Effects of Age, Gender and Soil-Transmitted Helminth Infection on Prevalence of Plasmodium Infection among Population Living in Bata District, Equatorial Guinea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Area
2.3. Study Population
2.4. Sample Size Calculation
2.5. Laboratory Analysis
2.5.1. Malaria Diagnosis
2.5.2. Kato–Katz Technique for Helminth Diagnosis
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Study Participants Demographic Characteristics
3.2. Distribution of Malaria and STH Infection
3.3. Prevalence of Malaria Infection and Age
3.4. Prevalence of Malaria Infection and STH Infections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monroe, A.; Williams, N.A.; Ogoma, S.; Karema, C.; Okumu, F. Reflections on the 2021 World Malaria Report and the future of malaria control. Malar. J. 2022, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- World Malaria Report 2021 [Internet]. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 13 August 2022).
- Nkumama, I.N.; O’Meara, W.P.; Osier, F.H.A. Changes in Malaria Epidemiology in Africa and New Challenges for Elimination. Trends Parasitol. 2017, 33, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berzosa, P.; Lucio, A.; Romay-Barja, M.; Herrador, Z.; González, V.; García, L.; Martínez, A.F.; Santana-Morales, M.; Ncogo, P.; Valladares, B. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malar. J. 2018, 17, 333. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, H.J.; Reddy, V.P.; Abaga, S.; Matias, A.; Reddy, M.R.; Kulkarni, V.; Schwabe, C.; Segura, L.; Kleinschmidt, I.; Slotman, M.A. Malaria transmission after five years of vector control on Bioko Island, Equatorial Guinea. Parasit. Vectors 2012, 5, 253. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.M.; Mann, A.G.; Schwabe, C.; Reddy, M.R.; Gomes, R.I.; Slotman, M.A. Five years of malaria control in the continental region, Equatorial Guinea. Malar. J. 2013, 12, 154. [Google Scholar] [CrossRef] [Green Version]
- Billingsley, P.; Maas, C.; Olotu, A.; Schwabe, C.; García, G.; Rivas, M.; Hergott, D.; Daubenberger, C.; Saverino, E.; Chaouch, A.; et al. The Equatoguinean Malaria Vaccine Initiative: From the Launching of a Clinical Research Platform to Malaria Elimination Planning in Central West Africa. Am. J. Trop. Med. Hyg. 2020, 103, 947. [Google Scholar] [CrossRef]
- Cook, J.; Hergott, D.; Phiri, W.; Rivas, M.R.; Bradley, J.; Segura, L.; Garcia, G.; Schwabe, C.; Kleinschmidt, I. Trends in parasite prevalence following 13 years of malaria interventions on Bioko island, Equatorial Guinea: 2004–2016. Malar. J. 2018, 17, 62. [Google Scholar] [CrossRef] [Green Version]
- Fuseini, G.; Phiri, W.; von Fricken, M.; Smith, J.; García, G. Evaluation of the residual effectiveness of Fludora fusion WP-SB, a combination of clothianidin and deltamethrin, for the control of pyrethroid-resistant malaria vectors on Bioko Island, Equatorial Guinea. Acta Trop. 2019, 196, 42–47. [Google Scholar] [CrossRef]
- Nchama, V.; Said, A.; Mtoro, A.; Bidjimi, G.; Eyang, M.; Maye, E.; Mangue, M.; Okomo, G.; Pasialo, B.; Ondo, D. Incidence of Plasmodium Falciparum Malaria Infection in 6-month- to 45-year-olds on Bioko Island, Equatorial Guinea. Malar. J. 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Birkholtz, L.M.; Bornman, R.; Focke, W.; Mutero, C.; de Jager, C. Sustainable malaria control: Transdisciplinary approaches for translational applications. Malar. J. 2012, 11, 431. [Google Scholar] [CrossRef] [Green Version]
- Shiff, C. Integrated Approach to Malaria Control. Clin. Microbiol. Rev. 2002, 15, 278–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, C.A.; Fuseini, G.; Donfack, O.T.; Smith, J.M.; Mifumu, T.A.O.; Akadiri, G.; Eyang, D.E.M.; Eburi, C.O.; Vaz, L.M.; Micha, M.V.; et al. Malaria outbreak in Riaba district, Bioko Island: Lessons learned. Malar. J. 2020, 19, 277. [Google Scholar] [CrossRef] [PubMed]
- Control I of M (US) C for the S on MP and Stanley Coaks; Oaks, S.C., Jr.; Mitchell, V.S.; Pearson, G.W.; Carpenter, C.C.J. Epidemiologic Approaches to Malaria Control [Internet]. Malaria: Obstacles and Opportunities. National Academies Press (US). 1991. Available online: https://www.ncbi.nlm.nih.gov/books/NBK234329/ (accessed on 16 September 2022).
- Makundi, E.A.; Mboera, L.E.G.; Malebo, H.M.; Kitua, A.Y. Priority Setting on Malaria Interventions in Tanzania: Strategies and Challenges to Mitigate against the Intolerable Burden [Internet]. Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene. American Society of Tropical Medicine and Hygiene; 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1702/ (accessed on 16 September 2022).
- Nalinya, S.; Musoke, D.; Deane, K. Malaria prevention interventions beyond long-lasting insecticidal nets and indoor residual spraying in low- and middle-income countries: A scoping review. Malar. J. 2022, 21, 31. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, M.; Xie, D.D.; Li, J.; Chen, J.T.; Eyi, U.M.; Monte-Nguba, S.; Ehapo, J.C.S.; Yang, H.; Yang, H.; et al. Prevalence of HIV and malaria: A cross-sectional study on Bioko Island, Equatorial Guinea. Afr. J. AIDS Res. AJAR 2017, 16, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barroso, D.; García-Carrasco, E.; Herrador, Z.; Ncogo, P.; Romay-Barja, M.; Mangue, M.E.; Nseng, G.; Riloha, M.; Santana, M.A.; Valladares, B.; et al. Spatial clustering and risk factors of malaria infections in Bata district, Equatorial Guinea. Malar. J. 2017, 16, 146. [Google Scholar] [CrossRef] [Green Version]
- Citron, D.T.; Guerra, C.A.; García, G.A.; Wu, S.L.; Battle, K.E.; Gibson, H.S.; Smith, D.L. Quantifying malaria acquired during travel and its role in malaria elimination on Bioko Island. Malar. J. 2021, 20, 359. [Google Scholar] [CrossRef]
- Bradley, J.; Monti, F.; Rehman, A.M.; Schwabe, C.; Vargas, D.; Garcia, G.; Hergott, D.; Riloha, M.; Kleinschmidt, I. Infection importation: A key challenge to malaria elimination on Bioko Island, Equatorial Guinea. Malar. J. 2015, 14, 46. [Google Scholar] [CrossRef] [Green Version]
- Ncogo, P.; Herrador, Z.; Romay-Barja, M.; García-Carrasco, E.; Nseng, G.; Berzosa, P.; Santana-Morales, M.A.; Riloha, M.; Aparicio, P.; Valladares, B. Malaria prevalence in Bata district, Equatorial Guinea: A cross-sectional study. Malar. J. 2015, 14, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, M.; de Sousa, B.; Ndong-Mabale, N.; Berzosa, P.; Arez, A.P. Malaria determining risk factors at the household level in two rural villages of mainland Equatorial Guinea. Malar. J. 2018, 17, 203. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, I.; Roca-Feltrer, A.; Griffin, J.T.; Smith, L.; Tanner, M.; Schellenberg, J.A.; Greenwood, B.; Schellenberg, D. Age-Patterns of Malaria Vary with Severity, Transmission Intensity and Seasonality in Sub-Saharan Africa: A Systematic Review and Pooled Analysis. PLoS ONE 2010, 5, e8988. [Google Scholar] [CrossRef] [Green Version]
- Pathak, S.; Rege, M.; Gogtay, N.J.; Aigal, U.; Sharma, S.K.; Valecha, N.; Bhanot, G.; Kshirsagar, N.A.; Sharma, S. Age-Dependent Sex Bias in Clinical Malarial Disease in Hypoendemic Regions. PLoS ONE 2012, 7, e35592. [Google Scholar] [CrossRef] [PubMed]
- Awosolu, O.B.; Yahaya, Z.S.; Farah Haziqah, M.T.; Simon-Oke, I.A.; Fakunle, C. A cross-sectional study of the prevalence, density, and risk factors associated with malaria transmission in urban communities of Ibadan, Southwestern Nigeria. Heliyon 2021, 7, e05975. [Google Scholar] [CrossRef]
- Okiring, J.; Epstein, A.; Namuganga, J.F.; Kamya, E.V.; Nabende, I.; Nassali, M.; Sserwanga, A.; Gonahasa, S.; Muwema, M.; Kiwuwa, S.M.; et al. Gender difference in the incidence of malaria diagnosed at public health facilities in Uganda. Malar. J. 2022, 21, 22. [Google Scholar] [CrossRef]
- Tadege, B.; Mekonnen, Z.; Dana, D.; Sharew, B.; Dereje, E.; Loha, E.; Verweij, J.J.; Casaert, S.; Vlaminck, J.; Ayana, M.; et al. Assessment of environmental contamination with soil-transmitted helminths life stages at school compounds, households and open markets in Jimma Town, Ethiopia. PLoS Negl. Trop Dis. 2022, 16, e0010307. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, P.M.; Lamberton, P.H.L.; Fenwick, A.; Addiss, D.G. Soil-transmitted helminth infections. Lancet 2018, 391, 252–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical dis-eases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartorius, B.; Cano, J.; Simpson, H.; Tusting, L.S.; Marczak, L.B.; Miller-Petrie, M.K.; Kinvi, B.; Zour, E.H.; Mwinzi, P.; Hay, S.I.; et al. Prevalence and intensity of soil-transmitted helminth infections of children in sub-Saharan Africa, 2000–2018: A geospatial analysis. Lancet Glob. Health 2021, 9, e52–e60. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, M.O.; Adebiyi, A.; Cano, J.; Sartorius, B.; Greenwood, B.; Johnson, O.; Wariri, O. Prevalence and distribution pattern of malaria and soil-transmitted helminth co-endemicity in sub-Saharan Africa, 2000–2018: A geospatial analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010321. [Google Scholar] [CrossRef]
- Jourdan, P.M.; Montresor, A.; Walson, J.L. Building on the success of soil-transmitted helminth control—The future of de-worming. PLoS Negl. Trop. Dis. 2017, 11, e0005497. [Google Scholar] [CrossRef] [Green Version]
- Udoidung, N.I.; Opara, K.N.; Yaro, C.A.; Okon, H.A.; Sampson, S.O.; Okoro, P.O.; Johnson, K.C.; Ekpo, E.E. Epidemiology of Co-Infection of Soil-Transmitted Helminths and Plasmodium Falciparum in School Children of Okorombokho, Eastern Obolo Local Government Area, Akwa Ibom State. WOJAST 2021, 13, 191–200. [Google Scholar]
- Degarege, A.; Animut, A.; Legesse, M.; Erko, B. Malaria severity status in patients with soil-transmitted helminth infections. Acta Trop. 2009, 112, 8–11. [Google Scholar] [CrossRef]
- Abbate, J.L.; Ezenwa, V.O.; Guégan, J.F.; Choisy, M.; Nacher, M.; Roche, B. Disentangling complex parasite interactions: Protection against cerebral malaria by one helminth species is jeopardized by co-infection with another. PLoS Negl. Trop. Dis. 2018, 12, e0006483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindawi. Malaria Parasitemia in Febrile Patients Mono and Co-infected with Soil-Transmitted Helminthiasis Attending Sanja Hospital, Northwest Ethiopia [Internet]. Available online: https://www.hindawi.com/journals/jpr/2020/9891870/ (accessed on 16 September 2022).
- Salazar-Castañon, V.H.; Legorreta-Herrera, M.; Rodriguez-Sosa, M. Helminth parasites alter protection against Plasmodium infection. BioMed Res. Int. 2014, 2014, 913696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulu, A.; Kassu, A.; Legesse, M.; Erko, B.; Nigussie, D.; Shimelis, T.; Belyhun, Y.; Moges, B.; Ota, F.; Elias, D. Helminths and malaria co-infections are associated with elevated serum IgE. Parasit. Vectors 2014, 7, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahittikorn, A.; Masangkay, F.R.; De Jesus Milanez, G.; Kuraeiad, S.; Kotepui, M. Prevalence and effect of Plasmodium spp. and hookworm co-infection on malaria parasite density and haemoglobin level: A meta-analysis. Sci. Rep. 2022, 12, 6864. [Google Scholar] [CrossRef] [PubMed]
- Mpina, M.; Stabler, T.; Schindler, T.; Bijeri, J.; Deal, A.; Pupu, L.A.; Nyakarungu, E.; Davis, M.C.O.; Urbano, V.; Mtoro, A.; et al. Diagnostic Performance and Comparison of Ultrasensitive and Conventional Rapid Diagnostic Test, Thick Blood Smear and Quantitative PCR for Detection of Low-Density Plasmodium Falciparum Infections During a Controlled Human Malaria Infection Study in Equatorial Guinea. Malar. J. 2022, 9921, 1–14. [Google Scholar]
- Shekalaghe, S.; Rutaihwa, M.; Billingsley, P.F.; Chemba, M.; Daubenberger, C.A.; James, E.R.; Mpina, M.; Juma, O.A.; Schindler, T.; Huber, E.; et al. Controlled Human Malaria In-fection of Tanzanians by Intradermal Injection of Aseptic, Purified, Cryopreserved Plasmodium falciparum Sporozoites. Am. J. Trop. Med. Hyg. 2014, 91, 471–480. [Google Scholar] [CrossRef]
- Katz, N.; Chaves, A.; Pellegrino, J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 1972, 14, 397–400. [Google Scholar]
- Nyasa, R.B.; Fotabe, E.L.; Ndip, R.N. Trends in malaria prevalence and risk factors associated with the disease in Nkongho-mbeng; a typical rural setting in the equatorial rainforest of the South West Region of Cameroon. PLoS ONE 2021, 16, e0251380. [Google Scholar] [CrossRef]
- Ramdzan, A.R.; Ismail, A.; Mohd Zanib, Z.S. Prevalence of malaria and its risk factors in Sabah, Malaysia. Int. J. Infect. Dis. 2020, 91, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Quaresima, V.; Agbenyega, T.; Oppong, B.; Awunyo, J.A.D.A.; Adu Adomah, P.; Enty, E.; Donato, F.; Castelli, F. Are Malaria Risk Factors Based on Gender? A Mixed-Methods Survey in an Urban Setting in Ghana. Trop. Med. Infect. Dis. 2021, 6, 161. [Google Scholar] [CrossRef]
- Jennifer, O.; Dogara, M.; Dogara, M. Prevalence of malaria and risk factors among patients attending Dutse General Hospital, Jigawa State, Nigeria. Int. Res. J. Public Environ. Health 2016, 3, 270–277. [Google Scholar]
- Manego, R.Z.; Koehne, E.; Kreidenweiss, A.; Mombo, B.N.; Adegbite, B.R.; Mbadinga, L.B.D.; Akinosho, M.; Matthewman, J.; Adegnika, A.A.; Ramharter, M.; et al. Description of Plasmodium falciparum infections in central Gabon demonstrating high parasite densities among symptomatic adolescents and adults. Malar. J. 2019, 18, 371. [Google Scholar] [CrossRef] [Green Version]
- Coulibaly, D.; Guindo, B.; Niangaly, A.; Maiga, F.; Konate, S.; Kodio, A.; Diallo, A.; Antar, A.T.M.; Kone, A.K.; Traore, K.; et al. A Decline and Age Shift in Malaria Incidence in Rural Mali following Implementation of Seasonal Malaria Chemoprevention and Indoor Residual Spraying. Am. J. Trop. Med. Hyg. 2021, 104, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Nwaneli, E.I.; Eguonu, I.; Ebenebe, J.C.; Osuorah, C.D.I.; Ofiaeli, O.C.; Nri-Ezedi, C.A. Malaria prevalence and its soci-odemographic determinants in febrile children—A hospital-based study in a developing community in South-East Nigeria. J. Prev. Med. Hyg. 2020, 61, E173–E180. [Google Scholar] [PubMed]
- White, M.; Watson, J. Age, exposure and immunity. eLife 2018, 7, e40150. [Google Scholar] [CrossRef]
- Ototo, E.N.; Zhou, G.; Kamau, L.; Mbugi, J.P.; Wanjala, C.L.; Machani, M.; Atieli, H.; Githeko, A.K.; Yan, G. Age-specific Plasmodium parasite profile in pre and post ITN intervention period at a highland site in western Kenya. Malar. J. 2017, 16, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muntari, B.; Tureta, B.A.; Muntari, B.; Tureta, B.A. Detection of Plasmodium species and parasite density among patients at-tending some selected hospitals in Sokoto Metropolis. GSC Biol. Pharm. Sci. 2019, 9, 001–007. [Google Scholar] [CrossRef] [Green Version]
- Doolan, D.L.; Dobaño, C.; Baird, J.K. Acquired Immunity to Malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef] [Green Version]
- Pinkevych, M.; Petravic, J.; Chelimo, K.; Kazura, J.W.; Moormann, A.M.; Davenport, M.P. The Dynamics of Naturally Acquired Immunity to Plasmodium falciparum Infection. PLoS Comput. Biol. 2012, 8, e1002729. [Google Scholar] [CrossRef] [Green Version]
- Ngwa, C.J.; Rosa TF de, A.; Pradel, G. The Biology of Malaria Gametocytes [Internet]. Current Topics in Malaria. IntechOpen; 2016. Available online: https://www.intechopen.com/chapters/undefined/state.item.id (accessed on 21 September 2022).
- Adomako-Ankomah, Y.; Chenoweth, M.S.; Tocker, A.M.; Doumbia, S.; Konate, D.; Doumbouya, M.; Keita, A.S.; Anderson, J.M.; Fairhurst, R.M.; Diakite, M.; et al. Host age and Plasmodium falciparum multiclonality are associated with gametocyte prevalence: A 1-year prospective cohort study. Malar. J. 2017, 16, 473. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Mitchell, R.; Kariuki, S.; Odero, C.; Otieno, P.; Otieno, K.; Onyona, P.; Were, V.; Wiegand, R.E.; Gimnig, J.E.; et al. Assessment of submicroscopic infections and gametocyte carriage of Plasmodium falciparum during peak malaria transmission season in a community-based cross-sectional survey in western Kenya 2012. Malar. J. 2016, 19, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, K.M.; Caicedo, M.A.; Gaitán, A.; Herrera-Varela, M.; Arce, M.I.; Vallejo, A.F.; Padilla, J.; Chaparro, P.; Pacheco, M.A.; Escalante, A.A.; et al. Characterizing the malaria rural-to-urban transmission interface: The importance of reactive case detection. PLoS Negl. Trop. Dis. 2017, 11, e0005780. [Google Scholar]
- Musa, J.J.; Moore, S.J.; Moore, J.; Mbuba, E.; Mbeyela, E.; Kobe, D.; Swai, J.K.; Odufuwa, O.G. Long-lasting insecticidal nets retain bio-efficacy after 5 years of storage: Implications for malaria control programmes. Malar. J. 2020, 19, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koudou, B.G.; Ghattas, H.; Essé, C.; Nsanzabana, C.; Rohner, F.; Utzinger, J.; Faragher, B.E.; Tschannen, A.B. The use of insecticide-treated nets for reducing malaria morbidity among children aged 6–59 months, in an area of high malaria transmission in central Côte d’Ivoire. Parasit. Vectors 2010, 3, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yukich, J.; Stuck, L.; Scates, S.; Wisniewski, J.; Chacky, F.; Festo, C.; Kabulika, G.; Dimoso, K.; Mandike, R.; Greer, G.; et al. Sustaining LLIN coverage with continuous distribution: The school net programme in Tanzania. Malar. J. 2020, 19, 158. [Google Scholar] [CrossRef] [Green Version]
- Dejon-Agobé, J.C.; Zinsou, J.F.; Honkpehedji, Y.J.; Ateba-Ngoa, U.; Edoa, J.R.; Adegbite, B.R.; Mombo-Ngoma, G.; Agnandji, S.T.; Ramharter, M.; Kremsner, P.G.; et al. Schistosoma haematobium effects on Plasmodium falciparum infection modified by soil-transmitted helminths in school-age children living in rural areas of Gabon. PLoS Negl. Trop. Dis. 2018, 12, e0006663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers [Internet]. Available online: https://apps.who.int/iris/handle/10665/43545 (accessed on 21 September 2022).
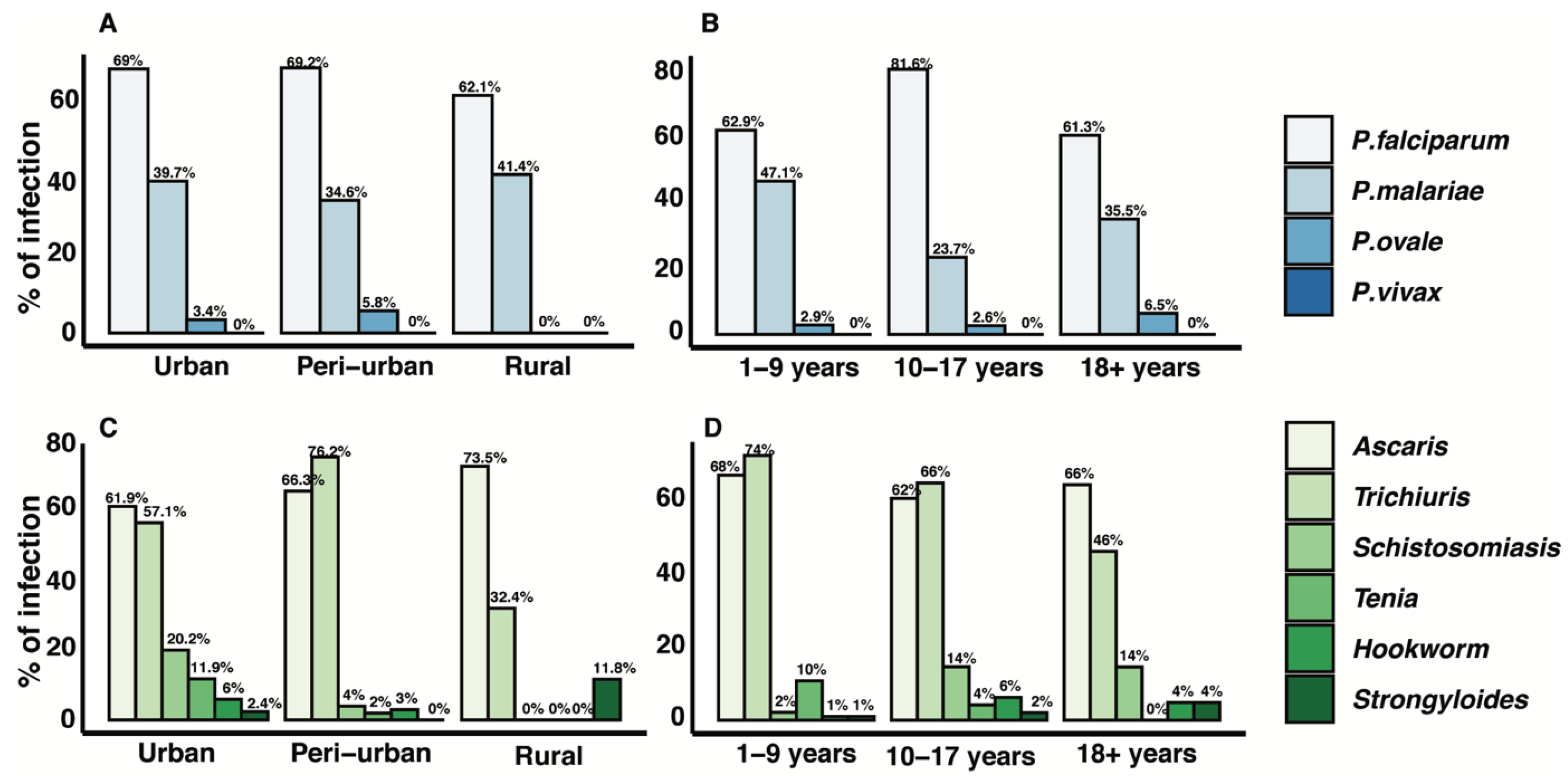


| Parameter | Age Group | All | ||
|---|---|---|---|---|
| 1–9 Years | 10–17 Years | ≥18 Years | ||
| Sex | ||||
| Female | 76 (45.8) | 36 (47.4) | 103 (64.4) | 215 (53.5) |
| Male | 90 (54.2) | 40 (52.6) | 57 (35.6) | 187 (46.5) |
| Mean age ± SD (range) years | 5.3 ± 2.5 (1.0, 9.8) | 12.4 ± 2.0 (10, 17.5) | 49.0 ± 16.8 (18.2, 86.1) | 24.0 ± 23.1 (1.0, 86.1) |
| LLINs | ||||
| Having LLINs | NA | NA | 83 (51.9) | |
| Area | ||||
| Urban | 72 (43.4) | 54 (71.1) | 52 (32.5) | 178 (44.3) |
| Peri-urban | 63 (37.9) | 16 (21.0) | 55 (34.4) | 134 (33.3) |
| Rural | 31 (18.7) | 6 (7.9) | 53 (33.1) | 90 (22.4) |
| Malaria Positive (%) | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Parameter | Odds Ratio (SE) | 95% CI | Odds Ratio (SE) | 95% CI | p-Value | |
| Age groups (years) | ||||||
| Age (year) | 0.98 (0.01) | 0.97, 0.99 | 0.98 (0.01) | 0.97, 0.99 | <0.001 | |
| 1–9 | 42.2 | Reference | ||||
| 10–17 | 50 | 1.37 (0.38) | 0.79, 2.36 | - | - | - |
| ≥18 | 20 | 0.34 (0.09) | 0.21, 0.56 | - | - | - |
| Sex | ||||||
| Female | 28.8 | Reference | Reference | |||
| Male | 41.7 | 1.77 (0.37) | 1.17, 2.67 | 1.6 (0.35) | 1.04, 2.46 | 0.031 |
| LLINs | ||||||
| No LLINs | 39.2 | Reference | Reference | |||
| With LLINs | 18.1 | 0.78 (0.31) | 0.36, 1.69 | - | - | - |
| Area | ||||||
| Urban | 32.6 | Reference | Reference | |||
| Peri-urban | 39.6 | 1.35 (0.32) | 0.85, 2.16 | 1.6 (0.4) | 0.98, 2.6 | 0.059 |
| Rural | 32.2 | 0.98 (0.27) | 0.57, 1.69 | 1.33 (0.39) | 0.75, 2.35 | 0.335 |
| Parameter | Number of Gametocytes (Gametocyte/µL of Blood) | ||
|---|---|---|---|
| 0 | 1–4 | >5 | |
| Age (years) | |||
| 1–9 | 84 (79.3) | 14 (13.2) | 8 (7.6) |
| 10–17 | 39 (79.6) | 8 (16.3) | 2 (4.1) |
| ≥18 | 38 (77.6) | 8 (16.3) | 3 (6.1) |
| Area | |||
| Urban | 59 (81.9) | 8 (11.1) | 5 (6.9) |
| Peri-urban | 65 (74.7) | 15 (17.2) | 7 (8.1) |
| Rural | 37 (82.2) | 7 (15.6) | 1 (2.2) |
| Soil-Transmitted Helminths | Malaria | |
|---|---|---|
| Negative (%) | Positive (%) | |
| Negative | 91 (75.8) | 29 (24.2) |
| Positive | 130 (59.4) | 89 (40.6) |
| Mono-infection | ||
| Ascaris lumbricoides | 37 (68.5) | 17 (31.5) |
| Ancylostoma spp. | 2 (66.7) | 1 (33.3) |
| Schistosoma intercalatum | 8 (80) | 2 (20) |
| Strongyloides stercoralis | 2 (66.7) | 1 (33.3) |
| Trichiuris trichiuras | 24 (49) | 25 (51) |
| Taenia solium | 3 (100) | 0 (0) |
| Double infections | ||
| Ascaris lumbricoides + Ancylostoma spp. | 1 (100) | 0 |
| Ascaris lumbricoides + Schistosoma intercalatum | 2 (66.7) | 1 (33.3) |
| Ascaris lumbricoides + Strongyloides stercoralis | 1 (100) | 0 (0) |
| Ascaris lumbricoides + Trichiuris trichiuras | 34 (48.6) | 36 (51.4) |
| Ascaris lumbricoides + Taenia solium | 3 (100) | 0 (0) |
| Ancylostoma spp. + Schistosoma intercalatum | 1 (100) | 0 (0) |
| Schistosoma intercalatum + Taenia solium | 1 (100) | 0 (0) |
| Schistosoma intercalatum + Trichiuris trichiuras | 1 (33.3) | 2 (66.7) |
| Strongyloides stercoralis + Trichiuris trichiuras | 0 (0) | 1 (100) |
| Tenia solium + Trichiuris trichiuras | 1 (100) | 0 (0) |
| Triple infections | ||
| Ascaris lumbricoides + Ancylostoma spp. + Trichiuris trichiuras | 2 (50) | 2 (50) |
| Ascaris lumbricoides + Schistosoma intercalatum + Trichiuris trichiuras | 3 (100) | 0 (0) |
| Ascaris lumbricoides + Strongyloides spp. + Trichiuris trichiuras | 0 (0) | 1 (100) |
| Ascaris lumbricoides + Taenia solium + Trichiuris trichiuras | 4 (100) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meñe, G.R.; Mpina, M.G.; Lopelo, A.; Nyakarungu, E.L.; Bijeri, J.R.; Elo, A.M.E.; Ondo, F.A.; Garcia, G.A.; Phiri, W.P.; Ali, A.M.; et al. Effects of Age, Gender and Soil-Transmitted Helminth Infection on Prevalence of Plasmodium Infection among Population Living in Bata District, Equatorial Guinea. Trop. Med. Infect. Dis. 2023, 8, 149. https://doi.org/10.3390/tropicalmed8030149
Meñe GR, Mpina MG, Lopelo A, Nyakarungu EL, Bijeri JR, Elo AME, Ondo FA, Garcia GA, Phiri WP, Ali AM, et al. Effects of Age, Gender and Soil-Transmitted Helminth Infection on Prevalence of Plasmodium Infection among Population Living in Bata District, Equatorial Guinea. Tropical Medicine and Infectious Disease. 2023; 8(3):149. https://doi.org/10.3390/tropicalmed8030149
Chicago/Turabian StyleMeñe, Gertrudis R., Maxmillian G. Mpina, Alejandro Lopelo, Elizabeth L. Nyakarungu, José Raso Bijeri, Antonio Martin Elo Elo, Florentino Abaga Ondo, Guillermo A. Garcia, Wonder P. Phiri, Ali Mohamed Ali, and et al. 2023. "Effects of Age, Gender and Soil-Transmitted Helminth Infection on Prevalence of Plasmodium Infection among Population Living in Bata District, Equatorial Guinea" Tropical Medicine and Infectious Disease 8, no. 3: 149. https://doi.org/10.3390/tropicalmed8030149
APA StyleMeñe, G. R., Mpina, M. G., Lopelo, A., Nyakarungu, E. L., Bijeri, J. R., Elo, A. M. E., Ondo, F. A., Garcia, G. A., Phiri, W. P., Ali, A. M., Agobé, J. C. D., Adegnika, A. A., & Abdulla, S. M. (2023). Effects of Age, Gender and Soil-Transmitted Helminth Infection on Prevalence of Plasmodium Infection among Population Living in Bata District, Equatorial Guinea. Tropical Medicine and Infectious Disease, 8(3), 149. https://doi.org/10.3390/tropicalmed8030149






