Tungiasis in the Sanumás Amerindians in the Amazon Rainforest, Brazil: Prevalence, Intensity and Morbidity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Population
2.2. Examination of Humans and Animals
2.3. Living Conditions
2.4. Examination of Soil
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Demographic, Environmental and Sanitation Characteristics
3.2. Prevalence and Univariate Analysis
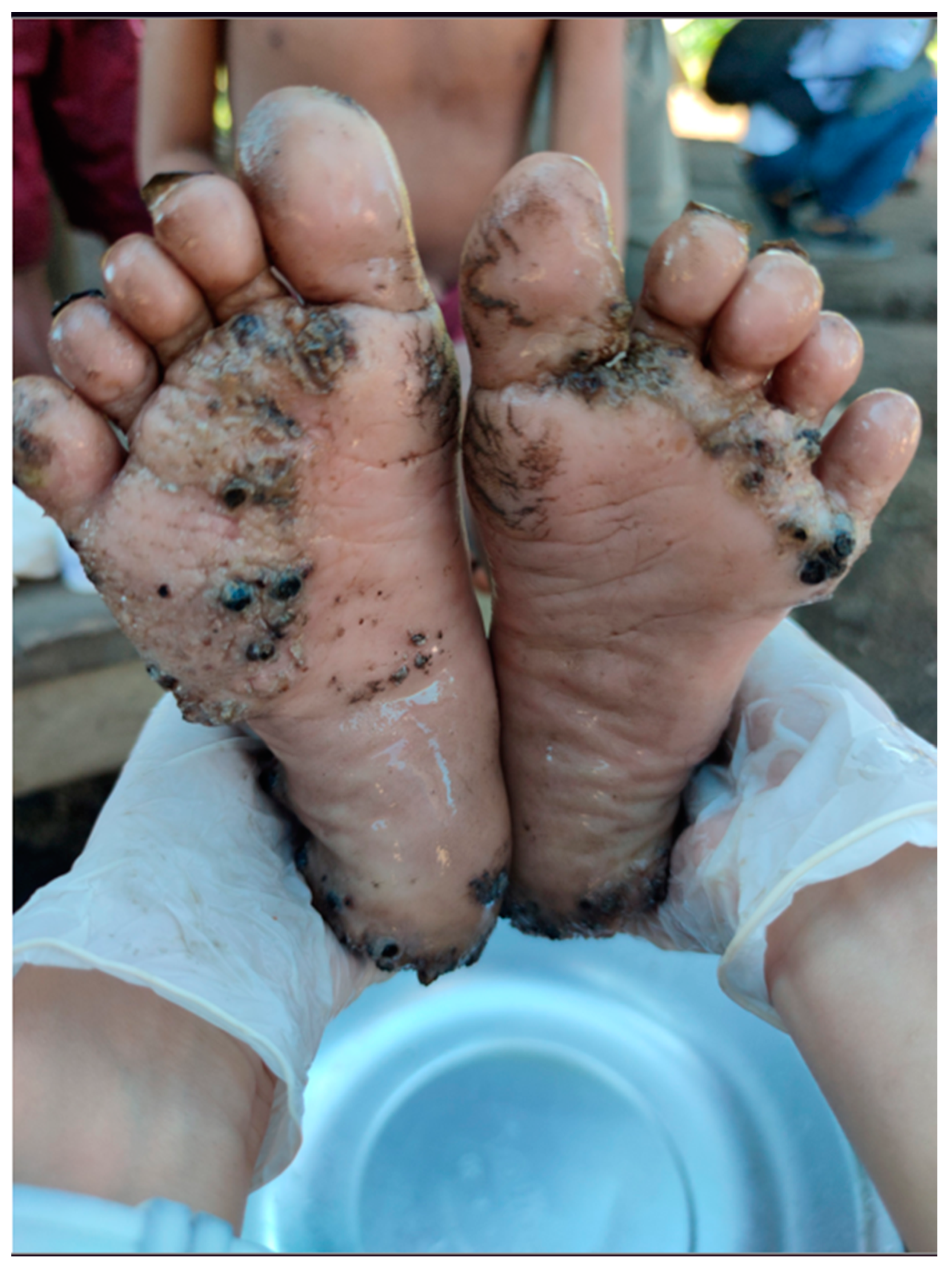
3.3. Intensity and Morbidity
3.4. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ariza, L.; Seidenschwang, M.; Buckendahl, J.; Gomide, M.; Feldmeier, H.; Heukelbach, J. Tungiasis: A neglected disease causing severe morbidity in a shantytown in Fortaleza, State of Ceará. Rev. Soc. Bras. Med. Trop. 2007, 40, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.W.; Thong, H.Y.; Jee, S.H. Tungiasis: A Case Report and Review of the Literature. Dermatol. Sin. 2011, 29, 29–31. [Google Scholar] [CrossRef]
- Deka, M.A.; Heukelbach, J. Distribution of Tungiasis in Latin America: Identification of Areas for Potential Disease Transmission Using an Ecological Niche Model. Lancet Reg. Health-Am. 2022, 5, 100080. [Google Scholar] [CrossRef] [PubMed]
- Eisele, M.; Heukelbach, J.; Van Marck, E.; Mehlhorn, H.; Meckes, O.; Franck, S.; Feldmeier, H. Investigations on the Biology, Epidemiology, Pathology and Control of Tunga Penetrans in Brazil: I. Natural History of Tungiasis in Man. Parasitol. Res. 2003, 90, 87–99. [Google Scholar] [CrossRef]
- Paranhos, L.S.; De Castro, P.A.S.V.; De Araújo, G.R.; Magalhães, F.D.C.; Bezerra, J.M.T. Prevalence of tungiasis in humans in brazil and in its federative units: A systematic review. J. Trop. Pathol. 2022, 51, 31–50. [Google Scholar] [CrossRef]
- Tardin Martins, A.C.; de Brito, A.R.; Kurizky, P.S.; Gonçalves, R.G.; Santana, Y.R.T.; de Carvalho, F.C.A.; Gomes, C.M. The Efficacy of Topical, Oral and Surgical Interventions for the Treatment of Tungiasis: A Systematic Review of the Literature. PLoS Negl. Trop. Dis. 2021, 15, e0009722. [Google Scholar] [CrossRef]
- Miller, H.; Rodríguez, G. Tungiasis in native Amerindians in Vaupés province: Epidemiology, clinical aspects, treatment, and prevention. Biomedica 2010, 30, 215–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, H.; Ocampo, J.; Ayala, A.; Trujillo, J.; Feldmeier, H. Very Severe Tungiasis in Amerindians in the Amazon Lowland of Colombia: A Case Series. PLoS Negl. Trop. Dis. 2019, 13, e0007068. [Google Scholar] [CrossRef]
- de Carvalho, R.W.; de Almeida, A.B.; Barbosa-Silva, S.C.; Amorim, M.; Ribeiro, P.C.; Serra-Freire, N.M. The Patterns of Tungiasis in Araruama Township, State of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2003, 98, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Romiti, R.; Martins, L.P.F.; Santana, Y.R.T.; Timbó, R.V.; Kurizky, P.S.; Barroso, D.H.; de Andrade, R.R.; Gomes, C.M.; Griffiths, C.E.M. Prevalence of Psoriasis and Other Autoimmune Skin Diseases in a Recently Contacted Amerindian Community in the Auaris Region, Yanomami Territory, Brazil. Br. J. Dermatol. 2023, ljad165. [Google Scholar] [CrossRef]
- Menezes, G.H. Yanomami Na Encruzilhada Da Conquista: Contato e Transformação Na Fronteira Amazônica. Ph.D. Thesis, (Doutorado em Antropologia Social)–Instituto de Ciências Sociais, Departamento de Antropologia, Universidade de Brasília, Brasília, Brazil, 2010. [Google Scholar]
- Guimarães, S. Cosmologia Sanumá: O Xamã e a Constituição Do Ser. Ph.D. Thesis, (Doutorado em Antropologia)—Universidade de Brasília, Brasília, Brazil, 2005. Volume 1. [Google Scholar]
- Guimarães, S.M.F. The Sanumá-Yanomami Medical System and Indigenous Peoples’ Health Policy in Brazil. Cad. Saude Publica 2015, 31, 2148–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, S. Corpos e Ciclos Da Vida Sanumá-Yanomami. Horiz. Antropológicos 2010, 16, 261–286. [Google Scholar] [CrossRef] [Green Version]
- Costa Da Silva, C.; de Magalhães Bethonico, M.B. O Conceito de Território Para o Povo Ye’kuana Que Habita a Região de Auaris-Terra Indígena Yanomami-Roraima. Rev. Estud. Pesqui. Sobre Américas 2017, 11, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Hakeem, M.J.M.L.; Morris, A.K.; Bhattacharyya, D.N.; Fox, C. Tungiasis—A Cause of Painful Feet in a Tropical Traveller. Travel Med. Infect. Dis. 2010, 8, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Linardi, P.M.; Beaucournu, J.C.; De Avelar, D.M.; Belaz, S. Notes on the Genus Tunga (Siphonaptera: Tungidae) II--Neosomes, Morphology, Classification, and Other Taxonomic Notes. Parasite 2014, 21, 68. [Google Scholar] [CrossRef]
- Tamhane, A.R.; Westfall, A.O.; Burkholder, G.A.; Cutter, G.R. Prevalence Odds Ratio versus Prevalence Ratio: Choice Comes with Consequences. Stat. Med. 2016, 35, 5730. [Google Scholar] [CrossRef] [Green Version]
- Nájera Villagrana, S.M.; García Naranjo Santisteban, A. Tungiasis: A Highly Neglected Disease among Neglected Diseases. Case Series from Nduta Refugee Camp (Tanzania). Oxf. Med. Case Rep. 2019, 2019, omz049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, A.C.T.; Martins, L.P.F.; Timbó, R.V.; Bezerra, N.V.F.; Urdapilleta, A.A.A.; Filho, F.M.P.; Gomes, C.M. Measuring Educational Neglect Using the Q Method: A Model Based on the Burden of Disseminated Tungiasis. Front. Epidemiol. 2022, 2, 1003102. [Google Scholar] [CrossRef]
- Nsanzimana, J.; Karanja, S.; Kayongo, M.; Nyirimanzi, N.; Umuhoza, H.; Murangwa, A.; Muganga, R.; Musafili, A. Factors Associated with Tungiasis among Primary School Children: A Cross-Sectional Study in a Rural District in Rwanda. BMC Public Health 2019, 19, 1192. [Google Scholar] [CrossRef]
- Arene, F.O.I. The Prevalence of Sand Flea (Tunga penetrans) among Primary and Post-Primary School Pupils in Choba Area of the Niger Delta. Public Health 1984, 98, 282–283. [Google Scholar] [CrossRef]
- Feldmeier, H.; Heukelbach, J.; Eisele, M.; Ribeiro, R.; Harms, G.; Mehlhorn, H.; Liesenfeld, O. Investigations on the Biology, Epidemiology, Pathology and Control of Tunga penetrans in Brazil: III. Cytokine Levels in Peripheral Blood of Infected Humans. Parasitol. Res. 2003, 91, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Thielecke, M.; Raharimanga, V.; Rogier, C.; Stauss-Grabo, M.; Richard, V.; Feldmeier, H. Prevention of Tungiasis and Tungiasis-Associated Morbidity Using the Plant-Based Repellent Zanzarin: A Randomized, Controlled Field Study in Rural Madagascar. PLoS Negl. Trop. Dis. 2013, 7, e2426. [Google Scholar] [CrossRef] [Green Version]
- McNeilly, H.; Thielecke, M.; Mutebi, F.; Banalyaki, M.; Reichert, F.; Wiese, S.; Feldmeier, H. Tungiasis Stigma and Control Practices in a Hyperendemic Region in Northeastern Uganda. Trop. Med. Infect. Dis. 2023, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Chadee, D.D.; Furlonge, E.; Naraynsingh, C.; Le Maitre, A. Distribution and Prevalence of Tunga Penetrans in Coastal South Trinidad, West Indies. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 549. [Google Scholar] [CrossRef]
- Kiesewetter, T.; Ariza, L.; Martins, M.M.; Limongi, J.E.; da Silva, J.J.; Mendes, J.; Calheiros, C.M.L.; Becher, H.; Heukelbach, J. In Vitro Efficacy of Four Insecticides Against Eggs of Tunga penetrans (Siphonaptera). Open Dermatol. J. 2013, 7, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Mutebi, F.; Krücken, J.; Feldmeier, H.; von Samsom-Himmelstjerna, G. Clinical Implications and Treatment Options of Tungiasis in Domestic Animals. Parasitol. Res. 2021, 120, 4113–4123. [Google Scholar] [CrossRef] [PubMed]





| Variable | Tungiasis Cases n (%) or Median (IQR) | Number of Persons Examined |
|---|---|---|
| Sex | ||
| Female | 14 (5.38%) | 260 |
| Male | 31 (10.51%) | 295 |
| Age group | ||
| Adult | 3 (1.09%) | 275 |
| Children | 42 (15.00%) | 280 |
| Median house location number | 4.00 (10.00) | -- |
| Median number of households in the community | 17.00 (2.00) | -- |
| Median number of inhabitants per house | 10.00 (4.00) | -- |
| Median severity of soil infestation in the patient’s home | 3.00 (1.00) | -- |
| Median number of dogs belonging to the household | 1.00 (1.00) | -- |
| Independent Variable | Odds Ratio (95% CI) | p Value |
|---|---|---|
| Age group (Children) | 15.77 (5.34–67.91) | <0.001 |
| Male sex | 1.68 (0.80–3.66) | 0.177 |
| House location number classification (≥ 4) | 0.92 (0.68–1.25) | 0.610 |
| Severity of soil infestation (=3 or not) | 12.29 (3.75–45.88) | <0.001 |
| Number of houses in the community (≥17) | 90.83 (9.26–2140.87) | 0.001 |
| Number of inhabitants in the same house (≥10) | 13.03 (2.00–102.58) | 0.010 |
| Number of dogs in the household (≥1) | 0.73 (0.51–0.96) | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, Y.R.T.; Oliveira, L.F.C.; Lima, G.M.; Timbó, R.V.; Pires, E.M.; de Brito, A.R.; Martins, A.C.T.; Magalhães, V.S.; de Faria, A.C.M.; Urdapilleta, A.A.A.; et al. Tungiasis in the Sanumás Amerindians in the Amazon Rainforest, Brazil: Prevalence, Intensity and Morbidity. Trop. Med. Infect. Dis. 2023, 8, 386. https://doi.org/10.3390/tropicalmed8080386
Santana YRT, Oliveira LFC, Lima GM, Timbó RV, Pires EM, de Brito AR, Martins ACT, Magalhães VS, de Faria ACM, Urdapilleta AAA, et al. Tungiasis in the Sanumás Amerindians in the Amazon Rainforest, Brazil: Prevalence, Intensity and Morbidity. Tropical Medicine and Infectious Disease. 2023; 8(8):386. https://doi.org/10.3390/tropicalmed8080386
Chicago/Turabian StyleSantana, Yago Ranniere Teixeira, Lucas Felipe Carvalho Oliveira, Gabriela Mafra Lima, Renata Velôzo Timbó, Eliane Mateus Pires, Amanda Ramos de Brito, Ana Carolina Tardin Martins, Vivyanne Santiago Magalhães, Ana Carolina Mota de Faria, Ada Amalia Ayala Urdapilleta, and et al. 2023. "Tungiasis in the Sanumás Amerindians in the Amazon Rainforest, Brazil: Prevalence, Intensity and Morbidity" Tropical Medicine and Infectious Disease 8, no. 8: 386. https://doi.org/10.3390/tropicalmed8080386
APA StyleSantana, Y. R. T., Oliveira, L. F. C., Lima, G. M., Timbó, R. V., Pires, E. M., de Brito, A. R., Martins, A. C. T., Magalhães, V. S., de Faria, A. C. M., Urdapilleta, A. A. A., Roger, I., de Andrade, R. R., Martins, L. P. F., Pellegrini, M., de Carvalho, F. C. A., Araújo, D. D., Barroso, D. H., Garcia, C. N., Feldmeier, H., & Gomes, C. M. (2023). Tungiasis in the Sanumás Amerindians in the Amazon Rainforest, Brazil: Prevalence, Intensity and Morbidity. Tropical Medicine and Infectious Disease, 8(8), 386. https://doi.org/10.3390/tropicalmed8080386







