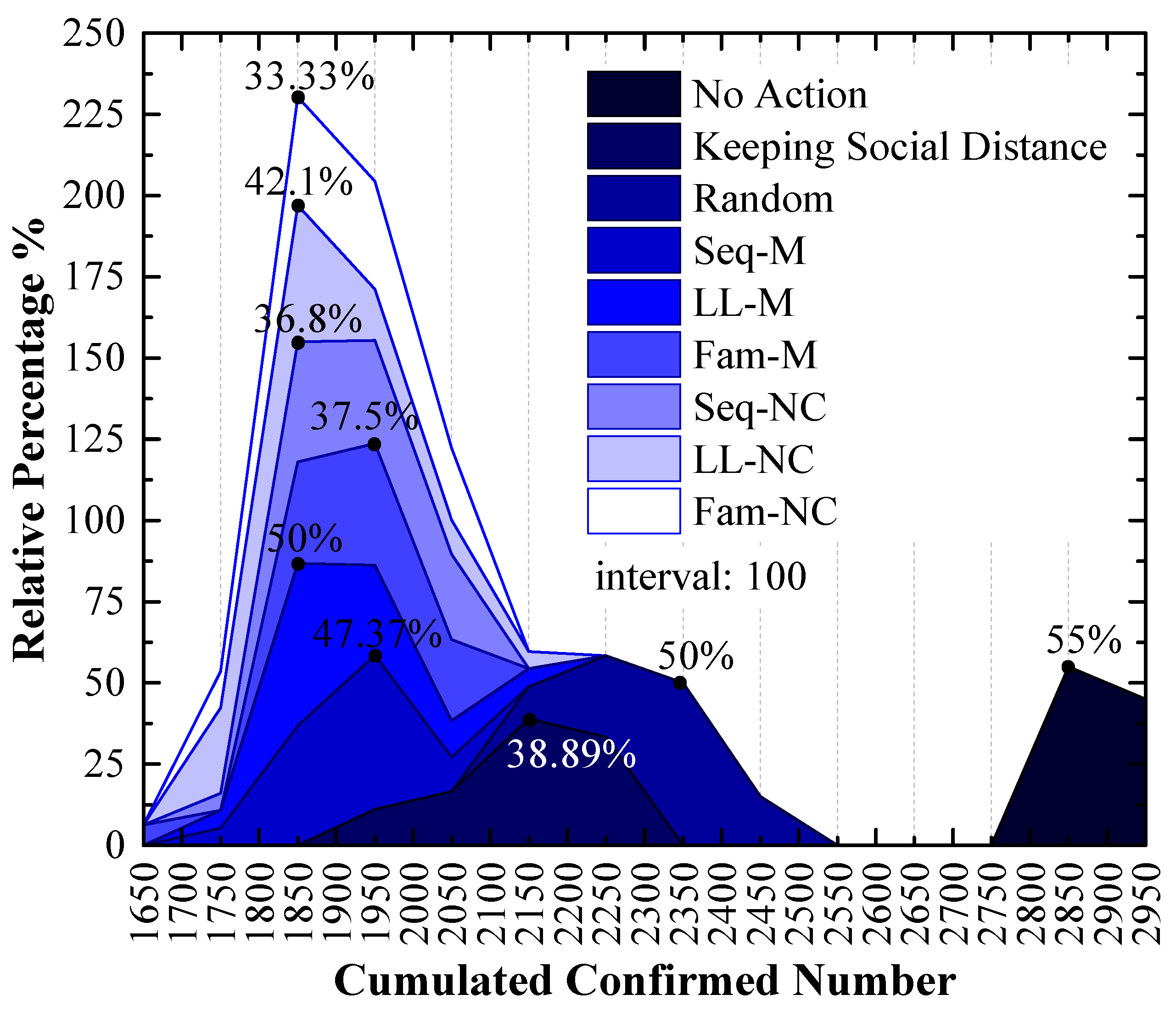
3.2. Experiment Results of the 2nd Scenario
4. Discussion
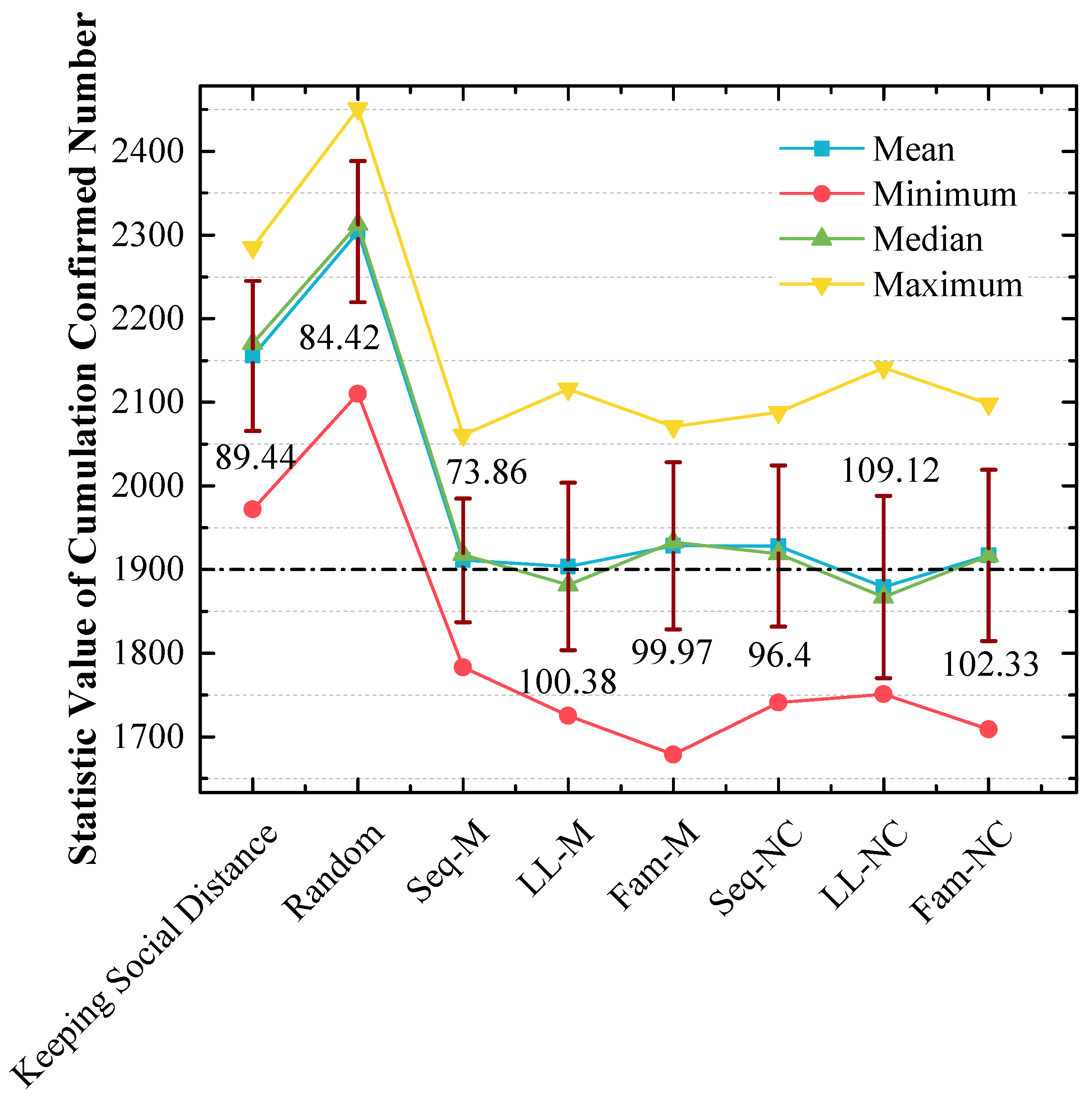
4.1. Seq-M Is the Most Efficient Strategy in the 1st Scenario
4.2. LL-NC Is the Most Efficient Strategy in the 2nd Scenario
4.3. Long-Line Screening Mode Does Not Perform Well in the 1st Scenario and Performs Well in the 2nd Scenario
4.4. Seq-M and Fam-M Perform Well in the 1st Scenario and Do Not Perform Well in the 2nd Scenario
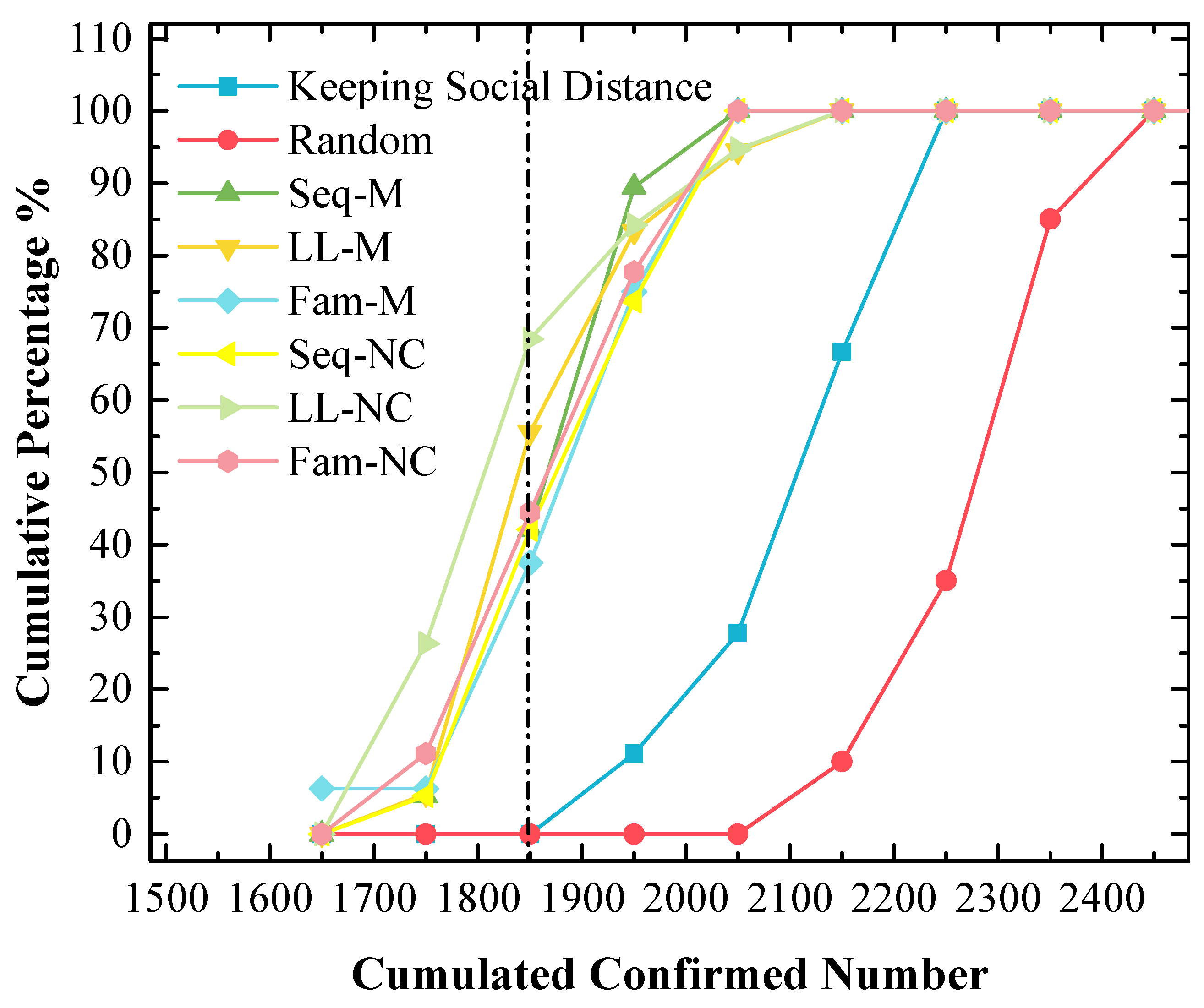
4.5. The Stabilities of the Screening Strategies That Perform Well Are Not Good
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H.; Stratton, C.W.; Tang, Y.-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020, 92, 401–402. [Google Scholar] [CrossRef]
- Liu, Y.; Morgenstern, C.; Kelly, J.; Lowe, R.; Jit, M. The impact of non-pharmaceutical interventions on SARS-CoV-2 transmission across 130 countries and territories. BMC Med. 2021, 19, 40. [Google Scholar] [CrossRef]
- Desforges, M.; Gurdasani, D.; Hamdy, A.; Leonardi, A.J. Uncertainty around the Long-Term Implications of COVID-19. Pathogens 2021, 10, 1267. [Google Scholar] [CrossRef]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- López Seguí, F.; Estrada Cuxart, O.; Mitjà i Villar, O.; Hernández Guillamet, G.; Prat Gil, N.; Maria Bonet, J.; Ara del Rey, J. A cost-benefit analysis of the COVID-19 asymptomatic mass testing strategy in the north Metropolitan area of Barcelona. Int. J. Environ. Res. Public Health 2021, 18, 7028. [Google Scholar] [CrossRef]
- Niu, Y.; Rui, J.; Wang, Q.; Zhang, W.; Chen, Z.; Xie, F.; Zhao, Z.; Lin, S.; Zhu, Y.; Wang, Y.; et al. Containing the Transmission of COVID-19: A Modeling Study in 160 Countries. Front. Med. 2021, 8, 1322. [Google Scholar] [CrossRef]
- Stratil, J.M.; Biallas, R.L.; Burns, J.; Arnold, L.; Geffert, K.; Kunzler, A.M.; Movsisyan, A. Non-pharmacological measures implemented in the setting of long-term care facilities to prevent SARS-CoV-2 infections and their consequences: A rapid review. Cochrane Database Syst. Rev. 2021, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Muscatello, D.; Tian, Y.; Chen, Y.; Li, S.; Duan, W.; Ma, C.; Sun, Y.; Wu, S.; Ge, L.; et al. Role of presymptomatic transmission of COVID-19: Evidence from Beijing, China. J. Epidemiol. Community Health 2020, 75, 84–87. [Google Scholar] [CrossRef]
- Niehus, R.; De Salazar, P.M.; Taylor, A.R.; Lipsitch, M. Quantifying bias of COVID-19 prevalence and severity estimates in Wuhan, China that depend on reported cases in international travelers. medRxiv 2020. [Google Scholar]
- Black, J.R.; Bailey, C.; Przewrocka, J.; Dijkstra, K.K.; Swanton, C. COVID-19: The case for health-care worker screening to prevent hospital transmission. Lancet 2020, 395, 1418–1420. [Google Scholar] [CrossRef]
- Gostic, K.; Gomez, A.C.; Mummah, R.O.; Kucharski, A.J.; Lloyd-Smith, J.O. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. Elife 2020, 9, e55570. [Google Scholar] [CrossRef]
- Hoyler, M.M.; Abramovitz, S.; Aaronson, J.; White, R.S. The importance of COVID-19 screening and testing in the obstetric patient population. J. Clin. Anesthesia 2020, 66, 109938. [Google Scholar] [CrossRef]
- Keeling, M.J.; Hollingsworth, T.D.; Read, J.M. Efficacy of contact tracing for the containment of the 2019 novel coronavirus (COVID-19). J. Epidemiol. Community Health 2020, 74, jech-2020-214051. [Google Scholar] [CrossRef]
- Staceviien, I.; Burokien, S.; Steponaviien, A.; Vaiiūnien, D.; Jankauskien, A. A cross-sectional study of screening for coronavirus disease 2019 (COVID-19) at the pediatric emergency department in Vilnius during the first wave of the pandemic. Eur. J. Pediatr. 2021, 180, 2137–2145. [Google Scholar] [CrossRef]
- Johanna, N.; Citrawijaya, H.; Wangge, G. Mass screening vs lockdown vs combination of both to control COVID-19: A systematic review. J. Public Health Res. 2020, 9, covidwho-1013434. [Google Scholar] [CrossRef]
- Chowdhury, R.; Heng, K.; Goh, G.; Okonofua, D.; Ochoa-Rosales, C.; Gonzalez-Jaramillo, V.; Bhuiya, A.; Reidpath, D.; Prathapan, S.; Shahzad, S.; et al. Dynamic interventions to control COVID-19 pandemic: A multivariate prediction modelling study comparing 16 worldwide countries. Eur. J. Epidemiol. 2020, 35, 389–399. [Google Scholar] [CrossRef]
- Peng, Z.; Song, W.; Ding, Z.; Guan, Q.; Yang, X.; Xu, Q.; Wang, X.; Xia, Y. Linking key intervention timings to rapid declining effective reproduction number to quantify lessons against COVID-19. Front. Med. 2020, 14, 623–629. [Google Scholar] [CrossRef]
- Jia, Q.; Guo, Y.; Wang, G.; Barnes, S.J. Big Data Analytics in the Fight against Major Public Health Incidents (Including COVID-19): A Conceptual Framework. Int. J. Environ. Res. Public Health 2020, 17, 6161. [Google Scholar] [CrossRef]
- Anttiroiko, A.-V. Successful government responses to the pandemic: Contextualizing national and urban responses to the COVID-19 outbreak in east and west. Int. J. E Plan. Res. IJEPR 2021, 10, 1–17. [Google Scholar] [CrossRef]
- Ferretti, L.; Wymant, C.; Kendall, M.; Zhao, L.; Nurtay, A.; Abeler-Dörner, L.; Fraser, C. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020, 368, eabb6936. [Google Scholar] [CrossRef]
- Baker, M.G.; Kvalsvig, A.; Verrall, A.J.; Wellington, N. New Zealand’s COVID-19 elimination strategy. Med. J. Aust. 2020, 213, 198–200. [Google Scholar] [CrossRef]
- Wang, C.J.; Ng, C.Y.; Brook, R.H. Response to COVID-19 in Taiwan: Big data analytics, new technology, and proactive testing. Jama 2020, 323, 1341–1342. [Google Scholar] [CrossRef]
- España, G.; Grefenstette, J.; Perkins, A.; Torres, C.; Carey, A.C.; Diaz, H.; de la Hoz, F.; Burke, D.S.; van Panhuis, W.G. Exploring scenarios of chikungunya mitigation with a data-driven agent-based model of the 2014–2016 outbreak in Colombia. Sci. Rep. 2018, 8, 12201. [Google Scholar] [CrossRef]
- Hoertel, N.; Blachier, M.; Blanco, C.; Olfson, M.; Massetti, M.; Limosin, F.; Leleu, H. Facing the COVID-19 epidemic in NYC: A stochastic agent-based model of various intervention strategies. medRxiv 2020. [Google Scholar]
- Lee, S.; Zabinsky, Z.B.; Wasserheit, J.N.; Kofsky, S.M.; Liu, S. COVID-19 Pandemic Response Simulation in a Large City: Impact of Nonpharmaceutical Interventions on Reopening Society. Med. Decis. Mak. 2021, 41, 419–429. [Google Scholar] [CrossRef]
- Ze, A.; Oma, B.; Ca, C. A decision analytic approach for social distancing policies during early stages of COVID-19 pandemic-ScienceDirect. Decis. Support Syst. 2022, 161, 113630. [Google Scholar]
- Iyaniwura, S.A.; Falcão, R.C.; Ringa, N.; Adu, P.A.; Spencer, M.; Taylor, M.; Colijn, C.; Coombs, D.; Janjua, N.Z.; Irvine, M.A.; et al. Mathematical modeling of COVID-19 in British Columbia: An age-structured model with time-dependent contact rates. Epidemics 2022, 39, 100559. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Gao, S.; Wang, H. Complex Contact Network of Patients at the Beginning of an Epidemic Outbreak: An Analysis Based on 1218 COVID-19 Cases in China. Int. J. Environ. Res. Public Health 2022, 19, 689. [Google Scholar] [CrossRef]
- Kumar, N.; Oke, J.; Nahmias-Biran, B.-H. Activity-based epidemic propagation and contact network scaling in auto-dependent metropolitan areas. Sci. Rep. 2021, 11, 1–14. [Google Scholar]
- Pechlivanoglou, T.; Li, J.; Sun, J.; Heidari, F.; Papagelis, M. Epidemic Spreading in Trajectory Networks. Big Data Res. 2021, 27, 100275. [Google Scholar] [CrossRef]
- Nagarajan, K.; Muniyandi, M.; Palani, B.; Sellappan, S. Social network analysis methods for exploring SARS-CoV-2 contact tracing data. BMC Med. Res. Methodol. 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soodejani, M.T.; Tabatabaei, S.M.; Dehghani, A.; McFarland, W.; Sharifi, H. Impact of Mass Screening on the Number of Confirmed Cases, Recovered Cases, and Deaths Due to COVID-19 in Iran: An Interrupted Time Series Analysis. Arch. Iran. Med. 2020, 23, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Lai, L.; Du, Z.; Huang, Y.; Su, J.; Wu, C.; Yang, S.; Jia, P. Risk assessment of imported COVID-19 in China: A modeling study in Sichuan Province. Transbound. Emerg. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, X.; Gong, L.; Huo, S.; Gao, X.; Nie, S.; Liu, Z. Epidemiological and clinical features of SARS-CoV-2 cluster infection in Anhui Province, Eastern China. Int. J. Infect. Dis. 2022, 117, 372–377. [Google Scholar] [CrossRef]
- If You Are a COVID-19 ‘Close Contact’. Healthdirect. Available online: https://www.healthdirect.gov.au/covid-19/close-contacts (accessed on 16 January 2023).
- Qiu, L.; Zhang, J.; Tian, X. Ranking influential nodes in complex networks based on local and global structures. Appl. Intell. 2021, 51, 4394–4407. [Google Scholar] [CrossRef]
- Ghalmane, Z.; Hassouni, M.E.; Cherifi, C.; Cherifi, H. Centrality in modular networks. arXiv 2018, arXiv:181005101. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, C.; Li, G.; Sun, D.; Ren, F.; Zomaya, A.; Ranjan, R. Detecting users’ anomalous emotion using social media for business intelligence. J. Comput. Sci. 2018, 25, 193–200. [Google Scholar] [CrossRef]
- Wei, S.; Cho, K.E.; Um, K.H. Top-down Behavior Planning for Real-life Simulation. J. Korea Multimed. Soc. 2007, 10, 1714–1725. [Google Scholar]
- Her, M. How is COVID-19 affecting South Korea? What is our current strategy? Disaster Med. Public Health Prep. 2020, 14, 684–686. [Google Scholar] [CrossRef]
- Goel, A.D.; Bhardwaj, P.; Gupta, M.; Kumar, N.; Jain, V.; Misra, S.; Saurabh, S.; Garg, M.K.; Nag, V.L. Swift contact tracing can prevent transmission—Case report of an early COVID-19 positive case. J. Infect. Public Health 2020, 14, 260–262. [Google Scholar] [CrossRef]








| No. | Parameters | Value | Remark |
|---|---|---|---|
| 1 | Family size | 1000 | |
| 2 | Population size | 2890–3100 | |
| 3 | Personnel density | 23,885 people per square kilometer | The personnel density of a city in China. |
| 4 | Initial infected person | 1 | There is 1 infected person at the beginning of the simulation. |
| 5 | Initial warning/rumor spreaders | uniform_discr (10, 20) | 10–20 people are warned at the beginning of the simulation. |
| 6 | Initial infected persons in a day | Poisson (1) | Before the control, 0–4 new initially infected people (whose distribution conforms to the Poisson distribution) may appear in the crowd every day. |
| 7 | New spreaders of warning/rumor in a day | Poisson (5) | After the control, 0–15 initial warning or rumor spreaders (whose distribution conforms to the Poisson distribution) may be generated in the crowd every day. |
| 8 | Detection reagents | adequate | Assumption. |
| 9 | Immunities of people | abs (normal (0.1, 0.7)) | People’s immunities are in the range of 0.2–1.2, which conforms to the normal distribution, and the asymptomatic peoples’ immunities exceed 0.9. |
| 10 | Incubation duration | this.immunity * 10 | People’s incubation durations are in the range of 2–12 days, which conform to the normal distribution, and the middle value is 7 days. |
| 11 | Infectious distance | abs (normal (1.2, 6)) | The distances that can create infections are in the range of 2–12 m. People can be infected if the real distance is smaller than this parameter. |
| 12 | Infectious rate | One time per 1 min | People can diffuse the virus every minute if they have contacts. |
| 13 | Mortality | uniform (0.002, 0.003) * (confirmed time -infected time) | Mortality is related to the time when the infected person is confirmed. We assume that mortality is approximately 0.03–0.05. |
| 14 | Treatment duration | abs (normal (1, 10)) | The treatment durations of confirmed people are in the range of 6–14 days, which conforms to the normal distribution. |
| 15 | Detection duration | abs (normal (0.2, 1)) | The detection durations of people are in the range of 0–2 days, which conforms to the normal distribution, and the middle value is 1 day. |
| 16 | Close contact tracing rate | 2 h | More details as follows. |
| Application Scenario | Application Strategy |
|---|---|
| / | No action |
| / | Only keeping social distance |
| After the first confirmed case appears (which is named the 1st scenario) | Keeping social distance and the random screening strategy |
| Keeping social distance and the Seq-M | |
| Keeping social distance and the LL-M | |
| Keeping social distance and the Fam-M | |
| Keeping social distance and the Seq-NC | |
| Keeping social distance and the LL-NC | |
| Keeping social distance and the Fam-NC | |
| Before the first confirmed case appears (which is named the 2nd scenario) | Keeping social distance and the random screening strategy |
| Keeping social distance and the Seq-M | |
| Keeping social distance and the LL-M | |
| Keeping social distance and the Fam-M | |
| Keeping social distance and the Seq-NC | |
| Keeping social distance and the LL-NC | |
| Keeping social distance and the Fam-NC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, Y.; Zhang, Q.; Lu, Q.; Liu, C.; Yi, F. A Strategy Formulation Framework for Efficient Screening during the Early Stage of a Pandemic. Trop. Med. Infect. Dis. 2023, 8, 78. https://doi.org/10.3390/tropicalmed8020078
Wang S, Zhang Y, Zhang Q, Lu Q, Liu C, Yi F. A Strategy Formulation Framework for Efficient Screening during the Early Stage of a Pandemic. Tropical Medicine and Infectious Disease. 2023; 8(2):78. https://doi.org/10.3390/tropicalmed8020078
Chicago/Turabian StyleWang, Shuangyan, Yuan Zhang, Qiang Zhang, Qibin Lu, Chengcheng Liu, and Fangxin Yi. 2023. "A Strategy Formulation Framework for Efficient Screening during the Early Stage of a Pandemic" Tropical Medicine and Infectious Disease 8, no. 2: 78. https://doi.org/10.3390/tropicalmed8020078
APA StyleWang, S., Zhang, Y., Zhang, Q., Lu, Q., Liu, C., & Yi, F. (2023). A Strategy Formulation Framework for Efficient Screening during the Early Stage of a Pandemic. Tropical Medicine and Infectious Disease, 8(2), 78. https://doi.org/10.3390/tropicalmed8020078








