One Health Surveillance System in Gujarat, India: A Health Policy and Systems Research Protocol for Exploring the Cross-Sectoral Collaborations to Detect Emerging Threats at the Human-Animal–Environment Interface
Abstract
:1. Introduction
1.1. Global and Indian Burden of Emerging and Re-Emerging Zoonotic Diseases
1.2. Importance of Early Warning and Surveillance Systems: The Role of the One Health Approach
1.3. Global Action for Cross-Sectoral Collaboration to Strengthen Surveillance and EWRS
1.4. Cross-Sectoral Collaborations across the Surveillance Systems in the Context of Gujarat, India
2. Experimental Design
2.1. Study Definitions
2.2. Study Design
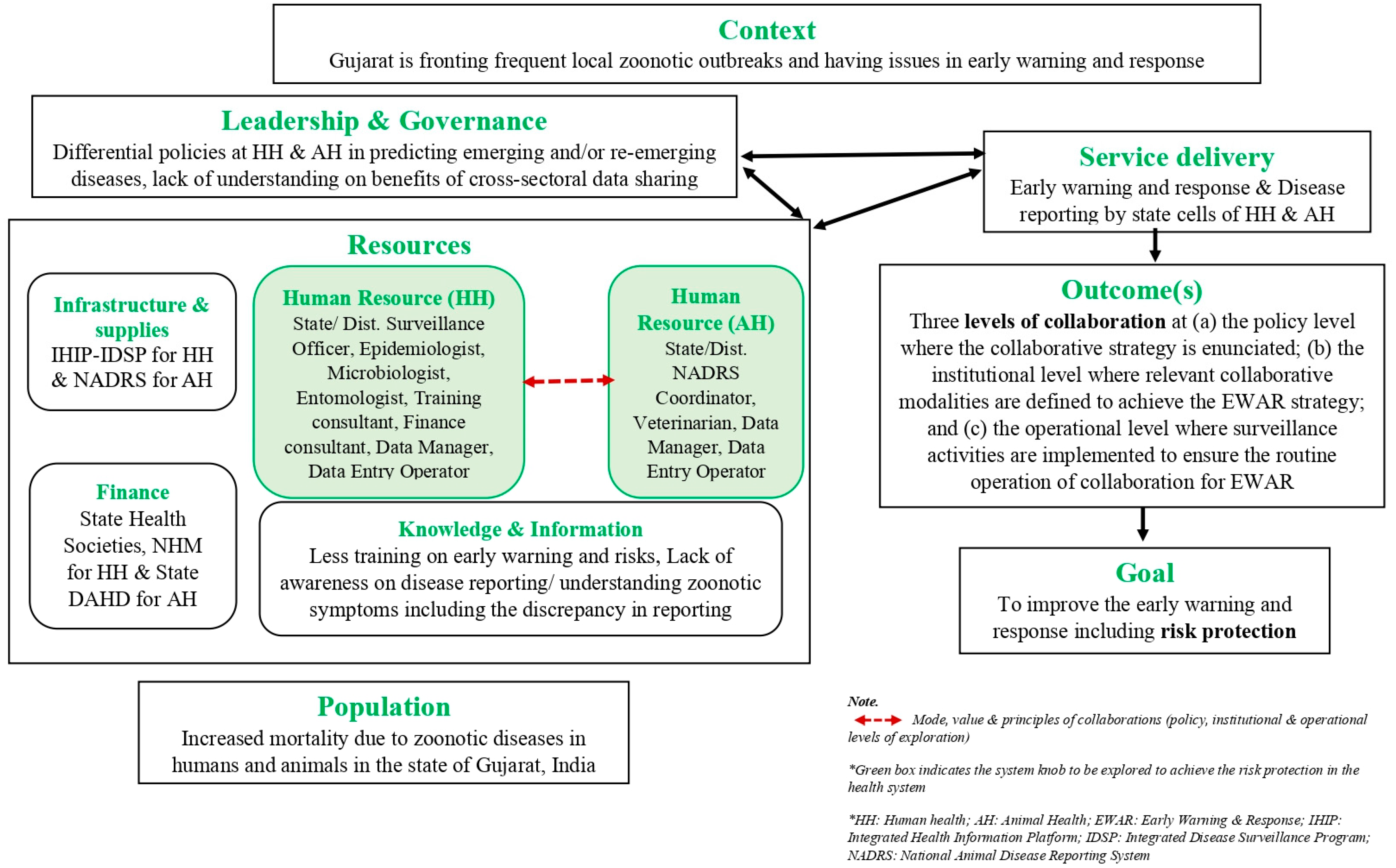
2.3. Conceptual Framework
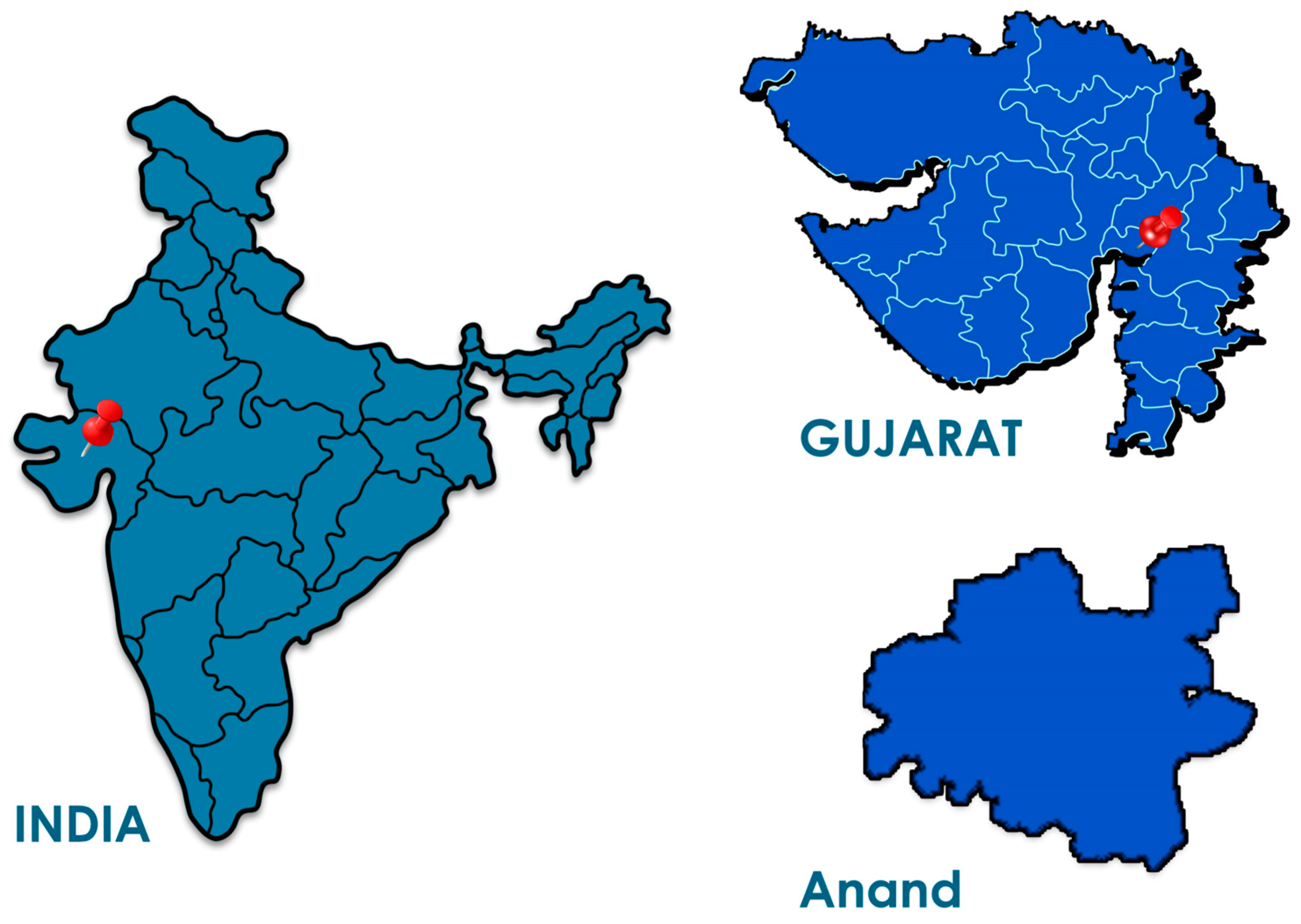
2.4. Study Setting
3. Materials and Equipment
3.1. Content Extraction Tool
3.2. Primary Data Collection
3.2.1. Qualitative Data Collection
3.2.2. Quantitative Tool
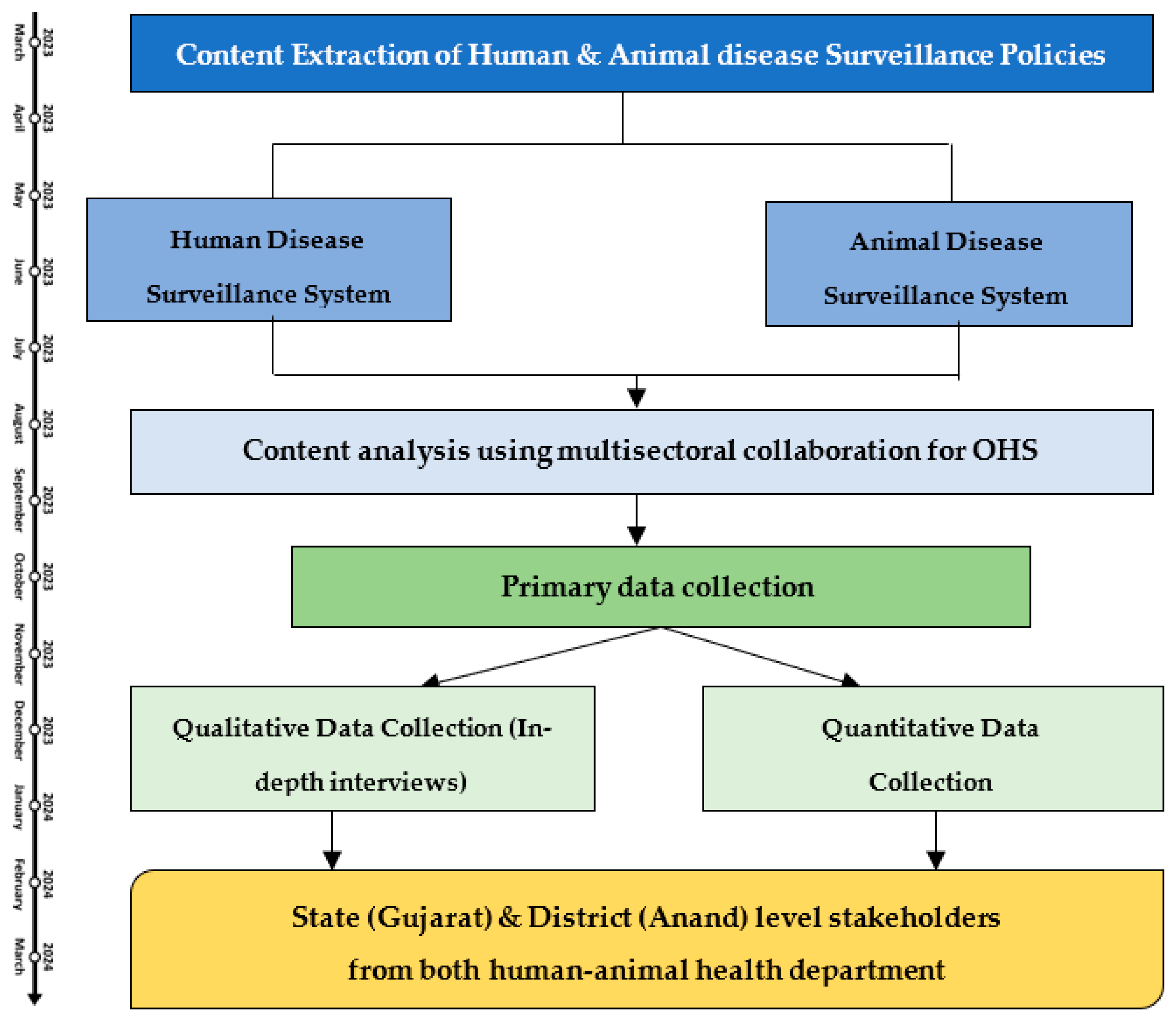
4. Detailed Procedure
4.1. Content Extraction and Analysis
4.2. Primary Data Collection and Analysis
4.2.1. Qualitative
4.2.2. Quantitative
4.3. Study Limitations
5. Expected Result
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Gebreyes, W.A.; Dupouy-Camet, J.; Newport, M.J.; Oliveira, C.J.B.; Schlesinger, L.S.; Saif, Y.M.; Kariuki, S.; Saif, L.J.; Saville, W.; Wittum, T.; et al. The global one health paradigm: Challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Negl. Trop. Dis. 2014, 8, e3257. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J.; Fooks, A.R.; van der Poel, W.H.M. The public health threat of new, reemerging, and neglected zoonoses in the industrialised world. Emerg. Infect. Dis. 2010, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salyer, S.J.; Silver, R.; Simone, K.; Barton Behravesh, C. Prioritizing Zoonoses for Global Health Capacity Building—Themes from One Health Zoonotic Disease Workshops in 7 Countries, 2014–2016. Emerg. Infect. Dis. 2017, 23 (Suppl. S1), S55–S64. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Swain, S.; Preetha, G.; Singh, B.; Aggarwal, D. Zoonotic Diseases in India. Indian J. Community Med. 2020, 45 (Suppl. S1), S1–S2. [Google Scholar] [CrossRef]
- Singhai, M.; Jain, R.; Jain, S.; Bala, M.; Singh, S.; Goyal, R. Nipah virus disease: Recent perspective and one health approach. Ann. Glob. Health 2021, 87, 102. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Achieving Sustainable Global Capacity for Surveillance and Response to Emerging Diseases of Zoonotic Origin. Diseases in Humans: Early Warning Systems; National Academies Press: Washington, DC, USA, 2008. [Google Scholar]
- Aarestrup, F.M.; Bonten, M.; Koopmans, M. Pandemics—One Health preparedness for the next. Lancet Reg. Health 2021, 9, 100210. [Google Scholar] [CrossRef]
- Keusch, G.T.; Pappaioanou, M.; Gonzalez, M.C.; Scott, K.A.; Tsai, P. Achieving an Effective Zoonotic Disease Surveillance System; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Pinto, J.; ben Jebara, K.; Chaisemartin, D.; de La Rocque, S.; Abela, B. The FAO/OIE/WHO global early warning system. In Challenges of Animal Health Information Systems and Surveillance for Animal Diseases and Zoonoses; FAO: Rome, Italy, 2011; pp. 39–40. [Google Scholar]
- Joint Tripartite (FAO-OIE-WHO) & UNEP. Tripartite and UNEP Support OHHLEP’s Definition of “One Health”. World Health Organization, 2021. Available online: https://www.who.int/news/item/01-12-2021-tripartite-and-unep-support-ohhlep-s-definition-of-one-health (accessed on 9 March 2022).
- Vandersmissen, A.; Welburn, S.C. Current initiatives in One Health: Consolidating the One Health Global Network. Rev. Sci. Tech. 2014, 33, 421–432. [Google Scholar] [CrossRef]
- Bidaisee, S.; Macpherson, C.N.L. Zoonoses and one health: A review of the literature. J. Parasitol. Res. 2014, 2014, 874345. [Google Scholar] [CrossRef]
- Mazet, J.A.K.; Clifford, D.L.; Coppolillo, P.B.; Deolalikar, A.B.; Erickson, J.D.; Kazwala, R.R. A “One Health” Approach to Address Emerging Zoonoses: The HALI Project in Tanzania. PLoS Med. 2009, 6, e1000190. [Google Scholar] [CrossRef]
- WHO. Early Warning Alert and Response in Emergencies: An Operational Guide; WHO: Geneva, Switzerland, 2022.
- FAO; OIE; WHO. The Global Early Warning and Response System for Major Animal Diseases including Zoonoses (GLEWS); 2006. Available online: https://www.woah.org/fileadmin/Home/eng/About_us/docs/pdf/GLEWS_Tripartite-Finalversion010206.pdf (accessed on 15 August 2023).
- Wacharapluesadee, S.; Ghai, S.; Duengkae, P.; Manee-Orn, P.; Thanapongtharm, W.; Saraya, A.W.; Yingsakmongkon, S.; Joyjinda, Y.; Suradhat, S.; Ampoot, W.; et al. Two decades of one health surveillance of Nipah virus in Thailand. One Health Outlook 2021, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Agbo, S.; Gbaguidi, L.; Biliyar, C.; Sylla, S.; Fahnbulleh, M.; Dogba, J.; Keita, S.; Kamara, S.; Jambai, A.; Harris, A.; et al. Establishing National Multisectoral Coordination and collaboration mechanisms to prevent, detect, and respond to public health threats in Guinea, Liberia, and Sierra Leone 2016–2018. One Health Outlook 2019, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture. 19th Livestock Census-2012 All India Report; Ministry of Agriculture: New Delhi, India, 2014.
- Ministry of Home Affairs. Census of India Website: Office of the Registrar General & Census Commissioner, India. 2011. Available online: https://censusindia.gov.in/census.website/ (accessed on 8 June 2023).
- Yasobant, S. Research to Explore Intersectoral Collaborations for One Health Approach (RICOHA): A Health System Study in Ahmedabad, India. Ph.D. Thesis, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Germany, 2021. [Google Scholar]
- Patel, A.K.; Patel, K.K.; Mehta, M.; Parikh, T.M.; Toshniwal, H.; Patel, K. First Crimean-Congo hemorrhagic fever outbreak in India. J. Assoc. Physicians India 2011, 59, 585–589. [Google Scholar]
- National Health Mission. IDSP (Integrated Disease Surveillance Project) Gujarat. State Health Society, Health and Family Welfare Department, Government of Gujarat, 2015; pp. 1–2. Available online: https://nhm.gujarat.gov.in/idsp1.htm (accessed on 23 April 2022).
- National Animal Disease Reporting System (NARDS) by Ministry of Fisheries, Animal Husbandry & Dairying. Available online: https://nadrsapps.gov.in/ (accessed on 8 June 2023).
- Ministry of Health and Family Welfare. Training Manual for Veterinary Consultants under IDSP; Ministry of Health and Family Welfare: New Delhi, India, 2016.
- Wadhwa, M. Revamping of Public Health Surveillance System in India; Columbia University, Earth Institute, Center for Sustainable Development (CSD): New York, NY, USA, 2021. [Google Scholar]
- Bedi, J.; Vijay, D.; Dhaka, P.; Singh Gill, J.; Barbuddhe, S. Emergency preparedness for public health threats, surveillance, modelling & forecasting. Indian J. Med. Res. 2021, 153, 287. [Google Scholar] [PubMed]
- Ariyari, S. Partnership in Surveillance: A Kerala model to Emerging Public Health Threats. Online J. Public Health Inform. 2019, 11, e257. [Google Scholar] [CrossRef]
- World Health Organization. Health Promotion and Disease Prevention through Population-Based Interventions, Including Action to Address Social Determinants and Health Inequity|Public Health Functions|About WHO. World Health Organization. Available online: https://www.emro.who.int/about-who/public-health-functions/health-promotion-disease-prevention.html (accessed on 4 October 2022).
- CDC. Introduction to Public Health Surveillance. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/training/publichealth101/surveillance.html (accessed on 4 October 2022).
- UNDRR. Early Warning System. United Nations Office for Disaster Risk Reduction. Available online: https://www.undrr.org/terminology/early-warning-system (accessed on 4 October 2022).
- Bordier, M.; Delavenne, C.; Nguyen, D.T.T.; Goutard, F.L.; Hendrikx, P. One health surveillance: A matrix to evaluate multisectoral collaboration. Front. Vet. Sci. 2019, 6, 109. [Google Scholar] [CrossRef]
- World Health Organization; Public Health Agency of Canada. An Analysis of 18 Country Case Studies Health Equity through Intersectoral Action; WHO: Geneva, Switzerland, 2008.
- World Health Organization. Health Promotion Glossary; WHO: Geneva, Switzerland, 1998.
- Mandell, M.; Keast, R. Evaluating the effectiveness of interorganizational relations through networks. Public Manag. Rev. 2008, 10, 715–731. [Google Scholar] [CrossRef]
- Von Bertalanffy, L. General System Theory: Foundations, Development, Applications; George Braziller: New York, NY, USA, 1969. [Google Scholar]
- Van Olmen, J.; Marchal, B.; Van Damme, W.; Kegels, G.; Hill, P.S. Health systems frameworks in their political context: Framing divergent agendas. BMC Public Health 2012, 12, 774. [Google Scholar] [CrossRef]
- Indian Institute of Science Education and Research Pune. Hazard Map of India. IISER Pune, 2021. Available online: http://sites.iiserpune.ac.in/~hazardmap/Anand/ (accessed on 5 January 2023).
- Willumsen, E.; Ahgren, B.; Odegård, A. A conceptual framework for assessing interorganizational integration and interprofessional collaboration. J. Interprof. Care 2012, 26, 198–204. [Google Scholar] [CrossRef]
- Glandon, D.; Leoutsakos, J.M.; Gupta, S.; Marsteller, J.; Paina, L.; Bennett, S. Development and psychometric testing of the FLW-MSC scale for measuring frontline worker multisectoral collaboration in rural India. BMJ Open 2021, 11, e037800. [Google Scholar] [CrossRef]
- Hennig, M.; Brandes, U.; Pfeffer, J.; Mergel, I. Studying Social Networks: A Guide to Empirical Research; Campus-Verlag: Frankfurt, Germany, 2012. [Google Scholar]
- Creswell, J.; Plano Clark, V. Designing and Conducting Mixed Methods Research; Sage Publications Ltd.: London, UK, 2007. [Google Scholar]
- Lincoln, Y.S.; Guba, E.G. Naturalistic Inquiry; SAGE Publications, Inc.: New York, NY, USA, 1985. [Google Scholar]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Scott, J. Social Network Analysis. Sociology 1988, 22, 109–127. [Google Scholar] [CrossRef]
- Borgatti, S.P.; Mehra, A.; Brass, D.J.; Labianca, G. Network Analysis in the Social Sciences. Science 2009, 323, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, K.; James, P. How to do (or not to do)… a social network analysis in health systems research. Health Policy Plan 2012, 27, 438–446. [Google Scholar] [CrossRef]
- Webb, C.; Bodin, Ö. A network perspective on modularity and control of flow in robust systems. In Complexity Theory for a Sustainable Future; Norberg, J., Cumming, G., Eds.; Columbia Press: New York, NY, USA, 2008; pp. 85–118. [Google Scholar]
- Borgatti, S.P.; Everett, M.G.; Freeman, L.C. Ucinet for Windows: Software for Social Network Analysis; Analytic Technologies: Harvard, MA, USA, 2002. [Google Scholar]
| Disease prevention [29] | Disease prevention is defined as specific, population-based and individual-based interventions for primary and secondary (early detection) prevention, aiming to minimise the burden of diseases and associated risk factors. |
| Disease control | Disease control is the reduction of disease incidence, prevalence, morbidity, or mortality to a locally acceptable level. |
| Disease surveillance system [30] | Disease surveillance is an information-based activity involving the collection, analysis and interpretation of large volumes of data from various sources. |
| Early warning system [31] | An early warning system is a warning system that can be implemented as a chain of information communication systems and comprises sensors, event detection and decision subsystems for the early identification of hazards. |
| One Health [11] | One Health is an integrated, unifying approach that aims to sustainably balance and optimise the health of people, animals and ecosystems. It recognises that the health statuses of humans, domestic and wild animals, plants, and the wider environment (including ecosystems) are closely linked and interdependent. The approach mobilises multiple sectors, disciplines and communities at varying levels of society to work together to foster well-being and tackle threats to health and ecosystems while addressing the collective need for clean water, energy and air, safe and nutritious food, action on climate change, and sustainable development. |
| One Health Surveillance [32] | One Health surveillance describes the systematic collection, validation, analysis, interpretation and dissemination of information collected on humans, animals and the environment to inform decisions for more effective, evidence- and system-based health interventions. The critical element of One Health surveillance is collaboration in planning, coordinating and implementing central functions across a wide range of sectors and disciplines. |
| Cross-sectoral collaborations [33,34] | In 1998, the Health Promotion Glossary was defined as “cooperation between different sectors of society, such as the public sector, civil society, and the private sector”. In 2008, it was defined as “actions undertaken by sectors outside the health sector, possibly, but not necessarily, in collaboration with the health sector, on health or health equity outcomes or the determinants of health or health equity.” |
| Levels of collaboration (environmental, organizational, operational) [35] | The environmental level refers to the impact of the external environment, including all relevant stakeholders surrounding the network and its operations. The organisational level refers to the effect of the structural characteristics of the different types of networks. The operating level relates to the interactions among the individual network participants. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasobant, S.; Saxena, D.; Tadvi, R.; Syed, Z.Q. One Health Surveillance System in Gujarat, India: A Health Policy and Systems Research Protocol for Exploring the Cross-Sectoral Collaborations to Detect Emerging Threats at the Human-Animal–Environment Interface. Trop. Med. Infect. Dis. 2023, 8, 428. https://doi.org/10.3390/tropicalmed8090428
Yasobant S, Saxena D, Tadvi R, Syed ZQ. One Health Surveillance System in Gujarat, India: A Health Policy and Systems Research Protocol for Exploring the Cross-Sectoral Collaborations to Detect Emerging Threats at the Human-Animal–Environment Interface. Tropical Medicine and Infectious Disease. 2023; 8(9):428. https://doi.org/10.3390/tropicalmed8090428
Chicago/Turabian StyleYasobant, Sandul, Deepak Saxena, Ravina Tadvi, and Zahiruddin Quazi Syed. 2023. "One Health Surveillance System in Gujarat, India: A Health Policy and Systems Research Protocol for Exploring the Cross-Sectoral Collaborations to Detect Emerging Threats at the Human-Animal–Environment Interface" Tropical Medicine and Infectious Disease 8, no. 9: 428. https://doi.org/10.3390/tropicalmed8090428
APA StyleYasobant, S., Saxena, D., Tadvi, R., & Syed, Z. Q. (2023). One Health Surveillance System in Gujarat, India: A Health Policy and Systems Research Protocol for Exploring the Cross-Sectoral Collaborations to Detect Emerging Threats at the Human-Animal–Environment Interface. Tropical Medicine and Infectious Disease, 8(9), 428. https://doi.org/10.3390/tropicalmed8090428








